Abstract
Preterm infants are extremely vulnerable to a range of morbidities and mortality. Underdeveloped cardiac, respiratory, gastrointestinal, and immune systems in the preterm period increase the risk of necrotizing enterocolitis (NEC), a serious disease of the gut. NEC affects 5–12% of very–low birth-weight infants, leads to surgery in 20–40% of cases, and is fatal in 25–50% of cases. There are multiple factors that may contribute to NEC, but the exact cause is not yet fully understood. Severe cases can result in intestinal resection or death, and the health care costs average >$300,000/infant when surgical management is required. Different types of nutrition may affect the onset or progression of NEC. Several studies have indicated that bovine milk–based infant formulas lead to a higher incidence of NEC in preterm infants than does human milk (HM). However, it is not clear why HM is linked to a lower incidence of NEC or why some infants fed an exclusively HM diet still develop NEC. An area that has not been thoroughly explored is the use of semielemental or elemental formulas. These specialty formulas are easy to digest and absorb in the gut and may be an effective nutritional intervention for reducing the risk of NEC. This review summarizes what is known about the factors that contribute to the onset and progression of NEC, discusses its health care cost implications, and explores the impact that different formulas and HM have on this disease.
Keywords: necrotizing enterocolitis, preterm infant, nutrition, breast milk, formula, elemental formula, semielemental formula, hydrolyzed formula
Introduction
Preterm infants: Prematurity and risk of mortality and morbidity
Prematurity in infants is defined as birth at <37 wk gestation (1). The preterm birth rate was 7.7% in Canada in 2010 (2), 9.57% in the United States in 2014 (3), and 11.1% worldwide in 2010 (4). Of the premature infants in Canada and the United States, 6% and 8% were low birth weight (LBW)8 (<2500 g), respectively (1, 3). Medical advancements have improved the rate of survival for preterm infants; however, survival in many cases has been coupled with health and developmental complications later in life (1). Preterm birth is a major cause of LBW (1), which is a preliminary indicator for health status. Although LBW is not a direct cause of mortality, the literature indicates that it is associated with adverse outcomes (e.g., respiratory distress syndrome, cardiovascular disorders, compromised immune system, limited ability to mitigate inflammation and infections, neurological impairments) that may lead to mortality (1, 5). A recent Japanese study found that the odds of mortality increase as the SD for birth weight decreases for growth-restricted extremely preterm infants (6). LBW (1500–2499 g), very low birth weight (VLBW) (1000–1499 g), and extremely low birth weight (<1000 g) have been linked to several morbidities, including chronic lung disease, retinopathy of prematurity, sepsis, and necrotizing enterocolitis (NEC) (1, 6–8). Preventing and ameliorating the effects of these morbidities is an ongoing challenge in neonatology.
NEC
Description and incidence of disease.
NEC is a serious intestinal inflammatory disease in neonates first described in 1965 by Mizrahi et al. (9). The disease is characterized by inflammation and injury of the gut wall barrier that may advance to necrosis and, potentially, perforation of the gut (10, 11). The diagnosis of NEC is commonly determined with the use of Bell’s modified staging criteria (12). Mild cases of NEC may be effectively treated by withholding enteral feeds, decompressing the stomach with a nasogastric tube, and starting broad-spectrum antibiotics. Advanced cases, however, may lead to surgery, extensive intestinal necrosis (NEC totalis), and death (10).
In Canada, 5.1% of infants aged <33 wk are affected by NEC (10). The incidence of NEC across developed countries is ∼5–12% for VLBW infants (13–18), depending on certain risk factors. Three major risk factors for NEC are <32 wk gestational age, <1500 g at birth, and cardiac complications (10). NEC is more prevalent in preterm infants (19), with ∼85% of cases occurring in infants born <35 wk gestation, whereas only 7–15% of cases occur in late-preterm (35–36 wk gestation) or term infants (37–42 wk gestation) (20–22). The incidence of NEC also drastically increases from 0.7% to 6.6% for infants with a birth weight <1500 g (10). NEC is less common in infants with a birth weight >1500 g, but the expected prognosis of larger infants is worse than smaller infants (23).
NEC has been studied for decades. Although some evidence has been found to elucidate the potential causes and progression of the disease, minimal advancements have been made in this field because of its complex nature. Clinical and theoretic knowledge of the disease mechanisms and interventions to protect an infant from NEC, including nutritional approaches, require further research.
Multifactorial causes of NEC.
Prematurity is a risk factor for poor health outcomes, largely because of the underdevelopment of cardiac, respiratory, gastrointestinal, and immune systems. Immaturity of the lungs, a problem especially affecting infants born <32 wk gestation, results in impaired gas exchange and insufficient oxygenation of tissues (24). Cardiac complications during the preterm period, such as a large patent ductus arteriosus, limit the availability of oxygen and nutrients to other tissues and organs (25). Immaturity of the gut is also a concern. The preterm gut is characterized by reduced peristalsis, a thin mucous layer, reduced tight junctions, increased enterocyte apoptosis, and impaired enterocyte regeneration (26, 27). These deficiencies may result in a “leaky” gut barrier, thereby facilitating the penetration of bacteria from the lumen (26, 27). Decreased structural integrity and functionality of the gut result in poor digestion and absorption of energy, protein, and other nutrients necessary for growth, the development of organs, and immunoprotection (26). Last, there are distinct differences between term and preterm infants in regard to the expression of immune cells and signaling pathways. A preterm immune system cannot readily detect pathogens and protect against infections due to multiple associated factors such as 1) the decreased production of IgA, IgM, IgG, and defensins; 2) changes in the expression of toll-like receptors (TLRs), especially TLR4 and TLR9, which are involved in pathogen recognition and the activation of the innate immune system (14, 28, 29); and 3) upregulation of proinflammatory TLRs (26) and/or proinflammatory cytokines such as TNF-α, IL-6, IL-8, and IL-1β (26, 27). The culmination of these factors increases a preterm infant’s vulnerability to infections and disease, particularly NEC.
