Abstract
Physiological responses to temperature are known to be a major determinant of species distributions and can dictate the sensitivity of populations to global warming. In contrast, little is known about how other major global change drivers, such as ocean acidification (OA), will shape species distributions in the future. Here, by integrating population genetics with experimental data for growth and mineralization, physiology and metabolomics, we demonstrate that the sensitivity of populations of the gastropod Littorina littorea to future OA is shaped by regional adaptation. Individuals from populations towards the edges of the natural latitudinal range in the Northeast Atlantic exhibit greater shell dissolution and the inability to upregulate their metabolism when exposed to low pH, thus appearing most sensitive to low seawater pH. Our results suggest that future levels of OA could mediate temperature-driven shifts in species distributions, thereby influencing future biogeography and the functioning of marine ecosystems.
Global warming is expected to lead to shifts in species' geographic ranges to track preferred temperatures. Here, the authors show that populations of the common periwinkle vary in their sensitivity to ocean acidification, another major global change driver, which could further restrict range shifts caused by warming.
Populations of the same species from different climatic regions often differ in their ability to withstand altered environmental conditions1,2. In the marine realm, diversification of populations along thermo-latitudinal gradients has been demonstrated for traits related to thermal physiology in several taxonomic groups1,2,3,4,5,6, dispelling the assumption that local adaptation in marine organisms is uncommon. However, there is little evidence as to whether a similar latitudinal trend in sensitivity exists for other environmental drivers, such as elevated partial pressure of CO2 (Pco2)7.
The anthropogenically driven increase in atmospheric Pco2 is causing a decrease in global ocean pH and altered carbonate chemistry8. In several marine invertebrate species, this combination of chemical changes, collectively known as ocean acidification (OA), has been shown to exert significant effects on organismal life history, ecology and behaviour9,10, and on fundamental physiological processes, resulting in imbalances in acid–base status, a reduction in aerobic scope and a shift in energy budget allocation11. These effects can reduce physiological performance, growth and reproductive output12 and can increase the risk of local extinction, as has been shown to occur in natural high Pco2 habitats13. These patterns appear to be partly explained by the ability of some taxa to colonize naturally elevated Pco2 areas by exhibiting greater metabolic control and capacity for acid–base regulation14,15,16. Although there is some evidence for interpopulation variation in the level of sensitivity to Pco2 (ref. 17) and adaptation potential to different Pco2 regimes18, there has been no large-scale integrative assessment of this variation along latitudinal gradients.
In the current study, we take an integrative approach using data from population genetics, population shell growth and mineralization, physiology and metabolomics to compare the OA responses of different populations of the common intertidal snail Littorina littorea (Linnaeus, 1758) sampled from its geographical range in the Northeast Atlantic (that is, from the Iberian Peninsula to the North of Norway19; see Fig. 1). Six populations were collected from three distinctive climatic regions: (1) warm temperate; (2) cold temperate; and (3) subpolar (Figs 1 and 2). Our results altogether suggest that different populations of L. littorea found across its geographical range of extension were differently sensitive to future OA conditions, with populations closer to the range edges being most sensitive. As a consequence, with increased OA, we may predict a further shift and possible reduction in the geographical range of L. littorea, with OA likely modulating in future the species response to ongoing warming. More generally, our study points to the fact that information from single population studies should be used with caution when aiming at predicting species global responses to global drivers. As a consequence, we may be currently over- or underestimating the impact of different environmental changes in different climatic regions, with this having important implications for the development of directives and policies to promote the preservation of marine biodiversity under the ongoing global change.
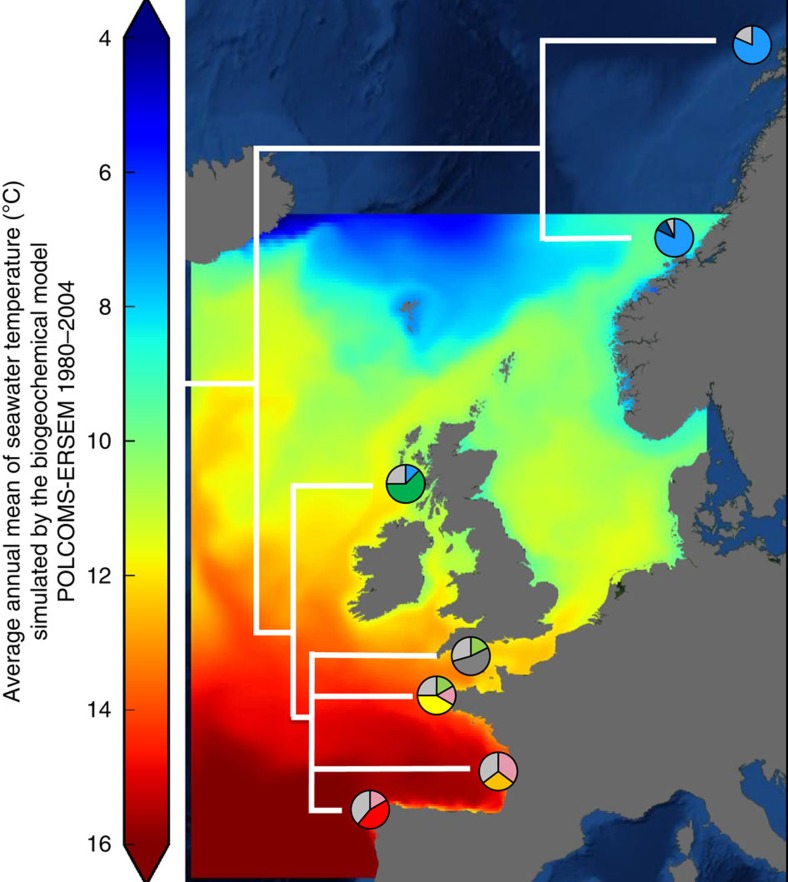
Figure 1. Genetic structure of Littorina littorea populations.
Thermal map of Europe from the POLCOMS-ERSEM biogeochemical model showing sampling locations and genetic structure of the six different populations of L. littorea sampled across the Northwest Atlantic latitudinal gradient (from 42° to 70°): pie chart position indicates location of collection of specimens, from south to north: Vigo (Spain), Île de Ré (France), Roscoff (France), Plymouth (UK), Millport (UK), Trondheim (Norway), Tromsø (Norway). Neighbour-joining population tree. Pie charts=proportion of common haplotypes (grey), shared between climatic regions (pastel colours, northern=blue, mid=green, southern=pink), private to individual sites (primary colours). Background image courtesy of NASA's Earth Observatory.
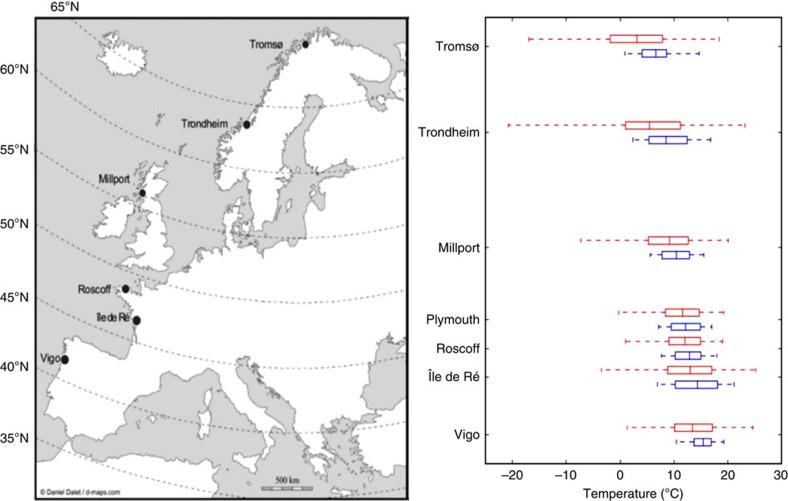
Figure 2. Collection locations for populations of Littorina littorea.
Map of Europe (left panel) with the locations (full circles) where populations of the common periwinkle Littorina littorea (Linnaeus, 1758) were collected from along the Western coastline of Europe during June to August 2010. Horizontal boxes indicate population mean values for temperature (central vertical line) of sea-surface temperature (SST): blue dashed line, and land-surface temperature (LST): red dashed line. The edges define the 25th and 75th percentiles, and error bars represent 95% CI. Temperature data were obtained from the National Oceanic and Atmospheric Administration/National Aeronautics and Space Administration (http://podaac.jpl.nasa.gov/) and comprised the mean annual SSTs for the 5-year period 2005–2009, measured across latitudes from 5.07 to 15.47 °C. Average annual (LSTs were obtained from World Climate (http://www.worldclimate.com/). Map outline courtesy of http://www.d-maps.com/.
Results
Sequence variability and population structure
Sequencing of a 598 bp section of COI from 238 individuals revealed 57 haplotypes (Supplementary Table 1). No saturation of substitution was detected in either the entire data set or third codon positions (ISS<ISS.C, P<0.001 in both cases, test of substitution saturation). Percent nucleotide composition was C: 21.9, T: 35.6, A: 22.2 and G: 20.2 and no insertions or deletions were detected. Base frequencies were found to be homologous among sequences at all sites (χ2=3.98, P=1.00, χ2 test), the 54 variable sites (χ2=21.59, P=1.00, χ2 test) and the third codon sites (χ2=0.13, P=1.00, χ2 test).
