Abstract
Major advances in resting-state functional magnetic resonance imaging (fMRI) techniques in the last two decades have provided a tool to better understand the functional organization of the brain both in health and illness. Despite such developments, characterizing regulation and cerebral representation of mind wandering, which occurs unavoidably during resting-state fMRI scans and may induce variability of the acquired data, remains a work in progress. Here, we demonstrate that a decrease or decoupling in functional connectivity involving the caudate nucleus, insula, medial prefrontal cortex and other domain-specific regions was associated with more sustained mind wandering in particular thought domains during resting-state fMRI. Importantly, our findings suggest that temporal and between-subject variations in functional connectivity of above-mentioned regions might be linked with the continuity of mind wandering. Our study not only provides a preliminary framework for characterizing the maintenance and cerebral representation of different types of mind wandering, but also highlights the importance of taking mind wandering into consideration when studying brain organization with resting-state fMRI in the future.
Over the past two decades, resting-state functional connectivity measured by functional magnetic resonance imaging (fMRI) has played an essential role in understanding brain functional networks in healthy and patient populations1,2,3,4,5. Resting-state functional connectivity is measured by the temporal co-activation level of spontaneous fMRI signals between spatially distinct brain regions in the absence of a perceptual or behavioral task6. Although the participants are not engaged in any particular task, there is increasing evidence that spontaneous thoughts (known as mind wandering, daydreaming, self-generated mental activity or task-unrelated thought) that are minimally constrained by external perception emerge during fMRI scans and may potentially affect resting-state fMRI data7,8. Mind wandering during resting-state fMRI has been assessed using different approaches. Questionnaires can be administered, following the resting-state fMRI scan, in which participants are asked to report the presence and frequency of spontaneous thoughts across various domains. Resting-state fMRI studies have employed several types of retrospective measures to assess spontaneous thoughts: Amsterdam Resting-State Questionnaire (ARSQ)9, New York Cognition Questionnaire (NYC-Q)10,11,12, and Resting-State Questionnaire (ReSQ)13,14. Alternatively, mind-wandering has been assessed using experience or thought sampling in conjunction with resting-state fMRI scanning15,16,17,18,19,20. While regions within the default mode network are involved in mind-wandering, a number of other brain regions outside the default mode network also show associations with various contents and forms of spontaneous thoughts10,15,16,21,22. These findings contribute to an increasingly diverse and complex understanding of the spontaneous thoughts that may occur during resting-state fMRI scans, and thus provoke more questions on the impact of mind-wandering on fMRI data.
For example, previous studies using the ReSQ have indicated that, on average, participants reported spending about 40% and 30% of time on visual and auditory mental imagery, respectively, during resting-state fMRI scans5,13,14. The remaining portion of the scan was filled with a variety of spontaneous thought domains including those pertaining to somatosensory awareness, inner musical experience, and manipulation of numbers13,14. This gives rise to the questions that form the analytical focus of our study. How is the continuity of spontaneous thoughts supported? Is the mechanism underlying the support of spontaneous thoughts comparable across different domains? Are different thought domains represented by divergent functional connections across the cerebral cortex? Recent studies have observed the non-static nature of resting-state functional connectivity across a single fMRI scan23,24,25,26,27. Will regulation of mind wandering contribute to the temporal changes in resting-state functional connectivity?
To address these questions, first, we employed multiple regression analyses to identify functional connections that exhibited a significant group difference in connectivity between participants who spent more time in a self-reported spontaneous thought and participants who spent less time in the same thought domain during resting-state fMRI (e.g., those who reported spending a lot of time in auditory mental imagery compared to those who reported spending little or no time on such wandering thought). The functional connections exhibiting a significant group difference in connectivity for a specific spontaneous thought domain would be indicative of the neural correlates associated with sustaining this spontaneous thought. Second, we investigated whether group effects on functional connectivity would vary between earlier and later parts of the resting-state fMRI data time points. Our goal is to provide a framework for studying the maintenance and cerebral representation of mind wandering, and understanding the impact of mind wandering on the acquired resting-state fMRI data.
Results
Behavioral Responses
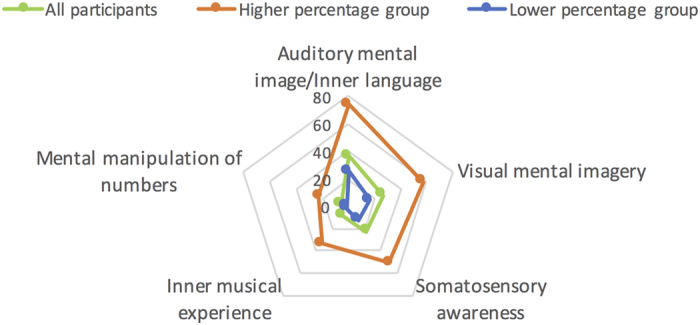
Each participant completed a post-resting-state-fMRI interview using the Resting-State Questionnaire (ReSQ)13 to assess spontaneous thoughts during the resting-state fMRI scans. Participants were asked to estimate the proportion of time (on a 0–100% scale) spent during the resting-state fMRI scans in each of the following five spontaneous thought domains: auditory mental imagery/inner language (AUDI/LANG), visual mental imagery (VIMG), somatosensory awareness (SEN), inner musical experience (MUS), and mental manipulation of numbers (NUM). Descriptions of each thought domain are included in the Methods section. On average, the participants reported spending the greatest amount of time in the AUDI/LANG (36.7%) domain, followed by VIMG (26.1%), SEN (22.5%), MUS (8.5%), and NUM (6.2%). For data analyses of each domain of spontaneous thought, participants were split into two groups (higher vs. lower percentage groups). The higher percentage group included participants whose estimated percentage of time spent in a specific thought domain was greater than the 75th percentile (i.e., upper quartile across all the participants), while the lower percentage group included the remainder of the participants. No significant differences in age were found between the two groups, for any of the individual thought domains. Figure 1 illustrates the estimated proportion of time spent in each thought domain for the higher and the lower percentage groups.
Figure 1. Estimated proportion of time spent in each thought domain of mind wandering.