Prematurity is a predominant risk factor for NEC, but several other medical risk factors have been identified. Infants with high clinical acuity or severe comorbidities may be at a greater risk for NEC. Low Apgar scores at birth, cardiac lesions, bowel obstruction, the use of ≥1 inotropes, and compromised respiratory function are a few indicators of clinical severity (26). Medical events or pharmaceuticals that reduce perfusion to the gut or oxygen saturation of the blood have also been linked to NEC (10, 26). Hypoperfusion or hypoxic conditions in the intestine occur when the metabolic requirements of epithelial cells are not met by the mesenteric blood supply. Incomplete reduction of oxygen in the mitochondria during hypoxic conditions produces reactive oxygen species, which in turn activate adenosine monophosphate-activated protein kinase through calcium-dependent channels (30). Adenosine monophosphate-activated protein kinase downregulates energy-consuming anabolic mechanisms such as Na+/K+-ATPase activity and favors catalytic processes in an effort to spare energy (30). These ensuing intracellular responses may set the stage for NEC. Over time, this catabolic, oxidative system may fail to maintain digestive and absorptive functionality and cellular integrity and increase the gut’s susceptibility to uncontrolled inflammation and necrosis.
For preterm infants, hypoxia-ischemia and respiratory complications such as bronchopulmonary dysplasia limit nutrient and oxygen delivery to the gut (9). Vasoconstrictive medications such as cyclooxygenase inhibitors (e.g., indomethacin), which are used in the treatment of patent ductus arteriosus in preterm infants, can also impair gut perfusion (10, 31). In a hypoxic gut environment, the introduction of enteral nutrients may cause oxygen to be preferentially used for digestion at the expense of maintaining the physical gut wall barrier (32). At the tissue level, hypoxic conditions or vasoconstrictive medications may lead to an inadequate supply of nutrients and oxygen needed to generate energy, produce immune cells, build membrane proteins to protect the integrity of the gut wall, and perform digestive and absorptive processes. Therefore, an inability to maintain the structure and function of the gut wall because of hypoperfusion may be an underlying catalyst for NEC.
Interestingly, NEC only occurs after infants have been enterally fed (26). This may be related to the gut microbiome. Preterm infants have a lower diversity of microbiota (10, 26) and higher proportion of potentially harmful species such as Proteobacteria (10, 33) than term infants. Disruptions of the microbiota have been attributed to the prophylactic use of antibiotics at birth, contact with harmful bacteria on the mother’s skin during a cesarean delivery, or the inability to transfer beneficial bacteria and prebiotics through breastfeeding or pasteurized donor human milk (DHM) shortly after birth. Ineffective digestion and absorption of enteral feeds in the lumen allows the microbiota to use these nutrients for their own growth and proliferation (27, 34). Bacterial overgrowth combined with an underdeveloped immune system and gut structure can facilitate bacterial adherence to the gut wall and increased mucosal permeability. Intestinal bacterial overgrowth, diagnosed by clinical symptoms (e.g., vomiting, diarrhea, gas, abdominal pain, etc.), breath testing measuring hydrogen and methane gas, or the aspiration and culture of intestinal fluids, is typically treated with antibiotics (35). Eradicating existing bacterial colonies by antibiotics combined with an underdeveloped immune system and gut structure can facilitate the adherence of successive bacterial colonies to the gut wall and mucosal permeability. The translocation of bacteria may, in turn, initiate the inflammatory processes involved in NEC.
Proton pump inhibitors or H2 blockers have been linked to NEC because of changes to the intestinal microbiota (27, 33). The mechanism of action is not clear, but researchers suspect that H2 blockers increase the intestinal pH, consequently promoting the growth of Proteobacteria and overgrowth of the microbiota. The interaction between these microbiota and the intestinal epithelium has been associated with increased leukocytes and calprotectin, indicating mucosal inflammation (36). This inflammation may predicate NEC.
Health consequences.
NEC is associated with widespread effects. The length of hospital stay (LOS) is considerably longer for NEC patients than infants without NEC. One study that evaluated 291 VLBW infants found that LOS was much longer for infants with NEC than without (85 ± 36 d compared with 70 ± 33 d, respectively) (13). Another study reported similar differences in LOS, in which infants with NEC had a mean incremental LOS of 11.7 d (95% CI: 6.9, 16.5) compared with infants without NEC (16). Prolonged hospital stay is often used as a proxy for illness severity, but it may also be a risk factor for nosocomial infections and further complications.
Severe forms of NEC lead to surgery in ∼20–40% of cases (37, 38). Surgery involves laparotomy (often with intestinal resection) and ostomy creation, with potential long-term health effects and a mortality rate of ≤50% (27, 37, 39). Surgical NEC survivors may be affected by short-bowel syndrome or intestinal failure, with attendant failure to thrive and postoperative complications such as intestinal strictures, bowel obstruction, enterocutaneous fistulas, intraabdominal abscess, wound dehiscence, central line sepsis, or poor neurodevelopmental outcomes (14, 19, 37, 40–42).
Long-term outcomes for NEC survivors are also concerning. Ganapathy et al. (17) found that surgical NEC survivors were much more likely to have feeding difficulties and gastrointestinal ostomies from chronological ages 6–36 mo than matched controls with no diagnosis of NEC during birth hospitalization. Medical NEC infants (those treated with nonsurgical approaches) were more likely to have a higher risk of failure to thrive, feeding difficulties, neurodevelopmental delay, and open gastrointestinal ostomies between 6 and 12 mo than matched controls with various chronic conditions (17).
Health care costs.
The health care costs associated with NEC are exponential. Data from the United States in 2011 and 2012 indicate that the cost of NEC is $180,000 to $198,000/infant (13, 16) and nearly doubles to $313,000/infant for surgically treated NEC (13). By comparison, the mean neonatal intensive care unit (NICU) hospitalization cost for infants without NEC is ∼$134,500/infant (13). In the first 3 y of life, NEC survivors also accrue substantially higher outpatient costs. Ganapathy et al. (17) determined that between 6 and 36 mo of age, the cost difference between surgical NEC survivors and matched controls (no diagnosis of NEC) was ∼$97,000/infant. Medical NEC survivors incurred a mean $5000 more in health care costs than controls from 6 to 12 mo (17).