Overall nucleotide diversity and haplotype diversity were both low and did not differ substantially among sampling sites (Supplementary Table 1). Of the 57 haplotypes detected, two haplotypes (Ll2 and Ll4) were the most common and found at all sites, in most cases constituting over 45% of the total individuals surveyed at each site. However, in the case of the Norwegian sites, Ll1 was the most numerous haplotype present, comprising over 62% of the individuals. In addition, 36 of the 57 haplotypes identified were exclusive (private) to a single sampling site (Supplementary Fig. 1). All of the private haplotypes occurred at low numbers, with only a few individuals present at each sampling site.
The sampled populations had a high level of genetic structuring (Fig. 1 and Supplementary Figs 1 and 2), with a clear genetic difference between the two subpolar and all other sites (FST values, P<0.05, analysis of molecular variance (AMOVA) test; Supplementary Table 2) and between the climatic regions (ΦCT=0.140, P<0.005, AMOVA test; Supplementary Table 3).
Metabolic responses under current P CO2 conditions
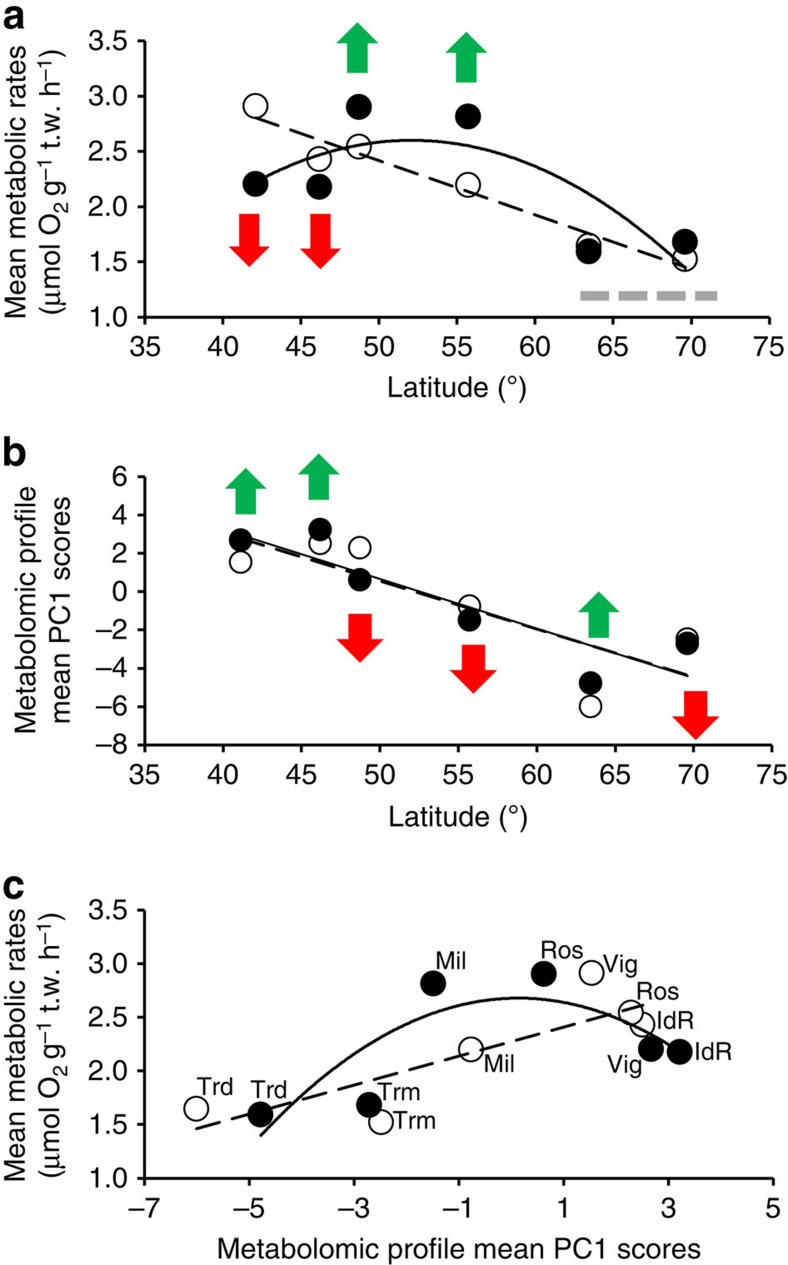
These genetic differences appeared to be related to the physiology of populations. Snails maintained under current Pco2 conditions showed a linear decline in mean metabolic rates (Fig. 3a) and mean metabolomic profiles (Figs 3c, 4 and 5) with increasing latitude. In addition, the presence of a positive linear relationship between mean population metabolomic PC1 scores and metabolic rate under control PCO2 conditions (r5=0.084, P=0.019, best-fit regression approach, see Fig. 3) suggests the existence of a clear mechanistic link between cellular and whole-organism responses (Fig. 3c). This relationship suggests the adaptation of the cellular physiology underpinning the observed whole-organism metabolic rate adaptation along the latitudinal cline investigated (Fig. 3a). The presence of a linear positive relationship between mean population metabolic rate and ATP+ADP levels under control PCO2 (r5=0.77, P=0.041, best-fit regression approach, y=427.820 × −1.078) further corroborate this idea, specifically suggesting the existence of a positive relationship between metabolic rate levels and cellular energy levels20. Subpolar (Norwegian) populations had the most divergent metabolomic profiles (Fig. 4a,b) with lower concentrations of homarine and ATP+ADP but higher concentrations of leucine, valine, glutamate, glutamine and formate compared with other sites (for formate and ATP+ADP see Fig. 5b,e). Amino acids are used extensively in energy metabolism21 and can function as an alternative carbon source in the citric acid cycle when ATP has been exhausted. The ratios of adenylates and amino acids seen here could indicate changes that have occurred in the underlying metabolic machinery of northern populations that have allowed snails to adapt metabolically to cooler climates (Fig. 3a). Furthermore, in marine molluscs, homarine and branched chain amino acids (such as leucine and valine) also underpin processes related to acid–base balance and immune function that are intertwined with energy metabolism. In comparison, glutamate has been highlighted as a possible excitatory neurotransmitter in molluscs22. Thus, our data suggest that compared with more southerly populations, subpolar snails significantly upregulate a host of physiological systems, including metabolism and acid–base balance and immune and neurological function to help maintain homeostasis. In summary, the trends observed under present-day PCO2 levels suggest that populations are metabolically adapted to the different environmental conditions found along the latitudinal cline investigated (Figs 1 and 3a,b)1,2,3.
Figure 3. Physiological responses of Littorina littorea populations.
(a) Mean metabolic rate plotted against the latitude of each population, current Pco2, best-fit regression approach r5=0.97, P<0.001, y=−0.049x+4.880, elevated Pco2, r5=0.76, P=0.047, y=−0.004x2+0.393x−7.644; population collection location latitudinal position provided along the x axis. (b) Mean metabolomic profile PC1 scores plotted against the latitude of each population, current Pco2, best-fit regression approach r5=0.82, P=0.024, y=−0.250x+13.058, elevated Pco2, r5=0.89, P=0.007, y=−0.258x+13.563; population collection location latitudinal position provided along the x axis. (c) Mean metabolic rate plotted against the metabolomics profile (PC1 score) of each population, current Pco2, best-fit regression approach r5=0.84, P=0.019, y=−0.135x+2.272, elevated Pco2, r5=0.89, P=0.007, y=−0.053x2+0.014x+2.677. Populations: Vig-Vigo, IdR-Île de Ré, Ros-Roscoff, Mil-Millport, Trd-Trondheim, Trm-Tromsø (n=6). White circles and dotted line indicate current Pco2 and black circles and full line indicate elevated Pco2. Arrows indicate within-population significant increases (green), decreases (red) (P<0.05) or no difference (grey) between different Pco2 using post hoc Bonferroni test of one-way ANOVA (P<0.05), according to 95% confidence interval test for estimate marginal means (EMM) with Bonferroni correction as reported by Melatunan59; t.w. in y-axis labels in a and c stands for ‘tissue wet'.
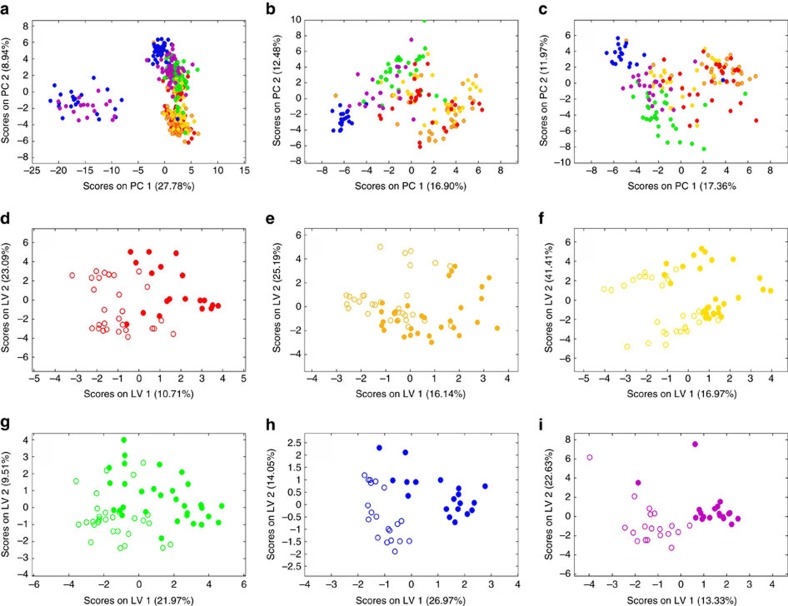
Figure 4. Metabolomic profiling of Littorina littorea populations.