Matrix-Based Connectivity Analysis Results
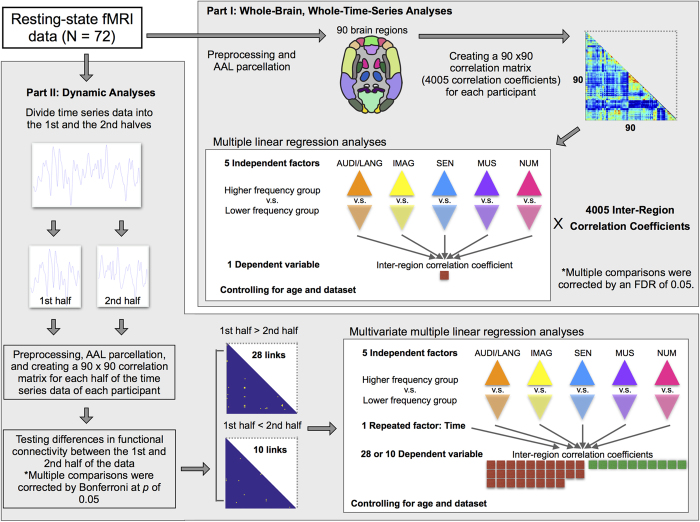
In contrast to seed-based analysis that relies on prior knowledge for choosing seed regions, the matrix-based approach employed in this study thoroughly examines functional connectivity between every pair of regions across the whole brain. Our matrix-based functional connectivity analysis procedures are described in detail in the Methods section and illustrated in Fig. 2.
Figure 2. Summary of functional connectivity analysis procedures.
Part I: Whole-brain, whole-time-series analyses
The first goal of our study was to identify functional connections that exhibited significantly different connectivity between groups of higher- and lower-frequency of mind wandering during fMRI scans (see Part I in Fig. 2). The resting-state fMRI data were preprocessed and parceled into a set of 90 brain regions using Automated Anatomical Labeling (AAL) template28. Inter-regional functional connectivity was estimated using the pairwise Pearson correlation statistics, resulting in 4005 ([90 × 89]/2) correlation coefficients for each participant. We examined group effects on functional connectivity of individual links by performing multiple linear regression analysis 4005 times. Each regression model included 5 independent factors (i.e., group effects of each thought domain), with each controlled for the others, and 1 dependent variable (i.e., the functional connectivity value of an individual link). Our resting-state data were aggregated from three unpublished datasets (see Methods section). Therefore, we added the “dataset” as a covariate in the regression models to control for any variability across datasets.
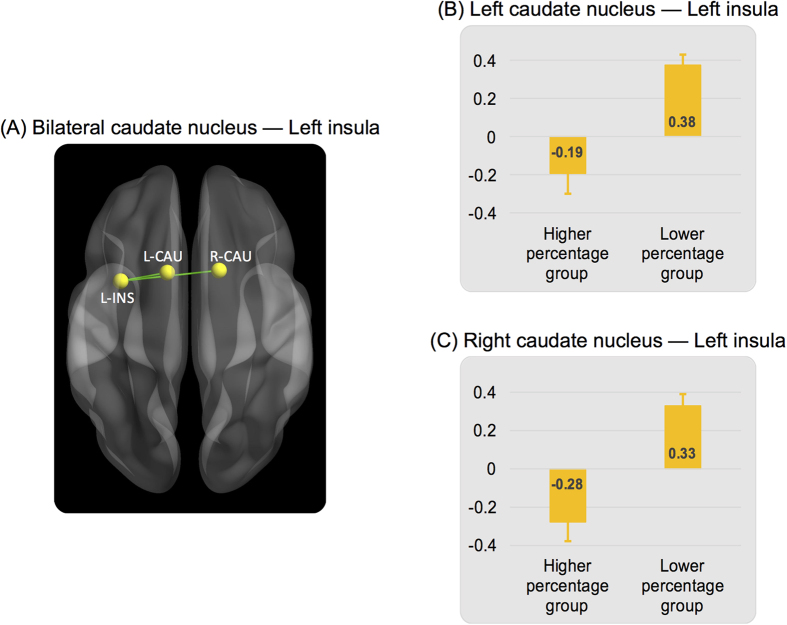
The analyses yielded 2 significant functional links for the thought domain of AUDI/LANG, corrected for multiple comparisons at a false discovery rate (FDR) of 0.0529. The two functional links (Fig. 3A) were connected between the left insula and the left caudate nucleus, t(65) = −4.64, p = 0.000017, and between the left insula and the right caudate nucleus, t(65) = −5.01, p = 0.000004. For both functional links, participants in the higher percentage group for AUDI/LANG exhibited a significantly more negative connectivity relative to the participants in the lower percentage group (Fig. 3B and C). As described in the Discussion section, bilateral caudate nuclei are brain regions involved in brain state maintenance, and the left insula supports switching between different mental states30. No significant associations with functional links were identified for other thought domains. The results suggest that the decrease in functional connectivity of connections between the left insula and bilateral caudate nuclei was associated with the continuity of spontaneous thought related to AUDI/LANG.
Figure 3.
(A) Functional connections between the left insula (L-INS) and bilateral caudate nuclei (CAU) were associated with the continuity of spontaneous thought for auditory mental imagery/inner language (AUDI/LANG). Data were derived from whole time series data. (B) and (C) Participants who reported spending more time in mind wandering associated with AUDI/LANG (higher percentage group) exhibited a more negative functional connectivity compared to participants who reported spending less time in AUDI/LANG (lower percentage group). Error bars denote standard errors.
Part II: Dynamic analyses
The second goal of our study was to investigate whether group effects on functional connectivity links would vary between earlier and later portions of the resting-state time series data (see Part II in Fig. 2). To this end, we examined whether there was an interaction effect between group and timing of the resting-state fMRI data time course profiles. First, we divided each participant’s time series data into halves (i.e., the 1st half and the 2nd half of the time series data, see Fig. 2). For each half, the preprocessed fMRI data were parceled using the AAL template28, and 4005 ([90 × 89]/2) correlation coefficients were estimated for each half of the time series data of each participant (as stated in the previous section). We then examined differences in functional connectivity between the 1st half and the 2nd half of the time series data by performing paired sample t tests on each connectivity value of the 4005 inter-regional functional links.