The type of enteral nutrition product used for preterm infants affects health care costs. Human milk (HM) may be supplied by a baby’s mother [mother’s own milk (MOM)] or a human donor (DHM). An exclusive HM diet for preterm infants weighing typically <1800 g and with a gestational age <32 wk at birth also includes an HM-based human milk liquid fortifier such as Prolact+ H2MF, which is manufactured by Prolacta (43). One study has found that an exclusive HM diet resulted in net hospital cost savings (excluding physician fees and posttreatment care costs) of $8167/extremely premature infant (95% CI: 4405, 11,930; P < 0.0001) and 3.9 fewer days in the NICU (95% CI: 3.25, 4.58; P < 0.0001) (16). However, an exclusive HM diet is substantially more expensive than a diet containing bovine milk–based products. The mean cost of 0.8 kcal enteral feed/mL that uses bovine milk–based products is $0.03/mL for preterm formula or ∼$0.05/mL MOM with human milk fortifier (16, 44) (Table 1). Alternatively, MOM with Prolact+ H2MF costs ∼$1.25/mL, and DHM with Prolact+ H2MF costs $1.33/mL for 0.8 kcal feed/mL (16, 43) (Table 1). Another retrospective study calculated hospital and physician costs for preterm infants ≤28 wk gestation and/or VLBW fed 4 different diets (45). The authors determined that total hospital charges per infant were much lower for the exclusive HM diet ($237,647) than diets consisting of MOM with a bovine milk–based fortifier ($265,035), formula only ($266,825), and a combination of MOM, bovine-based fortifier, and formula ($344,615). A caveat to this study is that selection bias may have been a concern given the single-center design and small sample size (n = 293). In addition, the study commenced in March 2009, the exclusive human milk diet was introduced in March 2012, and the study ended in March 2014. Confounding factors such as changes to clinical practices other than infant diets over the 5-y period may have affected the results. Nonetheless, these results suggest that nutritional interventions have an impact on service utilization and health care expenses. It is unknown how these costs compare to other nutritional products such as semielemental or elemental formulas because, to our knowledge, this topic has not yet been studied.
TABLE 1.
Cost comparison of Enfamil HMF (Mead Johnson Nutrition) and Prolact+ 4 (Prolacta Bioscience) H2MF to prepare enteral feeds of 0.8 kcal/mL for a preterm infant
| Enfamil HMF1 (powder) | Prolact+4 H2MF (liquid) | |
| Cost of fortifier | $1.20/sachet2 × 4 sachets = $4.80 | 20 mL Prolact+4 H2MF × $6.25/mL3 = $125 |
| Volume to prepare 0.8 kcal/mL | (0.71-g sachet2 × 4 sachets × 0.84 mL/g4 displacement) + 100 mL breast milk = 102.4 mL | 20 mL Prolact+4 H2MF + 80 mL breast milk5 = 100 mL |
| If mixed with MOM | $4.80/102.4 mL = $0.05/mL | $125/(100 mL) = $1.25/mL |
| If mixed with donor human milk | [$4.80 fortifier + ($0.10/mL3)(100 mL)]/102.4 mL = $0.14/mL | [$125 + ($0.10/mL3)(80 mL)]/100 mL = $1.33/mL |
Clearly, NEC is a multifactorial disease with substantial health consequences and costs. There are many research avenues available on this topic, but the focus of this review is on different types of enteral nutrition for the prevention of NEC.
Feeding protocols for preterm infants
Typical feeding progression.
Several challenges exist for preterm nutritional support. Many preterm infants, especially those born <1500 g and/or <34 wk gestation, are not able to breastfeed or start enteral feeds shortly after birth. The suck-swallow-breathe rhythm of oral feeding may not be possible for preterm infants because of coordination issues and/or low body stores of energy (27). Intense respiratory or cardiac support can limit or preclude an infant from oral or enteral feeds. The use of high-dose or multiple medications that compromise gut perfusion, cardiac lesions, substantial bladder pressure, acute abdominal issues, 48-h posthypoxic-ischemic encephalopathy or cardiopulmonary resuscitation, or persistent feeding intolerance are also contraindications for enteral feeds. Aggressive enteral feeding in the presence of ≥1 of these contraindications may potentiate NEC (46, 47). For these reasons, intravenous delivery of nutrients [parenteral nutrition (PN)] is often initiated for preterm infants after birth. PN is initiated slowly, individually prescribed to ensure tolerance and safety, and advanced to meet the infant’s nutritional and fluid needs. There are several risks associated with PN, such as line infections, liver damage, or gut atrophy (48, 49). A clinician’s aim is to wean PN and start enteral feeds as soon as possible while maintaining adequate energy and protein intake to promote appropriate growth velocity.
Nutritional practices of feeding initiation and advancement vary among neonatal practitioners, but enteral feeds typically follow a standard progression (50). Trophic feeding, also known as minimal enteral feeding or gut priming, of 10–24 mL ⋅ kg−1⋅d−1 HM is started for 1–4 d when appropriate to stimulate gastrointestinal functioning and promote endocrine and metabolic maturity (50–52). If tolerated, feeds are advanced by 20–30 mL ⋅ kg−1 ⋅ d−1 for VLBW infants and 15–25 mL ⋅ kg−1 ⋅ d−1 for extremely-low-birth-weight infants (50) or more slowly (10 mL ⋅ kg−1 ⋅ d−1) for infants with gastrointestinal or cardiac issues (11). Advancements continue until goal feeds are achieved. Enteral feeding goals are monitored daily and adjusted based on estimated energy requirements, fluid restrictions, medications, and clinical stability. PN is weaned as enteral intake increases to ensure nutritional goals are met. In general, caloric and protein goals for normal preterm development are 110–135 kcal ⋅ kg−1 ⋅ d−1 and 3–4.5 g protein ⋅ kg−1 ⋅ d−1 (8, 50, 53). Most preterm infants cannot meet these high needs through enteral intake of breast milk or standard formula alone (53–55). Therefore, fortification is required. Bovine milk–based and HM-based HM fortifiers (HMFs) contain additional energy, protein, fat, vitamins, and minerals (56) to ensure adequate growth, neurodevelopment, and bone mineralization (53). HMFs are typically added once enteral intake reaches 100 mL ⋅ kg−1 ⋅ d−1 to ensure the gut can tolerate more concentrated feeds. Some clinicians prefer to start the fortifier at 80 mL ⋅ kg−1 ⋅ d−1 or earlier to meet protein and energy goals sooner. HMFs are discontinued when the infant is 32–34 wk corrected gestational age and meeting growth expectations. Preterm infants are constantly monitored for feeding intolerance, including excessive gastric residuals, vomiting, diarrhea, distended abdomen, or bloody stools. If signs of feeding intolerance are observed, enteral feeds are either reduced or discontinued (50) to prevent exacerbating a problem that may trigger NEC.