PCA score plots for (a) all populations (both Pco2) (Vigo-red, Île de Ré-orange, Roscoff-yellow, Millport-green, Trondheim-blue, Tromsø-purple). (b) Control Pco2. (c) Elevated Pco2. (d) Vigo, (e) Île de Ré, (f) Roscoff, (g) Millport, (h)Trondheim, (i)Tromsø PLS-DA score plots for forward selected metabolomic profiles for each population (individual data, n=30). Open circles indicate current Pco2 and filled circles indicate elevated Pco2.
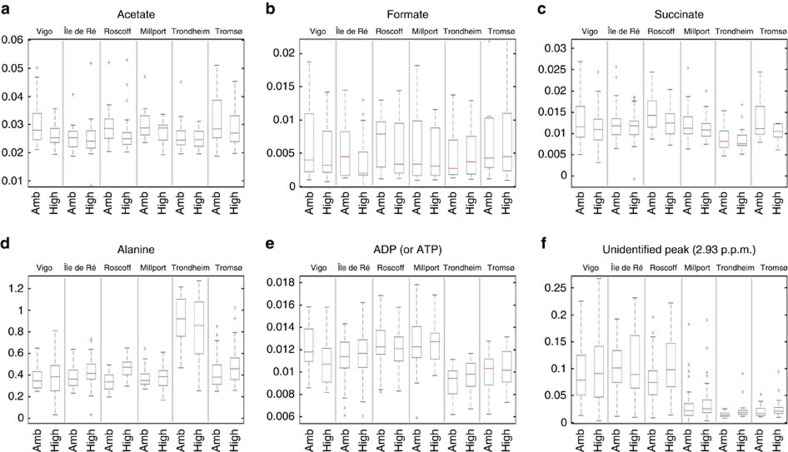
Figure 5. Metabolite concentrations of Littorina littorea populations.
(a) Acetate, (b) formate, (c) succinate, (d) alanine, (e) ADP (or ATP), (f) unidentified peak at 2.93 p.p.m. Boxplots describing the integrated peak areas for peaks of interest from each location sampled at different Pco2 conditions. Vertical boxes indicate population mean values for metabolites (central horizontal line), the edges define the 25th and 75th percentiles and error bars represent 95% CI. The symbol ‘+' represents outlier data. The y axis is given in mmol−1.
Metabolic responses under elevated P CO2 conditions
Overall, the metabolic rate response under elevated PCO2 was nonlinear, with warm-temperate populations (Vigo and Île de Ré) showing a strong downregulation of metabolic rates, cold temperate (Roscoff and Millport) showing upregulation and subpolar (Trondheim and Tromsø) showing no overall differences (Fig. 3a). These results suggest that population levels of metabolic adaptation to prevalent regional environmental conditions lead to different sensitivity to elevated Pco2 conditions, with populations found towards the range edges being more sensitive. Similarly, a greater degree of sensitivity to elevated Pco2 has been proposed for stenotherms compared with eurytherms, based on their different levels of thermal physiological adaptation23. In contrast to the pattern observed for metabolic rates, the latitudinal trend in metabolomic profiles under elevated Pco2 was identical to that observed under control PCO2 (Fig. 3b). In addition, the presence of a linear positive relationship between mean population metabolic rate and ATP+ADP levels under elevated PCO2 (r5=0.95, P=0.001, best-fit regression approach, y=691.970x−2.949), suggests that the positive relationship found under control PCO2 conditions between metabolic rate levels and cellular energy levels does not fundamentally change under future OA conditions across the latitudinal range of L. littorina. However, a positive binomial relationship was found between mean population metabolomic PC1 scores and metabolic rate under elevated PCO2 (r5=0.79, P=0.007, best-fit regression approach, see Fig. 3c), suggesting that the mechanistic relationship observed under control PCO2 conditions between the cellular and whole-organism compartments may change under future OA conditions. Some metabolomic differences for warm-temperate sites were also highlighted by partial least-squares discriminant analysis (PLS-DA) (Fig. 4d–i) under elevated Pco2: the Île de Ré population showed a significant decrease in acetate, an essential ingredient for the formation of the co-enzyme acetyl-co-A that is known to be implicated in anaerobic metabolism of organisms at their thermal limits, and enables energy production24; the Vigo population showed a significant decrease in ATP (or ADP) concentrations that is of fundamental importance for central metabolism25.
Shell responses under different P CO2 conditions
In order to determine whether differences in metabolic rates and metabolomic profiles following exposure to OA conditions were accompanied by changes in life-history responses26, growth and (empty) shell dissolution and mineralization status27 were examined in the six populations investigated (Fig. 6, Supplementary Figs 3–5 and Supplementary Table 4). Under current Pco2 conditions there was no relationship between growth rate and latitude (Fig. 6a). In contrast, under elevated Pco2, mean growth was significantly negative (between −0.45 and −3.60%) across all populations (Fig. 6a), with this decrease being most pronounced in subpolar and warm-temperate populations (mean reduction −3.25%) compared with cold-temperate populations (−0.46%). Tests of mean passive dissolution on empty shells showed that dissolution and net growth levels were generally comparable in warm- and cold-temperate populations, but were 2.5–4 times greater in subpolar populations (Fig. 6b). It should be noted that differences in the amount of mean shell loss across populations (Fig. 6b) cannot be wholly explained on the basis of differences in mean shell mineral status (that is, original concentration and ratios for shell Ca2+, Mg2+ and Sr2+) nor responses to elevated PCO2 (Supplementary Figs 3–5). Exposure to elevated PCO2 led to a change in empty shell mineral content of L. littorea, although responses varied among populations (see Supplementary Fig. 3), as indicated by the presence of a significant interaction between the factors ‘latitudinal position' and ‘PCO2' for [Ca2+] and [Sr2+] (see Supplementary Table 4). In more detail, snails from Roscoff (48.73°) showed the lowest [Sr2+] following exposure to elevated PCO2, whereas snails in Trondheim (63.45°) showed the least change. In addition, the populations in Millport (55.73°) and Tromsø (69.62°) showed the highest [Ca2+] after exposure to PCO2, whereas snails from Roscoff (48.73°) showed the least change. Shell [Mg2+] and [K+] were significantly lower in snail exposed to elevated PCO2 and both significantly decreased with increasing latitude (Supplementary Fig. 3 and Supplementary Table 4). The ratio [Mg2+]:[Ca2+] and [Ca2+]:[Sr2+] in shells of L. littorea decreased and increased significantly following exposure to elevated PCO2 respectively, but differently in different population as indicated by the presence of a significant interaction between the factors ‘latitudinal position' and ‘PCO2' (see Supplementary Table 4). In addition, the [Mg2+]:[Sr2+] ratio showed a significant decrease with increasing latitude, equally for control and acidified conditions (Supplementary Fig. 4 and Supplementary Table 4). Finally, the presence of a significantly negative relationship between [ATP] and shell [Mg2+], [K+], [Mg2+]:[Ca2+] and [Mg2+]:[Sr2+] (Supplementary Fig. 5), which appeared comparable at both control and elevated PCO2 conditions, could suggest that differences may exist in the way shells are built and in the costs associated with mineralization in each of the different populations of L. littorea (Fig. 6).