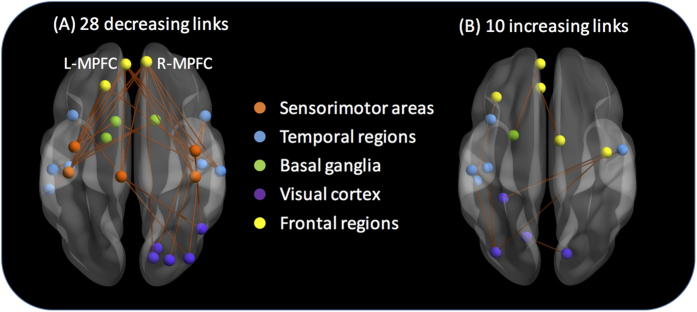
The analysis yielded 38 functional links for which mean connectivity differed significantly between the first and second halves of the scan, with Bonferroni correction for multiple comparisons (alpha = 0.05/4005 ≈ 0.000012) to minimize false positives. Among the 38 links, 28 links exhibited decreased functional connectivity from the 1st half to the 2nd half of the time series data. These links temporally changed their connectivity either from positive to negative, from more positive to less positive, or from less negative to more negative and we called these 28 links “decreasing links”. An additional 10 links exhibited increased temporal functional connectivity in the second half of the scan and they are termed “increasing links”. Among the decreasing links (Fig. 4A), most links were connected to the bilateral medial prefrontal cortex (MPFC), primary sensorimotor area, and temporal regions. For the increasing links (Fig. 4B), connections were dispersed among visual, temporal, and frontal areas. Additional details of the decreasing and increasing links are presented in Supplementary Table S1. We then converted the resultant 2 sets of links (i.e., decreasing and increasing links) into 2 binary matrices and used them as inclusive masks in the subsequent analysis.
Figure 4. Functional links that exhibited a significant difference in connectivity between the 1st and the 2nd halves of the fMRI time series data.
(A) The majority of the decreasing links were connected to the bilateral medial prefrontal cortex (MPFC), primary sensorimotor cortex, and temporal regions. (B) The increasing links were distributed among visual, temporal, and frontal areas.
Within each resultant set of links, we examined Group × Time interaction effects on functional connectivity using a multivariate multiple regression analysis, which estimated a single regression model with more than one dependent variable. The main advantage of the multivariate multiple regression analysis is that all the assessments can be performed in a single step, and thus the risk of false positives associated with repeated assessments (i.e., multiple comparisons) in conventional univariate multiple regression can be inherently eliminated. This analysis was chosen to account for the relationships among several dependent variables and conduct tests of the coefficients across different variables. Our model included 5 independent factors (i.e., group effects of each thought domain), 1 repeated factor (i.e., time: 1st half vs. 2nd half of the time series data), 1 covariate (i.e., dataset), and functional connectivity values for a set of links (either all decreasing links or all increasing links) as dependent variables. The multivariate multiple regression analysis yielded two outputs: 1) results of multivariate analysis of variance that tested the overall group effects on functional connectivity across all dependent variables; and 2) results of univariate analysis that examined the group effect on the functional connectivity of each individual dependent variable for each thought domain.
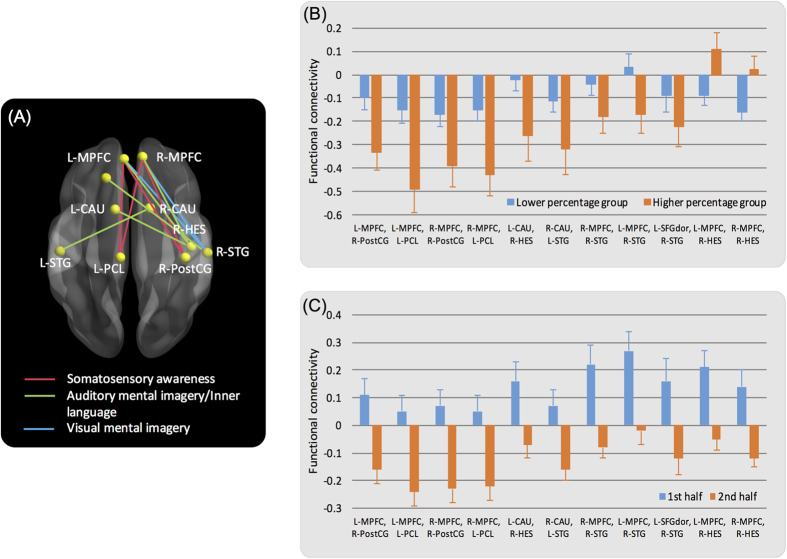
For the decreasing links (i.e., functional connectivity decreasing from the 1st to the 2nd half), the multivariate analysis yielded a significant Group × Time interaction effect, F (55, 3575) = 1.43, p = 0.02, for the AUDI/LANG, and an expected, significant time effect, F (55, 3575) = 2.16, p < 0.0001. Post-hoc analysis of the Group × Time interaction for the AUDI/LANG showed that the group effect was significant for the 2nd half of the time series data (p = 0.04), but was not significant for the 1st half of the data (p = 0.28). While the overall group effect across all dependent variables was not significant for the SEN and VIMG, partially due to a number of potentially less-relevant connections (i.e., not associated with mind wandering) being included in the model, the univariate analysis for the 2nd half of the data yielded 11 functional links that exhibited a significant group effect for SEN and VIMG in addition to the AUDI/LANG (Fig. 5A). Four links associated with the SEN were connected between bilateral MPFC, left paracentral lobule, and right post-central gyrus. Four links associated with the AUDI/LANG were connected between bilateral MPFC, right Heschl gyrus, left superior temporal gyrus, and bilateral caudate nucleus. For the VIMG, 3 links were connected between bilateral MPFC, right superior temporal gyrus, and left dorsolateral superior frontal gyrus. Thus, among the 11 decreasing links that were associated with particular thought domains, 8 were connected to the MPFC, consistent with previous studies indicating its extensive patterns of connectivity between MPFC and other sensory modalities31,32. These significant group effects on functional connectivity of the decreasing links for the 2nd half of the time series data indicated that participants in the higher percentage group exhibited more negative functional connectivity in the majority of the connections relative to participants in the lower percentage group (Fig. 5B and C). The exception to this pattern involved two links between right Heschl gyrus and bilateral MPFC in which higher functional connectivity was observed among participants who reported higher percentage of AUDI/LANG thought content. Additional statistical results of the univariate analysis are presented in Supplementary Table S2. For the increasing links (i.e., functional connectivity increasing from the 1st to the 2nd half), the expected time effect was significant, F (25, 1625) = 2.31, p = 0.0002. However, neither the group nor the Group × Time interaction effects were significant.