Growth and development goals.
A tool used to monitor and evaluate health and nutritional status for preterm infants is the Fenton preterm growth charts for boys and girls (57). Expected postnatal growth velocities of preterm infants are based on an intrauterine growth of ∼15 g ⋅ kg−1 ⋅ d−1 (8, 54). Although this approach may not be precise given the differences between intra- and extrauterine environments, to our knowledge there are currently no alternative standards (57).
Nutritional goals for clinically stable infants are set to help them reach their genetic growth potentials and track on corrected gestational age- and sex-specific Fenton growth chart centiles for weight, length, and head circumference. After birth, it is expected that infants lose ≤10% of their birth weight (mean: 5.7–6.6%), but this weight is normally regained within 2 wk (58). Preterm infants often require enteral or parenteral nutritional support to help them achieve their growth potential. There is no need, however, to accelerate weight gain beyond the centile that the infant is tracking provided that growth is meeting patient-specific expectations. Doing so may lead to further harm from overfeeding. The key message is that although many preterm infants have considerably lower birth weights than their term counterparts and LBW is a risk factor for morbidities and mortality, preterm infants can still grow and develop at a rate that tracks the preterm growth chart and is appropriate for each infant’s genetic and physiologic potential. The difficulty of nutritional support is balancing adequate growth while avoiding complications and comorbidities that may predispose an infant to diseases such as NEC.
Sources of nutrition.
MOM.
HM includes breast milk from an infant’s mother (MOM) or DHM. There are many benefits of HM, including improved gastrointestinal functioning, protection against respiratory illnesses and infections (e.g., sepsis, urinary tract infections), improved bonding between the mother and baby, faster achievement of full enteral feeds, shorter LOS, and improved cognitive and visual development (54, 59). Breast milk is a functional food that contains the appropriate proportion of macronutrients for the optimal growth and development of infants (60–62) and bioactive agents to help them grow and mount immunologic defenses against diseases such as NEC (26, 63). For instance, lactoferrin is a glycoprotein in breast milk that is believed to aid in iron transport, but it also has antimicrobial properties. Lactoferrin has been found to mitigate the release of proinflammatory cytokines from monocytic cells in the presence of lipopolysaccharides (11). Breast milk also contains a host of immune cells such as mucosal-protective IgA; growth factors to promote enterocyte development; a phospholipid mediator, platelet-activating factor acetylhydrolase, which may be protective against NEC; Igs, cytokines, chemokines, prostaglandins, neuropeptides, and nucleotides; an appropriate pH and osmolarity for a newborn’s naïve gut; microbiota to colonize the gut and establish a healthy mucosal layer; and probiotic human milk oligosaccharides to facilitate the colonization of beneficial bacteria such as Lactobacillus and Bifidobacterium (7, 64, 65). Together, these active breast milk components promote the proliferation of beneficial microbiota relative to enterobacteria and influence immune system responses to favor an anti-inflammatory environment that is suspected to be protective against NEC and other diseases (26, 33).
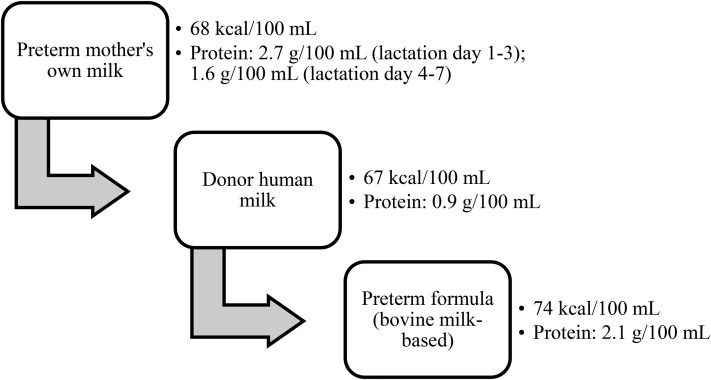
MOM has been recognized as the best source of nutrition for term and preterm infants (Figure 1) (7, 11, 54, 64, 66). The composition of breast milk changes over time to support an infant’s nutritional needs at different developmental stages. Notably, protein content in the first 8 wk of the preterm period is higher in preterm breast milk than in term breast milk (Table 2) (67). Colostrum contains the highest protein content in both term and preterm breast milk, with preterm colostrum having the highest concentration at 2.7 g compared with 2 g/100 mL in term colostrum (67). Higher protein intake is especially important to preterm infants given the accelerated rate of growth, anabolism, and brain development during the preterm period (55). Despite the benefits of preterm MOM, its macro- and micronutrient content alone is not sufficient to meet a preterm infant’s estimated high needs. As discussed previously, to meet the caloric, protein, and micronutrient requirements for most preterm infants, MOM must be fortified with HMF. Another consideration for preterm nutrition in the NICU is the availability of MOM. Delayed milk letdown, illness, psychologic stress, lack of understanding or social support (68), inability to put the baby to breast to stimulate milk production, or drug use may limit a mother’s supply. In these cases, alternative sources of nutrition are necessary.