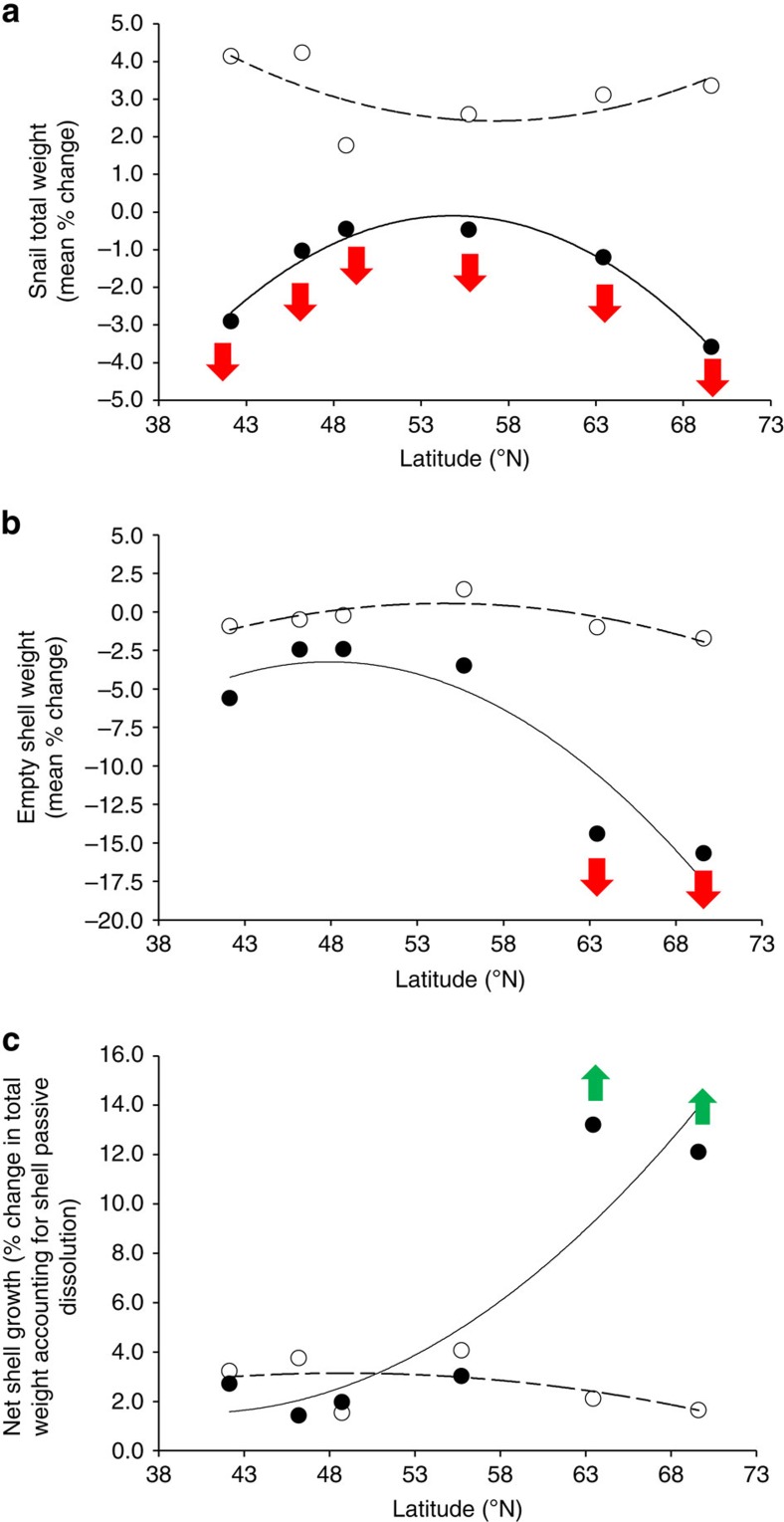
Figure 6. Relationship between latitude and growth of L. littorea populations.
(a) Mean total snail weight, current Pco2, best-fit regression approach r5=0.66, P=0.104, y=0.008x2−0.867x+27.242, elevated Pco2, r5=0.98, P<0.001, y=−0.016x2+1.753x−48.143. (b) Mean empty shell weight, current Pco2, best-fit regression approach r5=0.85, P=0.016, y=−0.011x2+1.210x−32.369, elevated Pco2, r5=0.93, P=0.002, y=−0.030x2+2.910x−72.874. (c) Growth effort (mean total snail weight–mean empty shell weight), current Pco2, best-fit regression approach r5=0.54, P=0.212, y=−0.004x2+0.340x−5.130, elevated Pco2, r5=0.91, P=0.004, y=0.010x2−1.150x+24.730. Population mean data, n=6; population collection location latitudinal position provided along the x axis. White circles and dotted line indicate current Pco2 and black circles and full line indicate elevated Pco2. Arrows indicate within-population significant increases (green) or decreases (red) (P<0.05, ANOVA test) between current and elevated Pco2, and maximum divergence from control conditions observed among all populations using post hoc Bonferroni test of one-way ANOVA (P<0.05), according to 95% confidence interval test for estimate marginal means (EMM) with Bonferroni correction as reported by Melatunan59.
Nonetheless, the increased shell dissolution rates, as a result of the exposure to sea water with low carbonate saturation states26, appear to eclipse any potential increase in calcium carbonate deposition resulting in a negative net calcification rate (Fig. 6b)—a mechanism that could explain the negative growth reported under OA conditions. Calculations of population net growth showed that this was 4–8 times greater in subpolar populations compared with the other two climatic regions (Fig. 6c), suggesting that the former upregulate shell mineralization to compensate for extreme levels of shell dissolution as a response to exposure to corrosive waters28 and, likely, with energetic and metabolic implications20,26,29.
Adaptive significance of physiological and life-history responses
The adaptive significance of the physiological and life-history responses of L. littorea populations is supported by the observation that ∼50% of each population comprises unique haplotypes (Fig. 1, Supplementary Figs 1 and 2 and Supplementary Tables 1–3)18. Adaptation to the warm and cold extremes of a species range appears to cause distinctive stress responses to future OA, confirming the idea that different levels of sensitivity of marine organisms to environmental change is likely based on extant differences in thermal physiological adaptation23,30. Under high Pco2, warm-temperate populations decreased metabolic rates (Fig. 3b) and had a reduced capacity for growth (Fig. 6a) that appear to be due to a reduction in energy metabolism, but could also be caused by an increase in maintenance costs20,26 and/or by homeostatic tradeoffs between energy metabolism and other fundamental functions. Metabolic depression has evolved as a strategy to minimize the negative effects of acute and chronic environmental extremes, including extreme and variable levels of pH (refs 31 and 32). In contrast, subpolar populations maintained their metabolic rates unchanged under OA conditions (Fig. 3b), but increased their mineralization effort, likely to compensate for a greater than threefold level of shell passive dissolution than that detected in other populations (Fig. 6c). These processes appear to be associated with altered metabolic functions, as suggested by the distinctiveness of the metabolomes of the sub-polar populations' (Figs 4 and 5), and it is reasonable to believe it is accompanied by a reallocation of the snails' energy budget largely towards growth and mineralization. Functional analyses of transcriptomes of sea urchins collected along a latitudinal pH gradient in the northeast Pacific have confirmed similar molecular patterns for energetically demanding, homeostatic processes in more northerly locations7. In contrast, cold-temperate populations showed metabolic rate upregulation when exposed to elevated Pco2 conditions (Fig. 3b). Such latitudinal differences in metabolic ‘strategies' may help explain the observed reduced growth towards range edges (Fig. 6b). Exposure to OA was shown to cause a reduction in the energy metabolism of L. littorina20, and it has been shown that such reductions can lead to a reallocation of the energy budget away from fundamental fitness-related functions, such as growth29, resulting in smaller adults16,33. More in general, our study indicates that a greater physiological ability to deal with environmental extremes may help in retaining higher fitness levels, and thus a species' geographical distribution in the face of environmental changes2,34, including in the context of OA14,15,16,35.
Discussion
Together, the findings of this study suggest that the relative sensitivity of different populations of L. littorea to future OA are likely to vary considerably across its geographical range of extension in the Northeast Atlantic through local and regional adaptation, with populations closer to the range edges being most sensitive. If OA selects against these sensitive, range-edge genotypes, it could cause a reduction of genetic diversity levels that could have far-reaching consequences for the ability of these populations to respond and further adapt to other local and global stressors. As a consequence, with increased OA, we may predict a further shift and possible reduction in the geographical range of L. littorea, as genotypes closer to the range edge appear at greater risk of extinction based on their specific metabolic abilities36. These genotypes could be replaced by central population genotypes, particularly towards the northern edge where snails appear to be metabolically less competent to deal with OA. Furthermore, the negative relationship between seawater pH and latitude37 may mean that southern genotypes would require to migrate southward in order to compensate for the negative effects of OA8, although this seems unlikely given the negative impact of global warming that has already caused a northward migration of L. littorea populations at this species' southern range edge38. In general, the negative effects of OA on L. littorea, a keystone species in rocky intertidal habitats, may have negative repercussions on the structure, dynamics and functioning of intertidal communities and ecosystems across the North Atlantic39,40, particularly towards the range edges of its distribution.
In conclusion, single population studies should be used with caution when aiming to predict species and community responses to global environmental drivers, such as OA, as phenotypic variation between populations may be considerable. As a consequence, modellers, conservationists, environmental managers, stakeholders and policy makers should be aware of the pitfalls of developing predictions, directives and policies solely based on single population observations, as has so far been the case41,42. Our results show that the effects of environmental changes on species may currently be over- or underestimated in different regions, particularly as, historically, most research is conducted in cold-temperate areas43, despite polar and tropical species remaining the most vulnerable to climate change44. The identification of which populations and genotypes will be more resilient to global change and which ones will be most at risk of extinction will improve conservation efforts and management of natural resources, if marine biodiversity and ecosystem functions are ultimately to be preserved39,45. This will be facilitated by the use of an integrated approach, as demonstrated in this study.
Methods
Species distribution and specimen collection
The common periwinkle L. littorea (Linnaeus, 1758) is an intertidal gastropod that has a wide latitudinal distribution along the coasts of the North Atlantic, between 42 and 70°N in the eastern North Atlantic (from the Iberian peninsula to the White Sea) and between 38 and 41°N in the Western North Atlantic (from southern New Jersey to northern Labrador)19,46,47.