Figure 5.
(A) Eleven functional connections were associated with the continuity of spontaneous thoughts for somatosensory awareness (red), auditory mental imagery/inner language (green), and visual mental imagery (blue). These connections were identified from the 2nd half time series data of the decreasing links. Spheres represent the centroids of the Automated Anatomical Labeling Template regions as estimated by the BrainNet Viewer70. (B) Participants in the higher percentage group (orange) exhibited more negative functional connectivity in the majority of links relative to the lower percentage group (blue). Functional connectivity was estimated from the 2nd half of the time series data. (C) Functional connectivity significantly decreased from the 1st half (blue) to the 2nd half (orange) of the time series data. Error bars denote standard errors. Abbreviations: L = left; R = right; MPFC = medial prefrontal cortex; PCL = paracentral lobule; PostCG = postcentral gyrus; HES = Heschl gyrus; CAU = caudate nucleus; STG = superior temporal gyrus; SFGdor = dorsolateral part of superior frontal gyrus.
To explore whether we were able to observe comparable findings using seed-based connectivity analysis, we chose the MPFC as the seed region of interest to assess the correspondence between voxel-wise fMRI signals and mind wandering of different domains. Methods and results of the seed-based analysis are shown in the Supplementary Information (in the section of “Seed-based analysis”). Overall, the seed-based connectivity analysis qualitatively reproduces the major findings from the matrix-based analysis.
Altogether, the results obtained from the multivariate multiple regression analysis suggest that 1) the functional connectivity of 11 links (the majority of which connected to the MPFC) was associated with sustaining the spontaneous thoughts in particular domains: AUDI/LANG, SEN, and VIMG, 2) participants who reported spending more time in the AUDI/LANG, SEN, or VIMG exhibited a more negative functional connectivity associated with several links, compared to participants who reported spending less time in each of those same thought domains, and 3) when we examined links for which the functional connectivity tended to decrease over the course of the scan, the relationship between reported thought content and functional connectivity varied between earlier and later portions of the resting-state time series data, with relationships more pronounced in the second half. It is worth noting that, without relying on any a priori hypothesis or pre-selected seeds, we were able to identify the important roles of MPFC for mind wandering, largely in agreement with previous studies15,16,22. We believe that our analytical approach is complementary to the methods used in previous studies, and our proposed discovery-driven matrix-based connectivity analysis is a powerful tool that can potentially add new knowledge to resting-state fMRI and mind wandering research.
Discussion
Negative functional connectivity and sustaining of spontaneous thoughts
What do these results suggest for the potential underlying mechanism responsible for sustaining spontaneous thoughts during resting-state fMRI? Overall, participants who reported spending more time in spontaneous thought domains tended to exhibit more negative functional connectivity in the majority of links compared to participants who reported spending less time in each of those same thought domains (Figs 3B,C and 5B). Furthermore, a number of connections from the decreasing links (i.e., functional connectivity decreasing from the 1st to the 2nd half) were related to the continuity of spontaneous thoughts (Fig. 5). The results suggest that this decrease in functional connectivity might be a potential mechanism underlying the maintenance of particular types of spontaneous thoughts during resting-state fMRI, and this mechanism appears to be comparable across different thought domains.
The observed negative functional connectivity associated with the maintenance of mind wandering might be explained by a recently proposed decoupling hypothesis, which postulates that our attention is decoupled or shifted from processing events in the external world to self-generated mental activity to ensure the continuity of mind wandering33,34,35,36. Previous support for the decoupling hypothesis primarily comes from electroencephalography (EEG) studies during cognitive tasks37,38,39. In these EEG studies, task-related attention during cognitive activity was characterized by amplitude of event-related potentials (ERPs)37,38 and phase-locking consistency across task trials39. The amplitude of ERPs was reduced for participants who engaged in greater amounts of task-unrelated thought37,38, and task-unrelated thought was associated with a reduction in the trial-to-trial phase-locking consistency to visual events39. The findings of these studies illustrate that mind wandering during cognitive tasks is accompanied by a decrease in the processing of task-related information.
Taken together with previous studies37,38,39,40, the negative connectivity between brain regions observed in the current study can be interpreted as evidence for increased functional segregation between cortical systems subserving opposite goals or competing representations41. The functional segregation might reflect reciprocal modulation or inhibition/suppression through direct or indirect anatomical connections42,43,44. It is to be noted that, while negative correlations have been associated with data preprocessing methods using global signal regression45,46, a number of studies42,47,48,49,50, including this current study, observed negative correlations even in the absence of global signal regression. Therefore, the negative connectivity observed in this study could not be an artifact introduced by a global signal regression procedure. Collectively, the evidence presented above has provided initial support for the associations between negative functional connectivity and the underlying neural processes for sustaining mind wandering during resting state fMRI scans.
Neural correlates of sustaining spontaneous thoughts during resting-state fMRI
In this study, we identified two sets of functional connections that were related to the continuity of spontaneous thoughts during resting-state fMRI. First, functional connections between the left insula and bilateral caudate nuclei, associated with AUDI/LANG, were identified from the whole-brain, whole resting-state fMRI time series data (Fig. 3A). Second, 11 connections with the majority of links connected to the MPFC, along with a number of domain-specific regions, were identified among the links which exhibited decreased connectivity in the 2nd half of the time series data (Fig. 5A). The connectivity of these links during the second half of the scan was related to sustaining of spontaneous thoughts in the AUDI/LANG, SEN, or VIMG domains.
Previous studies have shown that the insula is highly interconnected with the striatum, including the caudate nucleus51,52. Both the insula and striatum are complex structures and have been implicated in a wide range of autonomic, affective, sensorimotor, self-referential, and cognitive processes, including language-related function53,54,55. In an extensive review of previous neuroimaging data, Price56 described a set of brain regions that participate in regulating language-related function. Among the language-related regions, the left insula is specifically involved in articulatory planning, whereas the bilateral caudate nuclei are associated with suppression of unintended responses56. In addition to their involvement with language-related function, there is recent evidence indicating that the striatum plays a critical role in brain state maintenance, whereas the insula has a major role in switching between states30. In relation to our study, we found negative connectivity between bilateral caudate nuclei and the left insula in participants who reported spending more time in AUDI/LANG and positive connectivity among the same links in participants who reported spending less time in this spontaneous thought (Fig. 3B and C). It is possible that, to support the continuity of mind wandering state, the caudate nuclei might exert suppressive effects on the insula to prevent from switching between brain states. Future studies are warranted to elucidate the dynamics between these two brain regions involved maintaining a state of mind wandering.