FIGURE 1.
Preference for type of preterm nutrition. Mother’s own milk is the first choice of nutrition for preterm infants, followed by pasteurized donor human milk (66) and then bovine milk–based preterm formula. The composition of each nutrition source is different.
TABLE 2.
Nutritional comparison of preterm MOM, donor human milk, and preterm formula1
| Preterm MOM | Donor human milk | Preterm formula (bovine milk–based) | |
| Calories, kcal/100 mL (67) | 68 | 67 | 74 |
| Protein, g/100 mL (67) | 0.9 | 2.1 | |
| Days 1–3 | 2.7 | ||
| Days 4–7 | 1.6 | ||
| Week 2 | 1.3 | ||
| Weeks 3–4 | 1.1 | ||
| Fortification | Needed | More fortification needed than preterm breast milk given the low protein content | Concentrate as needed (patient-specific) |
| Bioactive components (e.g., immune cells, growth factors, prebiotics) | Present | Present but reduced by processing and pasteurization | Absent |
MOM, mother’s own milk.
DHM.
Pasteurized DHM is the next best source of nutrition for preterm infants if MOM is unavailable (Figure 1) (55, 66, 69). Compared with bovine milk–based formula, HM is efficiently digested and absorbed and contains immunologic cells and bioactive factors for infant growth and development. Although DHM shares some of the benefits of preterm MOM, its nutritional profile is different (Table 2). In accordance with the Human Milk Banking Association of North America, DHM is batched at human milk banks from 3 to 5 donors to maintain similar composition and quality of the milk across batches (70). Most donors have older infants or term infants and have been lactating for weeks or months (71). Because protein content decreases over time, the mean protein content of batched DHM is lower than preterm breast milk (67, 72). Adequate protein intake is essential for preterm infants given their rapid rate of weight gain and anabolism, so the limited protein content in DHM is a concern. As with preterm MOM, protein and energy deficits are corrected with fortification; however, more fortification may be required for DHM to compensate for its low mean protein content (73). Furthermore, the heating process of pasteurization can denature proteins and immunologic agents in DHM (74), possibly reducing the effectiveness of DHM in developing a preterm infant’s gut and immune systems. Despite these shortcomings, DHM is recommended as an alternative form of nutrition for preterm infants (66) because it is well-tolerated and still contains many beneficial bioactive components.
Standard infant formula.
Bovine milk–based preterm formulas are another option. The advantages of formula are that it provides a consistent amount of calories and macronutrients for adequate growth (75) and is less expensive than DHM (16). Several studies, however, have indicated that bovine milk–based products may increase the risk of NEC (8, 13, 18, 76). The mechanism of action is unclear. A possible explanation is that formula or bovine milk–based HMF does not contain oligosaccharides such as HM and that this deficiency may select for potentially pathogenic microbiota such as enterobacteria (26). The overgrowth of pathogenic microbiota and proinflammatory immune responses to the microbiota may contribute to the initiation of NEC (26).
Some researchers have suggested that casein rather than whey protein may be responsible for the gut lesions and proinflammatory immune responses that precede NEC (77). However, evidence regarding this hypothesis is conflicting. Thymann et al. (78) compared preterm piglets fed formula containing 100% whey to 40% whey and 60% casein for 30 h. Both formulas were isocaloric and equivalent with respect to the total amount of protein, maltodextrin, lactose, and fat. The piglets were killed after 30 h of feeding to determine NEC development and gut function. No significant difference was found with respect to the incidence and severity score of NEC, diversity of anaerobic and aerobic bacteria, glucose absorption, and lactase activity between the groups. The authors concluded that factors other than casein should be investigated in relation to NEC.
The processing of formula also leads to the removal of the milk fat globule membrane (79). One study found that the supplementation of the bovine milk fat globule membrane in infant formula for term infants led to several beneficial outcomes, including the decreased incidence of acute otitis media, decreased use of antipyretic medications, and increased production of serum IgG in response to the pneumococci vaccine (79). Effects from plant-based lipids such as soy oil added to infant formulas may also be problematic for the developing immune system of preterm infants. Higher ratios of ω-6 to ω-3 FAs and a higher proportion of arachidonic acid in soy oils are associated with proinflammatory responses (80, 81). The synthesis of leukotrienes and prostaglandins from arachidonic acid may propagate inflammation in response to cellular injury or infection (81), as seen with NEC. Therefore, the current composition or structure of bovine milk–based or artificial formulas may be unfavorable for certain infants, but again, this mechanism is not completely understood.
Hydrolyzed formula.