A total of six populations living along the European coastline of the north-east Atlantic from three distinctive climatic regions (that is, (1) warm temperate, (2) cold temperate and (3) subpolar) were sampled for this study covering a latitudinal range of 28° (42–70°), from Vigo in northern Spain to Tromsø in northern Norway, a distance of 3,780 km (Figs 1 and 2). During June–September 2010, adults of L. littorea were collected by hand from the intertidal zone of rocky shores at low tide. Approximately 250 adult individuals (shell width 12–15 mm) were collected per site and transported to the Marine Biology and Ecology Research Centre at Plymouth University (Plymouth, UK) within 48 h of collection. Before transport, live snails were placed into rectangular plastic containers (20 × 20 × 12 cm) with a perforated lid with 25 holes (ø 10 mm) to allow air circulation and reduce overheating during transportation. Approximately 50–75 individual snails were placed in each container with damp seaweed (Fucus serratus) to prevent desiccation and mechanical damage. No mortality was recorded during transport and snails were in good condition with normal activity levels upon arrival at the laboratory. Once in the laboratory, snails and algae were transferred to aerated filtered sea water (pH 8.01, salinity 35, 15 °C) and maintained for 14 days in four plastic aquaria (56 l, a maximum of 75 individuals per aquarium) before transfer to the experimental setup. Fourteen days of exposure to laboratory conditions was considered sufficient to standardize the snails' physiological condition following any stress from collection and transportation, and to minimize any ‘environmental signature' resulting from the local conditions at the collection site. Specimens were fed every second day ad libitum on F. serratus and 50% seawater volume was replaced every 4 days to maintain stable salinity and pH conditions and to eliminate any accumulated ammonia20.
All specimens were kept in a temperature-controlled room (12:12 h light/dark regime) at 15 °C. Despite the genetic distinctiveness of the populations of L. littorina investigated (Fig. 1), we cannot discount the possibility that the patterns observed in the snails' physiology were generated by exposing northern populations at an experimental temperature (15 °C) that is at the upper end of their natural range of seawater temperatures experienced (particularly for the Tromsø population), but not of air temperatures (Fig. 2). Both these Norwegian populations possess metabolomics fingerprints and metabolic rates (Fig. 3a,b) that are similar to each other, despite snails from these two populations experiencing thermal regimes that are somewhat different (Fig. 2). This suggests that the fairly significant temperature difference between these sites ultimately does not influence their metabolic responses to elevated PCO2, adding weight to the idea that the use of 15 °C did not cause bias by stressing the northernmost population.
Because of logistical limitations, population collection was staggered by a week so that no more than two populations were present in the laboratory at the same time. Where possible, collection was organized from south to north in an attempt to collect snails under comparable environmental conditions.
Experimental design and CO2 manipulation system
A factorial experimental design incorporating two levels of seawater PCO2 representing current conditions (385 μatm, pH 8.0) and predicted OA conditions for the year 2100 (1000 μatm, pH 7.6)8 was used to investigate the metabolic and metabolomics responses of the snails, and shell dissolution and mineralization to elevated PCO2 conditions in the selected populations.
For each population, individual snails were haphazardly allocated to each treatment (120 individuals per PCO2 treatment per population) and exposed for 14 days to control laboratory conditions to recover from transport and to adjust to laboratory conditions, before exposure for 14 days to experimental conditions. A CO2/air equilibration microcosm was set up for each treatment in a temperature-controlled room maintained at 15 °C (12 h:12 h, light/dark)20. Briefly, each CO2 equilibration system consisted of a header tank (80 l) in which the sea water was either aerated by bubbling normal air or acidified by bubbling pure CO2 gas. From each of the header tanks sea water was continuously gravity fed (600 ml min−1) to one of four holding tanks (60 × 35 × 15 cm, 32 l), containing each 30 plastic pots (80 ml). Plastic pots were each provided with 30 holes (4 mm in diameter) to ensure good water circulation, and containing a single snail (shell width 13.67±1.40 mm, mean±s.d.). The excess water from the four holding tanks flowed into a larger holding tray (simply used to help maintaining temperature stable). From the two holding trays excess water flowed into a sump (a large plastic container size 45 × 36 × 35 cm, 56 l), aerated vigorously to ensure CO2 degasification, and recirculated via a submersible pump (EP68, Hengtong Aquarium Co. Ltd, Hengtong, Taiwan) to both header tanks. CO2 gas was released into the header tank using a multi-stage CO2 regulator (EN ISO 7291, GCE, Worksop, UK) connected to a flip-flop control solenoid valve (ORIFICE 3/16 Closed System, Peter Paul Electronics Co. Inc., New Britain, CT, USA) controlled by a calibrated pH controller (pH-201 Digital, Dream Reef, Humberston, UK). Seawater temperature in the holding tanks was maintained at 15 °C in the temperature-controlled room where the experiment was conducted.
Aquaria environment monitoring and carbonate system profiles
Temperature, salinity and pHNBS of sea water in aquaria were measured daily during the experimental period using, respectively, a digital thermometer equipped with a thermocouple (HH806AU, Omega Ltd, Manchester, UK), a handheld refractometer (TA 197 LFMulti350, WTW, Weilheim, Germany) and a pH microelectrode (Seven Easy pH InLab, Mettler-Toledo Ltd, Beaumont Leys, UK) maintained at ambient seawater temperature coupled to a pH meter (Sevengo, Mettler-Toledo Ltd) calibrated using pH standards (pHNBS 4.01, 7.00 and 9.21 at 25 °C, Mettler-Toledo Ltd), also maintained at ambient seawater temperature (Supplementary Table 5). To determine seawater total alkalinity, samples of seawater (volume=120 ml) were also collected daily and transferred to borosilicate bottles with Teflon caps, poisoned with 30 μl of saturated HgCl2 solution (0.02% sample volume) and kept in the dark until measured by Gran titration (AS-ALK2, Apollo SciTech, Bogart, GA, USA)20.
Dissolved inorganic carbon, PCO2, calcite and aragonite saturation (Ωcalc and Ωara), bicarbonate and carbonate ion concentration ([HCO3–] and [CO32–]) were calculated from pH and total alkalinity measurements using the software program CO2SYS48 with dissociation constants49 refitted by50 and [KSO4] using51 (Supplementary Table 5).
Shell growth and empty shell passive dissolution
In order to determine shell growth under different PCO2 conditions the growth of the shell of 18 to 21 individuals per population per treatment was calculated as the proportional change between total weight measurements immediately before and at the end of 14 days of exposure. Snail shells were blotted dry using laboratory tissue paper, and specimens were placed aperture down for 10 min (ref. 52) to allow any remaining water to be expelled via gravity and absorbed with the use of a cotton bud by pressing down gently on the operculum. Each individual was then placed into a plastic vial and weighed using an analytical digital balance (Sartorius 1201 MP2, DWS Inc., Elk Grove, CA, USA)20. The percentage change in total weight of intact snails over the experimental exposure was calculated as a proxy for growth.
In order to determine empty shell passive dissolution under different PCO2 conditions, 30 snails were euthanized per population and 15 individuals were assigned to each PCO2 treatment, and shell passive dissolution was calculated as the proportional change between total weight measurements immediately before and at the end of 14 days of exposure. Tissues were immediately carefully removed to avoid damaging the shells that were blotted dry using laboratory tissue paper and placed aperture down for 10 min (ref. 52) to allow any remaining water to be expelled via gravity and absorbed with the use of a cotton bud by pressing down gently on the operculum. Each individual was then placed into a plastic vial and weighed using an analytical digital balance (Sartorius 1201 MP2, DWS Inc.)20. The percentage change in total weight of empty shells over the experimental exposure was calculated as a proxy for growth. This measure may represent an overestimate of snail shell passive dissolution but it still represents a reliable standardized estimate for this parameter27.
Metabolic rate determination
Metabolic rates of individual snails were measured at the end of exposure period (14 days) using O2 uptake as a proxy16. In order to collect metabolic rate data representative of both the spatiotemporal environmental variability that littorinids are exposed to and their physiological capacity during the phase of maximum stress for their respiratory physiology (that is, low tide), groups of six snails were selected haphazardly from each experimental tank (giving a total of 30 individuals per PCO2 treatment per population combination) and exposed in air in a custom-made incubator (Perspective cube, size 60 × 60 × 50 cm) equipped with an automatic heater (Microclimate Advance Heating System, 500 W, max heat 70 °C, Net Pet Shop Limited, Manchester, UK) that was set to produce one of five temperatures (15, 20, 25, 30 and 35 °C). Exposure lasted for 3 h to mimic an average tide duration. The CO2 in the incubator was adjusted manually according to the level of PCO2 in the treatment conditions either in 385 or 1000 μatm. Up- and downregulation of PCO2 levels was guided through a gas analyser (LI-7000 CO2/H2O, LI-COR Environmental Ltd, Cambridge, UK) connected to a PC. Following exposure to air each individual snail was placed in a blacked out glass jar (70 ml) for 5 min that was then submersed in a glass aquarium (49 × 23 × 28 cm, capacity 32 l) containing sea water at 15 °C. Initial O2 uptake was recorded just before the jar was sealed and after 1 h of incubation using a calibrated O2 meter (Model 781, Strathkelvin Instruments, Glasgow, UK) with an O2 electrode (1302 electrode, Strathkelvin Instruments). O2 uptake was expressed as μmol O2 g−1 wet weight h−1.
Metabolic profiling
To assess whether the metabolic profile of snails was influenced by exposure to elevated PCO2, 30 individuals from each of the two PCO2 treatments per population were selected for metabolomics analysis. Immediately following oxygen consumption trials, tissue samples (0.20–0.25 g) of foot muscle were dissected after the shell of each individual was quickly cracked, using a mini grip clamp (Quick-Grip Bar Clamp Q/G5122QC, Hyquip Ltd, Lancashire, UK), and removed. Shells were cleaned in fresh sea water, placed in a labelled plastic vial (Eppendorf tube 1.5 ml, Sigma-Aldrich Co. LLC., Dorset, UK), frozen in liquid nitrogen (temperature of −196 °C) and stored in a −80 °C freezer.