Among the additional 11 functional links that were related to greater continuity of specific domains of spontaneous thoughts, 8 of them were connected to the MPFC. The MPFC is a hub within the default mode network3 and has been implicated in self-related processing, such as the retrieval of remote and recent memory associated with autobiography or other self-referential processes, judgments about self and others, and simulations of social interaction21,57,58,59,60,61. Furthermore, the MPFC is characterized by a high-level interconnectivity with multiple sensory modalities such as primary/secondary auditory, somatosensory, and visual cortices31,32. This multi-modal convergence observed in the MPFC provides an anatomical ground to support the role of the MPFC together with other domain-specific brain regions in the maintenance and representation of spontaneous thoughts across different domains. Therefore, together with previous studies10,15,16,22, we propose that the MPFC is a nodal point that serves to support different domains of spontaneous thoughts during resting state.
Mind wandering and variance in resting-state functional connectivity
While we were able to identify functional links related to participant-reported mind wandering behavior from the whole fMRI time series data by assuming temporal uniformity of functional connectivity, we found significant temporal differences in a number of links (Fig. 4). Crucially, some of the decreasing links that were equivalently associated with multiple domains of spontaneous thoughts could only be identified from the 2nd half of the time series data. This indicates that differences in functional connectivity for the identified links not only manifest between individuals with different levels of self-reported spontaneous thought across specific domains, but also manifest throughout the fMRI time course profile within an individual. In the current study, the fMRI time points were divided into two halves, which revealed an overall trend of decreased functional connectivity for the 11 domain-specific links from the 1st to the 2nd half. Future research might include recently developed fMRI protocols of higher temporal sampling and advanced analytic procedures62 aimed at deconstructing time points into more non-overlapping segments, which may lead to more accurate assessment of dynamic changes in functional connectivity due to mind wandering.
As an exploratory analysis, we divided our dataset 2 (i.e., the one with the longest resting-state fMRI scan time of 520 sec: Supplementary Table S3) into four 130-sec segments for segment-specific connectivity measurement. Across the 11 domain-specific functional links of dataset 2, connectivity of the 1st quarter of time series data was significantly more positive (or less negative) compared to the other 3 quarters (p < 0.0001), and the connectivity was not significantly different among the 2nd, 3rd, and 4th quarters (Supplementary Fig. S1), suggesting that dynamic changes of functional connectivity in these links likely occurred between the 1st and 2nd quarters (i.e., centered around 130 sec after fMRI scans began). This observation could also explain why the maintenance of mind wandering was most pronounced in the 2nd half of the scan because the 2nd half of time series data for all 3 datasets began after 130 sec.
Overall, our findings associated with temporal differences in connectivity are consistent with the concept that non-static functional connectivity existed in a number of connections23,24,25,27. Further, our findings suggest that the temporal variations in functional connectivity are to some degree of neuronal origin and could be linked with the continuity of mind wandering. In addition, previous studies have found considerable between-subject variability in resting-state connectivity among healthy participants63,64. Our results suggest this between-subject variance in functional connectivity might be partially attributable to variations in spontaneous thought domain and the time spent in a specific spontaneous thought.
Limitations and Future Directions
Our findings provide a preliminary framework for characterizing the maintenance and cerebral representation of mind wandering during resting-state fMRI. These findings, although insightful and interesting, require replication in larger samples. Several limitations should be acknowledged while interpreting our results. First, the assessment of mind wandering did not account for when the reported mind wandering occurred in the course of the fMRI scan. Future studies that combine real-time resting-state fMRI and probe measures of mind wandering during fMRI scans will be needed to measure the association between transitions of functional connectivity (e.g., from more positive to more negative correlations) and the onset of mind wandering. Second, the mind wandering behavior was determined by self report after the fMRI scan; thus, it could potentially be subject to recall bias. Third, our current study focused on associations between functional connectivity and sustaining of mind wandering. Future investigations using non-invasive brain stimulation techniques (e.g., transcranial magnetic stimulation) to modulate brain activities involved in sustaining of mind wandering are warranted to establish causal relationships between functional connectivity and mind wandering65,66.
Conclusion
This study demonstrates that sustained mind wandering during resting-state fMRI is associated with decoupled or negative functional connectivity involving the caudate nucleus, insula, MPFC and other domain-specific brain regions. Our findings provide insights into mind wandering at the large-scale network level, and highlight the importance of including the estimated percentage of time and domain of spontaneous thoughts during resting-state fMRI into functional connectivity analyses. Future studies will need to account for these thought processes in analyses of fMRI signals during group comparisons and correlations between functional connectivity and behavioral measures. We expect that the power to detect group differences and to identify imaging-based biomarkers, especially for individual participants and clinical populations, can be enhanced by addressing the variability of mind wandering during resting-state fMRI.
Methods
Participants
The study’s participants included 72 right-handed healthy adults (mean age 55 ± 18 years, 37 males) from three unpublished datasets. Participants were excluded if they had a history of neurological or psychiatric disorder; unstable or untreated medical illness (including uncontrolled diabetes or treatment-resistant hypertension); any contraindication to MRI, such as claustrophobia or metallic implants; or raw score less than 27 on the Mini-Mental State Exam67. The institutional review board at Duke University Medical Center approved the study, and all data collection and analyses were carried out in accordance with the approved study protocol and the guidelines of the Helsinki Declaration. All of the participants provided written informed consent prior to participation.