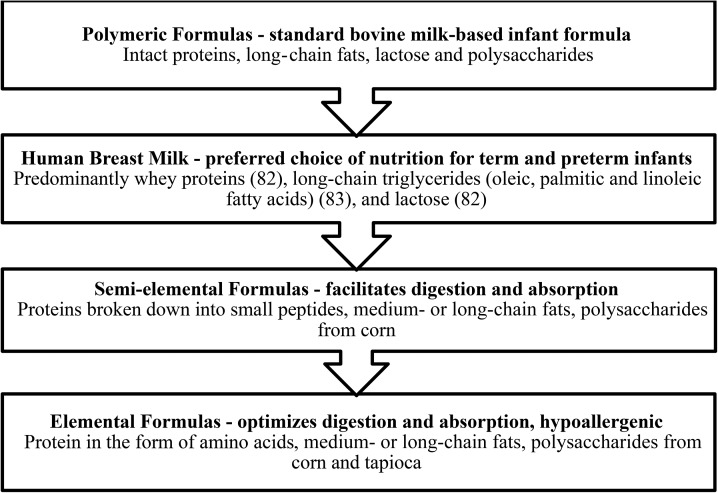
The other types of enteral formula for preterm infants are semielemental or elemental formulas. These formulas are primarily made from broken-down proteins (semielemental or protein-hydrosylated formula) or amino acids (elemental formula), medium-chain TGs, and a carbohydrate source (e.g., corn syrup solids) (Figure 2) (82, 83). The purpose of semielemental or elemental formulas is to facilitate nutrient digestion and absorption because there is minimal reliance on the gut to produce the enzymes, bile salts, and gastric juices needed to digest complex nutrients (84). Amino acids or small peptides, easily absorbed fats (e.g., medium-chain TG oil), and simple sugars (e.g., glucose) are especially beneficial for patients that are severely ill, have a feeding intolerance, or are at risk of gastrointestinal complications (84). In relation to NEC, semielemental or elemental formulas may protect against cytotoxicity of enterocytes and the propagation of proinflammatory processes. A cell-based study by Penn et al. (85) designed to test the cytotoxicity of enzymatically digested breast milk and infant formulas on intestinal epithelial cells offers a potential mechanism of action for the effectiveness of hydrolyzed formulas. The authors hypothesized that unbound free FAs (FFAs) produced by lipase digestion of standard formula would be cytotoxic to rat intestinal cells but that the digestion of fresh breast milk would not. Cytotoxicity was defined as the death of >5% of rat intestinal epithelial cells or >15% of neutrophil death. In total, 9 different infant formulas were tested, and all 9 resulted in significantly greater cytotoxicity after digestion with lipase or lipase plus proteases (P < 0.007; P < 0.025 was considered significant), as determined by greater epithelial cell death. Fresh breast milk digestion did not result in cytotoxicity. Interestingly, the addition of orlistat (a lipase inhibitor) (P < 0.0023; P < 0.017 was considered significant), bovine serum albumin (BSA) (P < 0.00008; P < 0.05 was considered significant) or proteases (P < 0.008; P < 0.025 was considered significant) reduced cytotoxicity significantly. The authors reasoned that the inhibition of lipase reduced the production of unbound FFAs and BSA bound and neutralized the unbound FFAs, thereby minimizing cell death. Similarly, proteases may help deactivate unbound FFAs by opening intact proteins, exposing their hydrophobic core, and increasing the ability of proteins to bind unbound FFAs. Fresh breast milk was suspected to resist cytotoxicity, potentially through the deactivation of pancreatic lipases and its lipid profile, which consists of fats that are less susceptible to lipase digestion.
FIGURE 2.
Breakdown of macronutrient composition in infant nutrition sources and corresponding uses (82, 83).
Studies in piglets that compared elemental diets with bovine milk–based diets have also uncovered potential benefits of elemental diets. Piglets are the best nonprimate model for studying neonatal nutrition because the gastrointestinal anatomy, physiology, and nutrient requirements of pigs are the most similar to humans (86). In one such study, Connor et al. (87) compared polymeric and elemental formulas for 3 surgically created short-bowel syndrome groups: 1) midintestinal resection with a jejunoileal anastomosis (equal amount of jejunum and ileum remaining) (n = 16); 2) distal intestinal resection, including the ileum, cecum, and 5 cm of the spiral colon, with a jejunocolic anastomosis (n = 17); and 3) sham surgery (n = 15). After surgical treatment, enteral nutrition was initiated with either a polymeric or isocaloric and isonitrogenous elemental formula on postoperative day 2. The polymeric formula contained nonfat milk and whey protein concentrate, lactose and glucose polymers, and high-fat oleic sunflower or safflower, soy, and coconut oils. The authors did not specify whether the polymeric formula was bovine milk–based, but this was likely the case. The outcomes of interest were functional and structural adaptations of the intestine, as well as glucagon-like peptide 2 (GLP-2), a gut-specific hormone that improves nutritional absorption and intestinal barrier function (87). No difference was found in structural measures such as intestinal lengthening, villus height, crypt depth, and colon weight between the diet groups. The concentration of plasma GLP-2 was higher at the end of the trial for the jejunocolic anastomosis piglets fed the polymeric formula than those on the elemental formula. The authors reasoned that higher GLP-2 concentrations for the polymeric diet may have resulted from undigested polymeric nutrients being used by bacteria in the colon that produced short-chain FAs and, in turn, GLP-2. However, in the same jejunocolic anastomosis group, the elemental diet led to significantly fewer days of diarrhea (9.9 ± 0.8 d on the elemental diet compared with 12.3 ± 0.4 d on the polymeric diet; P = 0.023) and PN support (12.7 ± 0.6 d on the elemental diet compared with 14.1 ± 0.1 d on the polymeric diet; P = 0.047). These improved functional measures with the elemental diet were deemed to be highly beneficial for an animal model with a surgically removed ileum.
With the use of a healthy piglet model, Stoll et al. (88) investigated the effects of bovine milk–based formula and an elemental formula fed over 6 d in piglets aged 3 wk. The elemental diet consisted of crystalline amino acids, glucose, and a lipid emulsion, and the polymeric diet was a bovine milk–based formula. Piglets on the elemental diet were fed intragastrically at a continuous rate, whereas piglets on the polymeric formula were fed orally 3 times/d. The elemental diet provided less calories and protein than the polymeric diet (165 kcal ⋅ kg−1 ⋅ d−1 and 10.6 g amino acids ⋅ kg−1 ⋅ d−1 compared with 195 kcal ⋅ kg−1 ⋅ d−1 and 12.5 g protein ⋅ kg−1 ⋅ d−1, respectively). The main purpose was to compare small intestinal growth and function between the 2 diet groups. There was no difference in total body weight or intestinal cell morphology (crypt depth, villus height, and muscle thickness) at the end of the 6-d trial. In contrast to the aforementioned piglet study (86), Stoll et al. (88) found that cell proliferation and protein synthesis, measured by the percentage of labeled crypt cells in the S-phase and ornithine decarboxylase activity, were considerably higher in the proximal jejunum and ileum of the piglets fed an elemental diet. Furthermore, concentrations of gut hormones GLP-2 and glucose-dependent insulinotropic polypeptide, but not peptide YY, were considerably higher in the elemental diet group. The authors concluded that an elemental diet matches a polymeric diet with respect to intestinal growth and cell morphology, with an added benefit of stimulating gut hormone production, cell proliferation, and protein synthesis. These conclusions should be interpreted with caution because of the difference in feeding protocols between the diet groups and short study duration (i.e., observations over several weeks would provide more robust results regarding cell morphology and gut function).