Metabolites were extracted from tissues and analysed using nuclear magnetic resonance (NMR) spectroscopy-based metabolomics, conducted at the Natural Environment Research Council (NERC) Biomolecular Analysis Facility at the University of Birmingham (NBAF-B). Twelve foot muscles were pooled from sample trays (two foot muscles per tray, in which each tray composed frozen foot tissues from one location), cut into small pieces (60–100 mg) and transferred to pre-cooled Precellys tubes containing steel balls (Precellys tube, cap. 7 ml, Bertin Technologies Corp., Saint Quentin en Yvelines Cedex, France). All procedures were carried out in <10 s. Next, the metabolites were extracted using a two-step solvent extraction protocol as described previously53. In brief, MeOH/H2O (HPLC grade) was added to frozen tissue and homogenized using a Precellys-24 bead-based homogenizer (Stretton Scientific, Stratton, UK; 6,800 r.p.m., 2 × 20 s cycles). Homogenized samples were transferred to 1.8 ml glass vials and then CHCl3 (pesticide grade) and H2O were added. Samples were vortexed for 30 s, cooled in an ice bath for 10 min and then centrifuged (Centrifuge Biofuge Primo-CFH-240–010A, Thermo Scientific Heraeus Corp., Leicestershire, UK; 4,000 g, 10 min, 4 °C). Polar and nonpolar phases were transferred to clean glass vials using a Hamilton syringe and then the polar fractions (400 μl) were dried in a rotary evaporator (SpeedVac SPD111VP115, Thermo Scientific Corp., Surrey, UK) and stored at −80 °C until analysis.
All 360 dried polar extracts were analysed using two-dimensional 1H, 1H J-resolved NMR spectroscopy-based metabolomics (2D pJRES)54,55. First, each dried extract was resuspended in sodium phosphate buffer in 90% H2O and 10% D2O (0.1 M, pH 7.0) containing 0.5 mmol−1 2,2,3,3-d4-3-(trimethylsilyl)propionic acid (TMSP) (GOSS Scientific Instruments). The 2D J-resolved NMR spectra were recorded at 500.11 MHz using a Bruker Avance DRX-500 (Bruker, Germany). Spectra were acquired using 8 transients per increment collected into 16k datapoints and a total of 32 increments. Data sets were zero-filled to 128 points in F1. The SEM window function was applied in the direct (chemical shift) dimension and sine-bell in the indirect dimension before Fourier transformation. The chemical shift axis was calibrated to TMSP at 0.0 p.p.m. and the skyline projections (pJRES) calculated54,55. All processing was conducted using TopSpin v3.0 (Bruker).
The residual water signal was excluded (4.38–5.22 p.p.m.) and the spectra were binned between 10 and 0.2 p.p.m. (bin width of 0.005 p.p.m.). Next, spectra were normalized using probabilistic quotient normalization and noise filtered (with noise threshold set to 3 times the s.d. of a region of known noise at 9.5–10 p.p.m.). For a bin to be retained, it was required that at least 10 samples have signal above this threshold, resulting in 1,103 bins. Finally, spectra were glog transformed (lambda=1.38 e−9). To assess the technical reproducibility of the analysis, the median relative s.d. for six technical replicates of one biological sample was calculated, equating to 12.7%, comparable with earlier analyses56. The relative concentrations of metabolites in the samples were calculated using the web-based automated identification and quantification data mining tool FIMA (Field Independent Metabolite Analysis), developed at the University of Birmingham (http://www.bml-nmr.org/). This software package uses a library of metabolite spectra55 and outputs a vector of relative metabolite concentrations for each sample.
Determination of shell mineral content
The mineral content of empty shells (see above) (for Ca2+, Mg2+, Sr2+, K+) of adult individuals of L. littorea exposed for 14 days to different PCO2 conditions was determined using an optical emission spectrometry technique. Shell fragments were meticulously cleaned of all tissue under low-power magnification (10–50, SZXI6 binocular microscope, Olympus, Tokyo) using only plastic or Teflon-coated dissection tools. Shell fragments were then weighed with a high-precision balance (AT201, Mettler-Toledo; degree of precision (dp) 0.01 mg) before being freeze-dried for 24 h (Modulyo freeze drier, Thermo Electron, Waltham, MA, USA) at −50 °C. The dry mass of each freeze-dried set of shell fragments was determined with a high-precision balance (AT201, Mettler-Toledo; dp 0.01 mg) before being digested in 2 ml nitric acid (79% concentration, trace analysis grade) in a microwave digestion unit (MarsXpress, CEM, Matthews, NC, USA). Digests were then diluted to 10 ml with ultrapure water and analysed for [Ca2+], [Mg2+] and [Sr2+] with an ICP-Optical Emission Spectrometer (Varian 725-ES, Agilent Technologies, Santa Clara, CA). Shell mineral content is expressed as mmol kg−1. Shell mineral content was determined for the populations of Île de Ré, Roscoff, Millport, Trondheim and Tromsø.
Genetic analyses
Genetic analyses utilized foot tissue from another 35 specimens of L. littorea collected per site in June–September 2010: Tromsø, Norway (Trm) (n=32), Trondheim, Norway (Trd) (n=35), Millport, UK (Mil) (n=36), Plymouth, UK (Ply) (n=34), Roscoff, France (Ros) (n=35), Île de Ré, France (IdR) (n=33) and Vigo, Spain (Vig) (n=33). Whole snails were preserved in 98% ethanol, shipped to the United Kingdom and stored at −20 °C before DNA extraction was carried out.
Total genomic DNA was isolated using a modified CTAB protocol57. Following DNA extraction, a fragment (∼600 base pairs) of the COI gene was amplified using two universal primers (that is, LCOI490, 5′-GGTCAACAAATCATAAAGATATTGG-3′ and HCO2198, 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′)58 using a standard PCR protocol. Sequencing was carried out using the same primers as the initial PCR reactions by Macrogen Inc. (Amsterdam, The Netherlands). Both forward and reverse sequences were generated.
Statistical analyses
The relationships between snail metabolic rates, metabolomics profiles and snail growth, empty shell dissolution and mineralization and their latitudinal position along the gradient examined, both under control and elevated PCO2 conditions, were investigated using a best-fit regression approach controlling for both R2 and Akaike information criterion values using a correlative approach59. More specifically, only best-fit models are shown. The relationship between latitude and the study variables was investigated under control conditions to shed light on the presence or absence of adaptation to current conditions in the populations investigated, and under future elevated PCO2 conditions to determine the relative sensitivity of the different populations to future PCO2 conditions.
Analyses on shell ion concentrations were conducted using three-way analysis of covariance tests, using the term ‘PCO2' and ‘latitudinal position' as fixed factors, whereas the term ‘tank' was set as a random factor that was nested within the PCO2 treatment. In addition, ‘snail total weight' or ‘tissue weight' were set as covariate. Data always met the assumptions for normality of distribution (in some cases following transformation) (minimum Z176=1.290, P=0.072, Shapiro–Wilk test) and homogeneity of variances (minimum F1,178=0.803, P=0.525, Leven test). The term ‘tank' was found to largely have no significant effect on the study variables, but even where it was found to cause a significant effect, removing it from the analyses caused no change to the significance patterns, and thus tank effect was considered marginal and ultimately removed from analyses. Finally, the relationship between the concentration of ATP and shell mineral content (see details on their estimation below) was explored with Pearson's correlation test26.
Principal components analyses of the processed pJRES NMR data were performed to assess the broad-scale variation between the various groups (three distinctive climatic regions: (1) warm temperate; (2) cold temperate; and (3) sub-polar, ambient and elevated PCO2 regimes, six field sites) considering all of the metabolites simultaneously. Principal components analyses were conducted using the PLS_Toolbox (version 5.5.1, Eigenvector Research, Manson, WA, USA) within Matlab (version 7.8; The MathsWorks, Natick, MA, USA) following mean centring of the processed NMR data. In order to discover the metabolic responses of the periwinkles to elevated PCO2, supervised multivariate analyses were performed using PLS-DA in the PLS_Toolbox. PLS-DA uses prior knowledge of sample group to maximize separation of the metabolic fingerprints of different groups and derive predictive models. Here, internal cross-validation and permutation testing were used (please see below) to define robust classification error rates associated with the prediction of a metabolic response. Results are presented as ‘balanced' error rates, that is, the average of false positive and false negative error rates60. Following this, an optimal PLS-DA model was built and a forward-selection approach (applied to the ranked bins, according to the Variable Importance on Projection (VIP)) was used to determine the smallest number of NMR bins that yielded the lowest classification error rate. This forward-selected PLS-DA model was then internally cross-validated (using venetian blinds; repeated 1,000 times) to yield the ‘optimal model' classification error rate. Then, in order to evaluate the statistical significance of these error rates, the group labels were randomly permuted and another PLS-DA model was constructed. Internal cross-validation was used to determine a classification error rate based on permutations (repeated 1,000 times). The statistical significance, for the prediction of each group, was assessed by comparing optimal model classification error rate with the null distribution of permuted error rates61. Specifically, the number of instances for which the permuted classification error rate was less than the optimal model error rate was calculated and then divided by the total number of permutations (1,000), generating a P value, with P<0.05 indicating that the metabolic fingerprint associated with that group could be discriminated from the other group. Finally, upon selection of the NMR bins of interest, those peaks were identified and then integrated using in-house peak-picking algorithms and the BML-NMR metabolite library62.
