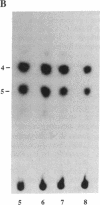
Abstract
The 5'----3' exonuclease activity of the thermostable enzyme Thermus aquaticus DNA polymerase may be employed in a polymerase chain reaction product detection system to generate a specific detectable signal concomitantly with amplification. An oligonucleotide probe, nonextendable at the 3' end, labeled at the 5' end, and designed to hybridize within the target sequence, is introduced into the polymerase chain reaction assay. Annealing of probe to one of the polymerase chain reaction product strands during the course of amplification generates a substrate suitable for exonuclease activity. During amplification, the 5'----3' exonuclease activity of T. aquaticus DNA polymerase degrades the probe into smaller fragments that can be differentiated from undegraded probe. The assay is sensitive and specific and is a significant improvement over more cumbersome detection methods.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberlain J. S., Gibbs R. A., Ranier J. E., Nguyen P. N., Caskey C. T. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988 Dec 9;16(23):11141–11156. doi: 10.1093/nar/16.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab F. F., Kan Y. W. Detection of specific DNA sequences by fluorescence amplification: a color complementation assay. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9178–9182. doi: 10.1073/pnas.86.23.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. Enzymic synthesis of DNA. 33. Hydrolysis of a 5'-triphosphate-terminated polynucleotide in the active center of DNA polymerase. J Mol Biol. 1969 Nov 14;45(3):513–531. doi: 10.1016/0022-2836(69)90309-x. [DOI] [PubMed] [Google Scholar]
- Hart C., Chang S. Y., Kwok S., Sninsky J., Ou C. Y., Schochetman G. A replication-deficient HIV-1 DNA used for quantitation of the polymerase chain reaction (PCR). Nucleic Acids Res. 1990 Jul 11;18(13):4029–4030. doi: 10.1093/nar/18.13.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Ehrlich G., Poiesz B., Kalish R., Sninsky J. J. Enzymatic amplification of HTLV-I viral sequences from peripheral blood mononuclear cells and infected tissues. Blood. 1988 Oct;72(4):1117–1123. [PubMed] [Google Scholar]
- Kwok S., Kellogg D. E., McKinney N., Spasic D., Goda L., Levenson C., Sninsky J. J. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 1990 Feb 25;18(4):999–1005. doi: 10.1093/nar/18.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist R. C., Olivera B. M. Transient generation of displaced single-stranded DNA during nick translation. Cell. 1982 Nov;31(1):53–60. doi: 10.1016/0092-8674(82)90404-4. [DOI] [PubMed] [Google Scholar]
- Miller H. Practical aspects of preparing phage and plasmid DNA: growth, maintenance, and storage of bacteria and bacteriophage. Methods Enzymol. 1987;152:145–170. doi: 10.1016/0076-6879(87)52016-x. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Ott J., Eckstein F. Protection of oligonucleotide primers against degradation by DNA polymerase I. Biochemistry. 1987 Dec 15;26(25):8237–8241. doi: 10.1021/bi00399a032. [DOI] [PubMed] [Google Scholar]
- Rayfield M., De Cock K., Heyward W., Goldstein L., Krebs J., Kwok S., Lee S., McCormick J., Moreau J. M., Odehouri K. Mixed human immunodeficiency virus (HIV) infection in an individual: demonstration of both HIV type 1 and type 2 proviral sequences by using polymerase chain reaction. J Infect Dis. 1988 Dec;158(6):1170–1176. doi: 10.1093/infdis/158.6.1170. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Walsh P. S., Levenson C. H., Erlich H. A. Genetic analysis of amplified DNA with immobilized sequence-specific oligonucleotide probes. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6230–6234. doi: 10.1073/pnas.86.16.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spector S. A., Rua J. A., Spector D. H., McMillan R. Detection of human cytomegalovirus in clinical specimens by DNA-DNA hybridization. J Infect Dis. 1984 Jul;150(1):121–126. doi: 10.1093/infdis/150.1.121. [DOI] [PubMed] [Google Scholar]