Overall, these cell and animal studies highlight the possible benefits of hydrolyzed formula in terms of intestinal structure, function, and absorption and provide insight for future clinical studies.
Current Status of Knowledge
In this section, we evaluate several randomized controlled trials (RCTs) and a Cochrane systematic review that compared the effect of different types of nutritional products (MOM, DHM, bovine milk–based formula, and an elemental fortifier) on the incidence of NEC in preterm infants.
MOM compared with preterm formula.
Sullivan et al. (18) conducted a multicenter RCT to evaluate the health effects of an exclusive HM diet compared with a diet containing both HM and bovine milk–based products. This study analyzed 207 preterm infants. Eligibility criteria included a birth weight between 500 and 1250 g, mothers’ intention to provide breast milk, enteral feedings started within 21 d of life, and PN started within 48 h of life. Infants with major congenital malformations were excluded from enrollment. The authors did not mention whether gastrointestinal comorbidities were considered a part of the eligibility criteria. Randomization to 3 groups occurred in blocks of 4 that were stratified by birth-weight categories (500–750, 751–1000, and 1001–1250 g) and whether the infants were appropriate or small for gestational age. Comparison groups were based on the type of enteral feeds and when fortifier was added. These groups were defined as follows: 1) HM100: HM-based fortifier was added once enteral feeds of MOM reached 100 mL ⋅ kg−1 ⋅ d−1, and DHM was used if MOM was unavailable; 2) HM40: same intervention as the HM100 group, except the fortifier was started once enteral feeds reached 40 mL ⋅ kg−1 ⋅ d−1; and 3) BOV: after enteral feeds of MOM were started, bovine milk–based fortifier was added once feeds reached 100 mL ⋅ kg−1 ⋅ d−1, and bovine milk-based preterm formula was used if MOM was unavailable. Standard feeding protocols were maintained for all infants. Outcomes were measured until the earlier of 91 d of life, hospital discharge, or 50% of oral feed goals were achieved.
No significant differences were found for days of PN, LOS, late-onset sepsis, or growth, although a subsequent analysis found that the probability of needing PN was significantly reduced by 11–14% for an exclusive HM diet (89). There were no differences between the HM100 and HM40 for any of the outcomes. After adjusting for confounding factors with the use of multivariate logistic regression, the OR for NEC was 0.23 (95% CI: 0.08, 0.66), or a 77% reduction in the odds of developing NEC, in favor of an exclusive HM diet.
A criticism of the study is that the method of randomization was not clear. The randomized block number (blocks of 4) was not divisible by the 3 comparison groups or 3 birth weight strata. This approach may have led to imbalances between the groups for known and unknown factors. A more transparent method would have been to create random blocks of a number divisible by 3 (90). Another important note is that 3 infants (4.5%) in the HM100 group and 5 (7.0%) in the HM40 group developed NEC (Table 3). Of these cases, NEC led to mortality for 1 infant in each of the HM groups, although the authors reported that both of these infants were protocol violators who had received some amount of bovine milk–based formula or fortifier during the study. Nonetheless, this finding reinforces that NEC is a multifactorial disease, and an exclusive HM diet may not fully protect infants from NEC.
TABLE 3.
Summary of studies that have evaluated the effect of infant diets on the incidence of NEC1
| References | Study design | Population | Duration of intervention | Comparison groups | NEC, n (%) |
| 182 | RCT | 500–1250 g | ≤91 d old, hospital discharge, or 50% oral feeds (4 complete feeds/d) achieved | HM100, HM40, and BOV | HM100: 3 (4.5%); HM40: 5 (7.0%); and BOV: 11 (15.9%)* |
| 76 | RCT | 500–1250 g | ≤91 d old, hospital discharge, or 50% oral feeds (4 complete feeds/d) achieved | HM and BOV | HM: 1 (3%); BOV: 5 (21%) |
| 75 | SR—4 RCTs of relevance (76, 91–93) | 500–1250, <1600, <1850, and <1500 g | ≤91 d old, hospital discharge, or 50% oral feeds achieved (76); until weight reached 1800 g (91); until discharge or transfer, or 2000 g (92); and from the 10th day of life until 2000 g or illness requiring intravenous nutrition (93) | DHM and BOV | DHM: 3 (1.6%); BOV: 13; (7.6%)** |
| 53 | RCT | <33 wk GA and 700–1500 g | Until 29 d after fortification or hospital discharge | LE-HMF and PI-HMF | LE-HMF: 1 (1.5%); PI-HMF: 2 (3.2%) |
*HM100 compared with BOV, P = 0.04; HM100 + HM40 compared with BOV, P = 0.02; and HM40 compared with BOV, P = 0.09. **DHM compared with BOV, P = 0.009. BOV, bovine milk–based preterm formula provided if MOM unavailable or bovine milk–based fortifier added when breast milk intake reached 100 mL/kg; DHM, donor human milk as sole diet; GA, gestational age; HM, pasteurized donor human milk plus human milk–based human milk fortifier; HM40, exclusive human milk diet, fortifier added when feeds reached 40 mL/kg; HM100, exclusive human milk diet, fortifier added when feeds reached 100 mL/kg; LE-HMF, liquid human milk fortifier with extensively hydrolyzed proteins; MOM, mother’s own milk; NEC, necrotizing enterocolitis; PI-HMF, powdered human milk fortifier with intact proteins; RCT, randomized controlled trial; SR, systematic review.
In both the HM100 and HM40 groups, 1 NEC case was a protocol violator that had received some amount of bovine milk–based formula or fortifier.
DHM compared with formula.