Phylogenetic analyses
Sequences were confirmed as L. littorea using the GenBank BLASTn search. Sequences were then aligned using ClustalW and edited using BIOEDIT 7.0.5.3 (ref. 63). All alignments and base substitutions were confirmed visually. Homogeneity of base frequencies among sequences was examined using χ2 tests in PAUP* 4.b.10 (ref. 64) and the degree of substitution saturation was examined, using the test of Xia in DAMBE 5.2.15 (ref. 65). The AIC in MODELTEST 3.7 (ref. 66) was used to specify the proportion of invariable sites for this test. Sequence divergences (uncorrected p-distance) between haplotypes were calculated using MEGA6 (ref. 67).
Standard population genetic analyses of the sequence data were carried out in ARLEQUIN 3.11 (ref. 68). For each sampling location and for the entire data set the following parameters were calculated: the number of haplotypes (Nhap), polymorphic sites (Nps), average number of nucleotide differences and haplotype (He) and nucleotide diversity.
Genetic differentiation among sampling locations was investigated using Φ indices in an AMOVA framework in ARLEQUIN68. Initially, each sampling site was tested individually for genetic subdivision between samples within populations relative to samples from the whole data set (ΦST) (tested by permuting haplotypes (10,000) between populations). Two further AMOVAs were calculated to examine genetic subdivision between samples within a group of populations relative to samples from the whole data set (ΦCT) and between samples within populations relative to samples from within the population subgroup (ΦSC). The first of these was on the basis of the three distinctive climatic regions (that is, (1) warm temperate, (2) cold temperate and (3) subpolar) investigated. Individuals from Norway were grouped as ‘northern samples', those from the United Kingdom and Roscoff as ‘mid-range samples' and those from Île de Ré and Spain as ‘southern samples'. The second AMOVA examined northern samples versus mid-range and southern samples combined.
A haplotype network was constructed using TCS 1.21 (ref. 69) to analyse evolutionary relationships among different haplotypes (gene genealogies) (Supplementary Fig. 1). Finally, to analyse genetic differentiation at the population level, a population tree was constructed using the pale lacuna sea snail Lacuna pallidula (Lacuninae: Littorinidae) as an outgroup. Genetic differentiation (Da distances) among populations were calculated using the program DnaSP 5.10.01 (ref. 70) and the population tree was constructed with the neighbour-joining algorithm in PAUP* (ref. 64) (Supplementary Fig. 2).
Data availability
COI sequences generated and analysed during this study are available from GenBank www.ncbi.nlm.nih.gov/genbank (accession numbers: KP221321 to KP221558). The data sets generated during and/or analysed during the current study are available on the British Oceanographic Data Centre (BODC) repository at www.bodc.ac.uk (doi:10.5285/40b332e8-e719-40a6-e053-6c86abc012b3).
Additional information
How to cite this article: Calosi, P. et al. Regional adaptation defines sensitivity to future ocean acidification. Nat. Commun. 8, 13994 doi: 10.1038/ncomms13994 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures, Supplementary Tables and Supplementary References
Acknowledgments
We thank Marie Palmer, Roger Haslam, Ann Torr, Richard Ticehurst, Evelyn Van der Ent and Ulf Sommer for their technical support, and Sophie Martin, Ines Le Fur as well as Maria Elena Alvarez for helping to collect specimens in France and Spain, respectively. We thank Evelyn Van der Ent for her assistance with the collection of genetic data and shell mineralogy data. P.C. acknowledges the great value of many discussions he has had with John Spicer, Dave Bilton and Francisco ‘Pancho' Bozinovic over the years on macrophysiology and evolutionary physiology of ectotherms that influenced the development of some of the ideas tested here. We also thank NASA and ECMWF for sharing climatological and oceanographic data. This work is a contribution to the NERC UK. Ocean Acidification Research Programme Benthic Consortium task 1.2 and 1.4. P.C., Y.A. and S.W. were funded by the NERC UK. Ocean Acidification Research Programme (NE/H017127/1). S.M. was funded by the Indonesian Government. L.M.T. was supported by Plymouth University internal funding. P.C., S.M., S.W. and S.D.R. were supported for the metabolomic analyses by the NERC Biomolecular Analysis Facility at the University of Birmingham (R8-H10-61). P.C. is supported by an NSERC Discovery Grant Program and a FRQ-NT New University Researchers Start Up Program Grant. Finally, we acknowledge that our research benefited greatly from the free circulation of people and ideas across the borders of many countries belonging to the European Union.
Footnotes
Author contributions S.M., P.C., S.W. and S.D.R. contributed to the rationale for this work and designed the field sampling and experimental work. S.M. carried out the experiments, collected and analysed physiological and growth data supported by P.C., S.W. and S.D.R. M.R.V. led the metabolomic component and S.M., R.L.D. and J.J.B. collected and analysed that dataset. L.M.T. collected and analysed genetic data. P.C. collected and analysed shell mineralogy data. Y.A. produced the biogeochemical model runs used to produce the environmental map in Fig. 1 and Supplementary Fig. 1. P.C., L.M.T. and S.D.R. wrote the first draft of this manuscript, and all authors contributed to the final write up.
References
- Gaston K. J. et al. Macrophysiology: a conceptual reunification. Am. Nat. 174, 595–612 (2009). [DOI] [PubMed] [Google Scholar]
- Bozinovic F., Calosi P. & Spicer J. I. Physiological correlates of geographic range in animals. Annu. Rev. Ecol. Evol. Syst. 42, 155–179 (2011). [Google Scholar]
- Dam H. G. Evolutionary adaptation of marine zooplankton to global change. Annu. Rev. Mar. Sci. 5, 349–370 (2013). [DOI] [PubMed] [Google Scholar]
- Bennett S., Wernberg T., Joy B. A., de Bettignies T. & Campbell A. H. Central and rear-edge populations can be equally vulnerable to warming. Nat. Commun. 6, 10280 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley S. A., Lurman G. J., Skepper J. N., Pörtner H.-O. & Peck L. S. Thermal plasticity of mitochondria: a latitudinal comparison between Southern Ocean molluscs. Comp. Biochem. Phys. A 152, 423–430 (2009). [DOI] [PubMed] [Google Scholar]
- Lucassen M., Koschnick N., Eckerle L. G. & Pörtner H.-O. Mitochondrial mechanisms of cold adaptation in cod (Gadus morhua L.) populations from different climatic zones. J. Exp. Biol. 209, 2462–2471 (2006). [DOI] [PubMed] [Google Scholar]
- Evans T. G. et al. Ocean acidification research in the 'post-genomic' era: Roadmaps from the purple sea urchin, Strongylocentrotus purpuratus. Comp. Biochem. Phys. A 185, 33–42 (2015). [DOI] [PubMed] [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press (2014). [Google Scholar]
- Nienhuis S., Palmer A. R. & Harley C. D. G. Elevated CO2 affects shell dissolution rate but not calcification rate in a marine snail. Proc. R. Soc. Lond. B. Biol. 277, 2553–2558 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S.-A. et al. Marine mollusc predator-escape behaviour altered by near-future carbon dioxide levels. Proc. R. Soc. B 281, 20132377 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpp M. et al. Resource allocation and extracellular acid-base status in the sea urchin Strongylocentrotus droebachiensis in response to CO2 induced seawater acidification. Aquat. Toxicol. 110, 194–207 (2012). [DOI] [PubMed] [Google Scholar]
- Dupont S., Ortega-Martinez O. & Thorndyke M. Impact of near-future ocean acidification on echinoderms. Ecotoxicology 19, 449–462 (2010). [DOI] [PubMed] [Google Scholar]
- Hall-Spencer J. M. et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99 (2008). [DOI] [PubMed] [Google Scholar]
- Maas A. E., Wishner K. F. & Seibel B. A. The metabolic response of pteropods to acidification reflects natural CO2-exposure in oxygen minimum zones. Biogeosciences 9, 747–757 (2012). [Google Scholar]
- Calosi P. et al. Distribution of sea urchins living near shallow water CO2 vents is dependent upon species acid-base and ion-regulatory abilities. Mar. Pollut. Bull. 73, 470–484 (2013). [DOI] [PubMed] [Google Scholar]
- Calosi P. et al. Adaptation and acclimatization to ocean acidification in marine ectotherms: an in situ transplant experiment with polychaetes at a shallow CO2 vent system. Philos. T. Roy. Soc. B 368, 20120444 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardies M. A. et al. Differential response to ocean acidification in physiological traits of Concholepas concholepas populations. J. Sea. Res. 90, 127–134 (2014). [Google Scholar]
- Pespeni M. H. et al. Evolutionary change during experimental ocean acidification. Proc. Natl Acad. Sci. USA 110, 6937–6942 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panova M. et al. Glacial history of the North Atlantic marine snail, Littorina saxatilis, inferred from distribution of mitochondrial DNA lineages. PLoS ONE 6, e17511 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melatunan S., Calosi P., Rundle S. D., Moody A. J. & Widdicombe S. Exposure to elevated temperature and PCO2 reduces respiration rate and energy status in the periwinkle Littorina littorea. Physiol. Biochem. Zool. 84, 583–594 (2011). [DOI] [PubMed] [Google Scholar]