Resting-state questionnaire
Each participant completed a post-resting-state-fMRI interview using the adapted ReSQ13 to assess the content of spontaneous thoughts during the resting-state-fMRI scan. Participants were asked to estimate the proportion of time (on a 0–100% scale) spent during the resting-state-fMRI scan in each of the following five mental activities: AUDI/LANG, VIMG, SEN, MUS, and NUM. AUDI/LANG is defined as remembering/imagining words, sentences or conversations spoken by oneself or others, or talking to oneself with one’s own voice without overt production. VIMG refers to having thoughts in the form of visual images. These visual images could be associated with memory, ongoing learning, or planning of upcoming events. SEN is related to attention attracted by somatosensory sensations such as sensory information from the face or body, or position and movement of our body parts. MUS and NUM is defined as thinking related to music and numbers, respectively. For each participant, the data were log-transformed to normalize population variance. The questionnaires were completed within 30–60 minutes after the resting-state-fMRI scan.
Imaging protocols
Resting-state-fMRI was performed on a 3 Tesla GE scanner. Each participant was scanned on a single occasion, lying still with eyes fixated on a centrally located crosshair. Participants were scanned using three echo planar imaging (EPI) protocols summarized in Supplementary Table S3. The potential influence of confounders (i.e., dataset and age) was controlled in the following analysis.
fMRI data analysis
The preprocessing of fMRI data was conducted through the Duke Brain Imaging and Analysis Center preprocessing pipelines based on the tools from the Oxford Centre for Functional MRI of the Brain’s Software Library (FSL version 5.0.1, www.fmrib.ox.ac.uk/fsl) and locally developed Matlab codes (Mathworks, Natick, MA, USA). The first 4 volumes were discarded in order to reach the T1 steady state. The data were corrected for slice-timing differences and motion (six parameters: three translations and three rotations), and were registered to the Montreal Neurological Institute (MNI) 152 template using a 12 degrees of freedom affine transformation implemented in FSL’s Linear Image Registration Tool. All subsequent analyses were conducted in the MNI standard space. We regressed out the 6-parameter rigid body head motion (obtained from motion correction), the averaged time course profiles in the white matter, and the averaged time course profiles in the cerebrospinal fluid regions to reduce non-neuronal contributions to BOLD correlations68. We also removed constant offsets and linear drift. Time domain signals with their frequencies less than 0.08 Hz were retained.
A schematic diagram of our matrix-based functional connectivity analysis procedures is illustrated in Fig. 2. For each participant, the preprocessed low-frequency fMRI data were parceled into a set of 90 brain regions using the AAL template28. Each participant’s BOLD time series was averaged within each brain region. We used Pearson correlation as the metric of association between the time series for each pair of the 90 brain regions. This resulted in a 90 × 90 correlation matrix with 4005 ([90 × 89]/2) unique inter-regional correlation coefficients (r). These inter-regional r values were transformed to the normal distribution by Fisher’s z transform for further statistical inference69. Because age varied widely across participants, we regressed out age-related signals of functional connectivity and then used the residuals for the following statistical analysis.
Statistical analyses
The first goal of the study was to identify functional connections that exhibited a significant group effect (i.e., higher percentage group vs. lower percentage group) on functional connectivity derived from the whole resting-state fMRI time series data (see Part I in Fig. 2). For each individual connection, we performed a multiple linear regression analysis to examine the group effects on functional connectivity. Each regression model included 5 independent factors (i.e., group effects of each thought domain), 1 covariate (i.e., dataset), and 1 dependent variable (i.e., functional connectivity value). The independent factor was coded as −1 (lower percentage group) and 1 (higher percentage group). The regression model was performed 4005 times, and multiple comparisons were corrected by an FDR of 0.0529.
The second goal of the study was to investigate whether there was an interaction effect between group and timing of the resting-state fMRI time series (see Part II in Fig. 2). First, we divided each participant’s time series data into halves (i.e., the 1st half and the 2nd half of the time series data). For each half, as for the whole time series data, the preprocessed fMRI data were parceled using the AAL template28, and 4005 ([90 × 89]/2) correlation coefficients were estimated for each half of the time series data of each participant. We then examined differences in functional connectivity between the 1st half and the 2nd half of the time series data by performing paired sample t tests on each connectivity value of the 4005 inter-regional functional links. The significance criterion was set at a p-value of 0.05 applying Bonferroni correction for multiple comparisons (i.e., alpha = 0.05/4005 ≈ 0.000012), minimizing false positives. The resultant networks were converted into 2 binary matrices (increasing and decreasing links) and used as inclusive masks in the subsequent analysis. Within each resultant set of links, the Group × Time interaction effects on functional connectivity were examined using multivariate multiple regression analysis. The multivariate multiple regression estimated a single regression model with more than one dependent variable (in a single step, where false positives associated with repeated assessments in conventional univariate multiple regression could be inherently eliminated). This approach allowed us to take into account the relationships among several dependent variables and conduct tests of the coefficients across different variables. The regression model included 5 independent factors (i.e., group effects of each thought domain), 1 repeated factor (i.e., time: 1st half vs. 2nd half of the time series data), 1 covariate (i.e., dataset), and functional connectivity values of a set of links (decreasing or increasing links) as dependent variables. The multivariate multiple regression analysis yielded two outputs: 1) results of multivariate analysis of variance that tested the overall group effects on functional connectivity across all dependent variables; and 2) results of univariate analysis that examined the group effect on functional connectivity of each individual dependent variable for each thought domain.
Additional Information
How to cite this article: Chou, Y.-h. et al. Maintenance and Representation of Mind Wandering during Resting-State fMRI. Sci. Rep. 7, 40722; doi: 10.1038/srep40722 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This research was supported by NIH grants R01 NS074045 (NkC), R01 MH098301 (DJM and LW), R01AG043438 (HEW), and R01 AG039684 (DJM).
Footnotes
Author Contributions Study concept and design: Y.C., N.C., and P.G. Acquisition, analysis, or interpretation of data: Y.C., M.S., H.W., P.G., M.C., C.W., D.M., L.W., I.K., M.J., M.D., Y.L., A.S., and N.C. Drafting of the manuscript: Y.C. and N.C. Critical revision of the manuscript for important intellectual content: Y.C., M.S., H.W., P.G., M.C., C.W., D.M., L.W., I.K., M.J., M.D., Y.L., A.S., and N.C. Statistical analysis: Y.C., M.S., Y.L. and H.W. Obtained funding: N.C., D.M., L.W., A.S., and H.W. Administrative, technical, or material support: N.C. and M.S. Study supervision: N.C., D.M., and L.W.