- Viant M. R., Rosenblum E. S. & Tjeerdema R. S. NMR-based metabolomics: a powerful approach for characterizing the effects of environmental stressors on organism health. Environ. Sci. Technol. 37, 4982–4989 (2003). [DOI] [PubMed] [Google Scholar]
- Krajcs N., Hernadi L., Elekes K., Kimura S. & Kiss T. Excitatory neurotransmitters in the tentacle flexor muscles responsible for space positioning of the snail olfactory organ. Invertebr. Neurosci. 14, 59–69 (2014). [DOI] [PubMed] [Google Scholar]
- Pörtner H.-O. & Farrell A. P. Physiology and climate change. Ecology 322, 690–692 (2008). [DOI] [PubMed] [Google Scholar]
- Johnston I. A. Cold adaptation in marine organisms. Philos. T. Roy. Soc. B 326, 655–667 (1990). [DOI] [PubMed] [Google Scholar]
- Starai V. J. & Escalante-Semerena J. C. Acetyl-coenzyme A synthetase (AMP forming). Cell Mol. Life Sci. 61, 2020–2030 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melatunan S., Calosi P., Rundle S. D., Widdicombe S. & Moody A. J. Effects of ocean acidification and elevated temperature on shell plasticity and its energetic basis in an intertidal gastropod. Mar. Ecol. Prog. Ser. 472, 155–168 (2013). [Google Scholar]
- Lischka S. & Riebesell U. Synergistic effects of ocean acidification and warming on overwintering pteropods in the Arctic. Glob. Chang. Biol. 18, 3517–3528 (2012). [Google Scholar]
- Rodolfo-Metalpa R. et al. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Change 1, 308–312 (2011). [Google Scholar]
- Findlay H. S., Kendall M. A., Spicer J. I. & Widdicombe S. Relative influences of ocean acidification and temperature on intertidal barnacle post-larvae at the northern edge of their geographic distribution. Estuar. Coast. Shelf. S 86, 675–682 (2010). [Google Scholar]
- Wernberg et al. Decreasing resilience of kelp beds along a latitudinal gradient: Potential implications for a warmer future. Ecol. Lett. 13, 685–694 (2000). [DOI] [PubMed] [Google Scholar]
- Reipschlager A. & Pörtner H.-O. Metabolic depression during environmental stress: The role of extracellular versus intracellular pH in Sipunculus nudus. J. Exp. Biol. 199, 1801–1807 (1996). [DOI] [PubMed] [Google Scholar]
- Guppy M. & Whithers P. Metabolic depression in animals: physiological perspectives and biochemical generalisations. Biol. Rev. Camb. Philos. Soc. 74, 1–40 (1999). [DOI] [PubMed] [Google Scholar]
- Garilli V. et al. Physiological advantages of dwarfing in surviving extinctions in high-CO2 oceans. Nat. Clim. Change 5, 678–682 (2015). [Google Scholar]
- Pörtner H.-O. & Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97 (2007). [DOI] [PubMed] [Google Scholar]
- Turner L. M. et al. Energy metabolism and cellular homeostasis trade-offs provide the basis for a new type of sensitivity to ocean acidification in a marine polychaete at a high CO2 vent: adenylate and phosphagen energy pools versus carbonic anhydrase. J. Exp. Biol. 218, 2148–2151 (2015). [DOI] [PubMed] [Google Scholar]
- Razgour O. et al. The shaping of genetic variation in edge-of-range populations under past and future climate change. Ecol. Lett. 16, 1258–1266 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (2005). [DOI] [PubMed] [Google Scholar]
- Rubal M., Veiga P., Cacabelos E., Moreira J. & Sousa-Pinto I. Increasing sea surface temperature and range shifts of intertidal gastropods along the Iberian Peninsula. J. Sea Res. 77, 1–10 (2013). [Google Scholar]
- Christen N., Calosi P., McNeill C. L. & Widdicombe S. Structural and functional vulnerability to elevated pCO2 in marine benthic communities. Mar. Biol. 160, 2113–2128 (2013). [Google Scholar]
- Connell S. D. et al. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Phil. Trans. R. Soc. B 368, 20120442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiros A. M. et al. Scaling up experimental ocean acidification and warming research: from individuals to the ecosystem. Glob. Chang. Biol. 21, 130–143 (2015). [DOI] [PubMed] [Google Scholar]
- Fernandes J. A. et al. Estimating the ecological, economic and social impacts of ocean acidificaton and warming on UK fisheries. Fish Fish doi:; DOI: 10.1111/faf.12183 (2016). [DOI] [Google Scholar]
- Addo-Bediako A., Chown S. L. & Gaston K. J. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. B Biol. Sci. 267, 739–745 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C. A. et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson K. The paradox of Rockall: why is a brooding gastropod (Littorina saxatilis) more widespread than one having a planktonic larval dispersal stage (L. littorea)? Mar. Biol. 99, 507–513 (1988). [Google Scholar]
- Reid D. G. Systematics and evolution of Littorina Vol. 164 (Ray Soc. (1996). [Google Scholar]
- Sokolova I. M. & Pörtner H.-O. Metabolic plasticity and critical temperatures for aerobic scope in a eurythermal marine invertebrate (Littorina saxatilis, Gastropoda: Littorinidae) from different latitudes. J. Exp. Biol. 206, 195–207 (2003). [DOI] [PubMed] [Google Scholar]
- Pierrot D. E., Lewis E. & Wallace D. W. R. MS Excel Program Developed for CO2 System Calculations. ORNL/CDIAC-105a. (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee (2006).
- Mehrbach C., Culberson C. H., Hawley J. E. & Pytkowicz R. M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973). [Google Scholar]
- Dickson A. G. & Millero F. J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 34, 1733–1743 (1987). [Google Scholar]
- Dickson A. G. Thermpodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep-Sea Res. 37, 755–766 (1990). [Google Scholar]
- Sokolova I. M. & Pörtner H.-O. Physiological adaptation to high intertidal life involves improved water conservation abilities and metabolic rate depression in Littorina saxatilis. Mar. Ecol. Prog. Ser. 224, 171–186 (2001). [Google Scholar]
- Wu H. F., Southam A. D., Hines A. & Viant M. R. High-throughput tissue extraction protocol for NMR- and MS-based metabolomics. Anal. Biochem. 372, 204–212 (2008). [DOI] [PubMed] [Google Scholar]
- Viant M. R. Improved methods for the acquisition and interpretation of NMR metabolomic data. Biochem. Bioph. Res. Co. 310, 943–948 (2003). [DOI] [PubMed] [Google Scholar]
- Ludwig C. & Viant M. R. Two-dimensional J-resolved NMR spectroscopy: review of a key methodology in the metabolomics toolbox. Phytochem. Anal. 21, 22–32 (2010). [DOI] [PubMed] [Google Scholar]
- Parsons H. M., Ekman D. R., Collette T. W. & Viant M. R. Spectral relative standard deviation: a practical benchmark in metabolomics. Analyst 134, 478–485 (2009). [DOI] [PubMed] [Google Scholar]
- Sokolov E. P. An improved method for DNA isolation from mucopolysaccharide-rich molluscan tissues. J. Mollus. Stud. 66, 573–575 (2000). [Google Scholar]
- Folmer O., Black M., Hoen W., Lutz R. & Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 3, 294–299 (1994). [PubMed] [Google Scholar]
- Melatunan S. Biochemical, metabolic and morphological responses of the intertidal gastropod Littorina littorea to ocean acidification and increased temperature. PhD thesis, Plymouth University, UK (2012).
- Fleuret F. Fast binary feature selection with conditional mutual information. J. Mach. Learn. Res. 5, 1531–1555 (2004). [Google Scholar]
- Westerhuis J. A. et al. Assessment of PLSDA cross validation. Metabolomics. 4, 81–89 (2008). [Google Scholar]
- Ludwig C. et al. Birmingham Metabolite Library: a publicly accessible database of 1-D 1H and 2-D 1H J-resolved NMR spectra of authentic metabolite standards (BML-NMR). Metabolomics 8, 8–18 (2012). [Google Scholar]
- Hall T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999). [Google Scholar]
- Swofford D. L. PAUP*, Version 4b10 Sinauer Associates Inc. (2002).
- Xia X. & Xie Z. DAMBE: data analysis in molecular biology and evolution. J. Hered. 92, 371–373 (2001). [DOI] [PubMed] [Google Scholar]
- Posada D. & Crandall K. A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 14, 817–818 (1998). [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A. & Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L., Laval G. & Schneider S. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinformatics Online 1, 47–50 (2005). [PMC free article] [PubMed] [Google Scholar]
- Clement M., Posada D. & Crandall K. A. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 9, 1657–1659 (2000). [DOI] [PubMed] [Google Scholar]
- Librado P. & Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures, Supplementary Tables and Supplementary References
Data Availability Statement
COI sequences generated and analysed during this study are available from GenBank www.ncbi.nlm.nih.gov/genbank (accession numbers: KP221321 to KP221558). The data sets generated during and/or analysed during the current study are available on the British Oceanographic Data Centre (BODC) repository at www.bodc.ac.uk (doi:10.5285/40b332e8-e719-40a6-e053-6c86abc012b3).