References
- Biswal B. B. et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA 107, 4734–4739, doi: 10.1073/pnas.0911855107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. D., Krasnow B., Reiss A. L. & Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100, 253–258 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E. et al. A default mode of brain function. Proc Natl Acad Sci USA 98, 676–682, doi: 10.1073/pnas.98.2.676 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E. The restless brain: how intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci 370, doi: 10.1098/rstb.2014.0172 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B. et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54, 287–298 (2001). [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F. Z., Haughton V. M. & Hyde J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34, 537–541 (1995). [DOI] [PubMed] [Google Scholar]
- Christoff K., Gordon A. M., Smallwood J., Smith R. & Schooler J. W. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America 106, 8719–8724, doi: 10.1073/pnas.0900234106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M. F. et al. Wandering minds: the default network and stimulus-independent thought. Science 315, 393–395, doi: 10.1126/science.1131295 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffers D. et al. Resting-State fMRI Functional Connectivity Is Associated with Sleepiness, Imagery, and Discontinuity of Mind. PLoS One 10, e0142014, doi: 10.1371/journal.pone.0142014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K. J. et al. A correspondence between individual differences in the brain’s intrinsic functional architecture and the content and form of self-generated thoughts. PLoS One 9, e97176, doi: 10.1371/journal.pone.0097176 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K. J. et al. A high resolution 7-Tesla resting-state fMRI test-retest dataset with cognitive and physiological measures. Sci Data 2, 140054, doi: 10.1038/sdata.2014.54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A. et al. Dynamic network participation of functional connectivity hubs assessed by resting-state fMRI. Front Hum Neurosci 8, 195, doi: 10.3389/fnhum.2014.00195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamillieure P. et al. The resting state questionnaire: An introspective questionnaire for evaluation of inner experience during the conscious resting state. Brain Res Bull 81, 565–573, doi: 10.1016/j.brainresbull.2009.11.014 (2010). [DOI] [PubMed] [Google Scholar]
- Doucet G. et al. Patterns of hemodynamic low-frequency oscillations in the brain are modulated by the nature of free thought during rest. Neuroimage 59, 3194–3200, doi: 10.1016/j.neuroimage.2011.11.059 (2012). [DOI] [PubMed] [Google Scholar]
- Tusche A., Smallwood J., Bernhardt B. C. & Singer T. Classifying the wandering mind: revealing the affective content of thoughts during task-free rest periods. Neuroimage 97, 107–116, doi: 10.1016/j.neuroimage.2014.03.076 (2014). [DOI] [PubMed] [Google Scholar]
- Smallwood J. et al. Representing Representation: Integration between the Temporal Lobe and the Posterior Cingulate Influences the Content and Form of Spontaneous Thought. PLoS One 11, e0152272, doi: 10.1371/journal.pone.0152272 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlburt R. T., Alderson-Day B., Fernyhough C. & Kuhn S. What goes on in the resting-state? A qualitative glimpse into resting-state experience in the scanner. Front Psychol 6, 1535, doi: 10.3389/fpsyg.2015.01535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S., Fernyhough C., Alderson-Day B. & Hurlburt R. T. Inner experience in the scanner: can high fidelity apprehensions of inner experience be integrated with fMRI? Front Psychol 5, 1393, doi: 10.3389/fpsyg.2014.01393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan C., Shine J. M., Lewis S. J., Andrews-Hanna J. R. & Irish M. Shaped by our thoughts–a new task to assess spontaneous cognition and its associated neural correlates in the default network. Brain Cogn 93, 1–10, doi: 10.1016/j.bandc.2014.11.001 (2015). [DOI] [PubMed] [Google Scholar]
- Van Calster L., D’Argembeau A., Salmon E., Peters F. & Majerus S. Fluctuations of Attentional Networks and Default Mode Network during the Resting State Reflect Variations in Cognitive States: Evidence from a Novel Resting-state Experience Sampling Method. J Cogn Neurosci, 1–19, doi: 10.1162/jocn_a_01025 (2016). [DOI] [PubMed] [Google Scholar]
- Fox K. C., Spreng R. N., Ellamil M., Andrews-Hanna J. R. & Christoff K. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage 111, 611–621, doi: 10.1016/j.neuroimage.2015.02.039 (2015). [DOI] [PubMed] [Google Scholar]
- Smallwood J. et al. The default modes of reading: modulation of posterior cingulate and medial prefrontal cortex connectivity associated with comprehension and task focus while reading. Front Hum Neurosci 7, 734, doi: 10.3389/fnhum.2013.00734 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. & Glover G. H. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50, 81–98, doi: 10.1016/j.neuroimage.2009.12.011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E. A. et al. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24, 663–676, doi: 10.1093/cercor/bhs352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz S. D. The neural basis of time-varying resting-state functional connectivity. Brain Connect 4, 769–779, doi: 10.1089/brain.2014.0250 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A. & Davis K. D. Dynamic functional connectivity of the default mode network tracks daydreaming. Neuroimage 100, 471–480, doi: 10.1016/j.neuroimage.2014.06.044 (2014). [DOI] [PubMed] [Google Scholar]
- Hutchison R. M. et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80, 360–378, doi: 10.1016/j.neuroimage.2013.05.079 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002). [DOI] [PubMed] [Google Scholar]
- Benjamini Y. & Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soc B Met 57, 289–300 (1995). [Google Scholar]
- Tang Y. Y., Rothbart M. K. & Posner M. I. Neural correlates of establishing, maintaining, and switching brain states. Trends in cognitive sciences 16, 330–337, doi: 10.1016/j.tics.2012.05.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover W. B. & Vertes R. P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212, 149–179, doi: 10.1007/s00429-007-0150-4 (2007). [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull 52, 319–330 (2000). [DOI] [PubMed] [Google Scholar]
- Schooler J. W. et al. Meta-awareness, perceptual decoupling and the wandering mind. Trends in cognitive sciences 15, 319–326, doi: 10.1016/j.tics.2011.05.006 (2011). [DOI] [PubMed] [Google Scholar]
- Smallwood J. et al. Pupillometric evidence for the decoupling of attention from perceptual input during offline thought. PLoS One 6, e18298, doi: 10.1371/journal.pone.0018298 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J. Distinguishing how from why the mind wanders: a process-occurrence framework for self-generated mental activity. Psychol Bull 139, 519–535, doi: 10.1037/a0030010 (2013). [DOI] [PubMed] [Google Scholar]
- Smallwood J. & Schooler J. W. The science of mind wandering: empirically navigating the stream of consciousness. Annu Rev Psychol 66, 487–518, doi: 10.1146/annurev-psych-010814-015331 (2015). [DOI] [PubMed] [Google Scholar]
- Barron E., Riby L. M., Greer J. & Smallwood J. Absorbed in thought: the effect of mind wandering on the processing of relevant and irrelevant events. Psychol Sci 22, 596–601, doi: 10.1177/0956797611404083 (2011). [DOI] [PubMed] [Google Scholar]
- Kam J. W. et al. Slow fluctuations in attentional control of sensory cortex. J Cogn Neurosci 23, 460–470, doi: 10.1162/jocn.2010.21443 (2011). [DOI] [PubMed] [Google Scholar]
- Baird B., Smallwood J., Lutz A. & Schooler J. W. The decoupled mind: mind-wandering disrupts cortical phase-locking to perceptual events. J Cogn Neurosci 26, 2596–2607, doi: 10.1162/jocn_a_00656 (2014). [DOI] [PubMed] [Google Scholar]
- Christoff K. Undirected thought: neural determinants and correlates. Brain Res 1428, 51–59, doi: 10.1016/j.brainres.2011.09.060 (2012). [DOI] [PubMed] [Google Scholar]
- Fox M. D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102, 9673–9678 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath K., Krishnamurthy V., Cabanban R. & Crosson B. A. Hubs of Anticorrelation in High-Resolution Resting-State Functional Connectivity Network Architecture. Brain Connect 5, 267–275, doi: 10.1089/brain.2014.0323 (2015). [DOI] [PubMed] [Google Scholar]
- Yatsenko D. et al. Improved estimation and interpretation of correlations in neural circuits. PLoS Comput Biol 11, e1004083, doi: 10.1371/journal.pcbi.1004083 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. et al. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. Neuroimage 54, 2571–2581, doi: 10.1016/j.neuroimage.2010.11.018 (2011). [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R. M., Handwerker D. A., Jones T. B. & Bandettini P. A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44, 893–905, doi: 10.1016/j.neuroimage.2008.09.036 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A. et al. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage 47, 1408–1416, doi: 10.1016/j.neuroimage.2009.05.005 (2009). [DOI] [PubMed] [Google Scholar]
- Chen G., Chen G., Xie C. & Li S. J. Negative functional connectivity and its dependence on the shortest path length of positive network in the resting-state human brain. Brain Connect 1, 195–206, doi: 10.1089/brain.2011.0025 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp 26, 15–29, doi: 10.1002/hbm.20113 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L. Q., Kelly A. M., Biswal B., Castellanos F. X. & Milham M. P. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp 30, 625–637 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. & Glover G. H. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage 47, 1448–1459, doi: 10.1016/j.neuroimage.2009.05.012 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge J. L., Breitbart M. A., Danish M. & Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol 490, 101–118, doi: 10.1002/cne.20660 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikama M., McFarland N. R., Amaral D. G. & Haber S. N. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci 17, 9686–9705 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn F. G. Anatomy of the insula functional and clinical correlates. Aphasiology 13, 55–78 (1999). [Google Scholar]
- Christopher L., Koshimori Y., Lang A. E., Criaud M. & Strafella A. P. Uncovering the role of the insula in non-motor symptoms of Parkinson’s disease. Brain 137, 2143–2154, doi: 10.1093/brain/awu084 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. D. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10, 59–70, doi: 10.1038/nrn2555 (2009). [DOI] [PubMed] [Google Scholar]
- Price C. J. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 1191, 62–88, doi: 10.1111/j.1749-6632.2010.05444.x (2010). [DOI] [PubMed] [Google Scholar]
- D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Front Hum Neurosci 7, 372, doi: 10.3389/fnhum.2013.00372 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny B. T., Kober H., Wager T. D. & Ochsner K. N. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci 24, 1742–1752, doi: 10.1162/jocn_a_00233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D. A., Akbudak E., Shulman G. L. & Raichle M. E. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America 98, 4259–4264, doi: 10.1073/pnas.071043098 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. et al. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage 31, 440–457, doi: 10.1016/j.neuroimage.2005.12.002 (2006). [DOI] [PubMed] [Google Scholar]
- Turk D. J., Heatherton T. F., Macrae C. N., Kelley W. M. & Gazzaniga M. S. Out of contact, out of mind: the distributed nature of the self. Ann N Y Acad Sci 1001, 65–78 (2003). [DOI] [PubMed] [Google Scholar]
- Lee H. L., Zahneisen B., Hugger T., LeVan P. & Hennig J. Tracking dynamic resting-state networks at higher frequencies using MR-encephalography. Neuroimage 65, 216–222, doi: 10.1016/j.neuroimage.2012.10.015 (2013). [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Panych L. P., Dickey C. C., Petrella J. R. & Chen N. K. Investigation of long-term reproducibility of intrinsic connectivity network mapping: a resting-state FMRI study. Am J Neuroradiol 33, 833–838, doi: 10.3174/ajnr.A2894 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. et al. Individual variability in functional connectivity architecture of the human brain. Neuron 77, 586–595, doi: S0896-6273(13)00004-4 [pii]10.1016/j.neuron.2012.12.028 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod V., Rees G., Lavidor M. & Bar M. Increasing propensity to mind-wander with transcranial direct current stimulation. Proceedings of the National Academy of Sciences of the United States of America 112, 3314–3319, doi: 10.1073/pnas.1421435112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S., Kochiyama T., Nakai R., Abe N. & Nomura M. Causal relationship between effective connectivity within the default mode network and mind-wandering regulation and facilitation. Neuroimage 133, 21–30, doi: 10.1016/j.neuroimage.2016.03.009 (2016). [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E. & McHugh P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975). [DOI] [PubMed] [Google Scholar]
- Van Dijk K. R. et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103, 297–321, doi: 10.1152/jn.00783.2009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. A. On the probable error of a coefficient of correlation deduced from a small sample. Metron 1, 3–32 (1921). [Google Scholar]
- Xia M., Wang J. & He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One 8, e68910, doi: 10.1371/journal.pone.0068910 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.