Cristofalo et al. (76) performed an RCT that paralleled Sullivan et al. (18) in objectives and methodology. The difference in Cristafalo et al. (76) was that MOM was not used—only DHM. In this multicenter blinded trial, 53 preterm infants weighing between 500 and 1250 g at birth were randomly assigned to 2 groups: DHM with HM-based fortifier (concentration not reported) (n = 29) or preterm formula concentrated to 0.8 kcal/mL (n = 24).
Unlike Sullivan et al. (18), Cristafalo et al. (76) found a significant reduction in the days of PN (27 compared with 36; P = 0.04) in favor of the HM group. Surgical NEC was significantly lower in the HM group (0 compared with 4 cases; P = 0.036), but the incidence of NEC (1 compared with 5 cases; P = 0.08) (Table 3) and NEC and/or death (1 compared with 5 cases; P = 0.08) were not significant. The findings were affirmed even after controlling for race, antenatal steroids, Apgar score, and age at the first enteral feed. Note that because the study was powered on the duration of PN as the primary outcome, it may not have been adequately powered to detect differences between the groups on NEC outcomes. The authors acknowledged that a potential issue with the study was that eligibility included no intention to provide MOM. The unavailability of MOM may have been caused by exposure to medications or medical problems, mother’s absence, or illicit drug use. These variables may have been confounders for NEC.
On the whole, the study found no significant difference between the DHM and preterm formula on the incidence of NEC (possibly because of the smaller sample size), but the incidence of surgical NEC supported the previous study. Both Sullivan et al. (18) and Cristofalo et al. (76) recommended an exclusive HM diet as a strategy for improving clinical outcomes, namely to reduce the incidence of NEC.
In 2014, a Cochrane systematic review compared bovine milk–based formula with DHM for feeding preterm or LBW infants (75). Nine RCTs, including the RCT conducted by Cristofalo et al. (76), involving 1070 infants were analyzed. The included RCTs compared formula with DHM in preterm or LBW infants in regard to short- and long-term (6 mo postterm) growth and neurodevelopmental outcomes. Secondary outcomes were all-cause mortality, NEC, days to full enteral feeds, feeding intolerance, and invasive infections. Most studies analyzed included patients who were stable, aged <2 wk, and weighed <1800 g at birth. Four trials compared term formula with DHM, and 5 trials compared preterm formula with DHM. One trial used unpasteurized DHM.
A meta-analysis that included 5 studies (n = 802 patients) on preterm formula and 1 study (n = 67 patients) on term formula determined that formula had a 2.77 greater risk of NEC than DHM (95% CI: 1.4, 5.46; I2 = 0). There was a slightly lower risk for preterm formula-only compared with DHM (RR: 2.61; 95% CI: 1.27, 5.35; I2 = 0). A subgroup analysis of 360 patients further examined the effect of preterm formula as a sole source of nutrition or supplemental nutrition. Preterm formula as a sole source of nutrition was associated with a significantly higher risk of NEC (RR: 4.62; 95% CI: 1.47, 14.56). The CI around the risk ratio was wide, suggesting either a small sample size or considerable heterogeneity within the sample with respect to the treatment effect. As supplemental nutrition, there was no significant difference between DHM and preterm formula for the incidence of NEC (RR: 1.96; 95% CI: 0.82, 4.67); however, there were twice as many NEC cases in the formula group (n = 15) than there were in the DHM group (n = 7) (Table 3).
A limitation of this evidence is the unclear or high selection bias for nearly half of the included studies and unclear performance and detection bias for most. Unclear allocation concealment and lack of blinding may have influenced the results; therefore, the findings should be interpreted with caution. The authors also noted that several included studies were conducted >20 y ago, but formula, DHM technologies, and clinical practice have evolved since that time. Outdated evidence poses even more questions for clinical practice. This limitation emphasizes the need for more trials to accurately assess the harms and benefits of current nutritional products.
Hydrolyzed nutrition products.
Kim et al. (53) conducted a nonblinded, multicenter, noninferiority RCT that involved protein-hydrosylated HMF. The trial compared liquid HMF with extensively hydrolyzed proteins (LE-HMF) to powdered HMF with intact proteins (PI-HMF) for enterally fed preterm infants. All infants were born <33 wk gestation, had a birth weight between 700 and 1500 g, and were fed MOM. DHM was not used during the study unless indicated by the clinician or principal investigator. HMF was added once feeds reached 100 mL ⋅ kg−1 ⋅ d−1. The HMFs were similar in caloric density, fat, carbohydrate, phosphorus, and vitamin D content. However, LE-HMF had more protein (3.6 compared with 3 g/100 kcal), twice the amount of docosahexaenoic acid, less calcium (153 compared with 175 mg/100 kcal), higher osmolality (450 compared with 385 mOsm water/kg), and added lutein (23 μg/100 kcal). Infants were followed for 29 d after HMF was started or until hospital discharge. The primary outcome was weight gain per day.
There were 63 and 66 infants included in the intention-to-treat analysis for the PI-HMF and LE-HMF groups, respectively. Noninferiority was achieved for the primary outcome, weight gain, because there was no significant difference between the study groups when the intention-to-treat analysis was used. However, the analysis that compared only the strict protocol followers found a substantially higher weight for the infants fed LE-HMF in the last 14 d of the study. Both HMFs were well-tolerated. There were no significant differences between the groups for length and head circumference gain, stool characteristics, and energy intake. The LE-HMF contained more protein than the PI-HMF and, as expected, infants in the LE-HMF group had higher protein intake (3.9 compared with 3.3 g ⋅ kg−1 ⋅ d−1; P < 0.0001), blood urea nitrogen (9.31 ± 0.53 compared with 5.81 ± 0.38 mg/dL), and prealbumin concentrations (10.01 ± 0.35 compared with 9.08 ± 0.35 mg/dL). All biochemistries were within normal limits. NEC incidence was low in the LE-HMF and PI-HMF groups (1.5% and 3.2% of infants, respectively). The incidence of sepsis was also low in both groups (4.5% of infants fed LE-HMF and 3.2% of infants fed PI-HMF). Of note, significantly fewer infants discontinued HMF because of feeding intolerance in the LE-HMF (2% of infants) than the PI-HMF (10% of infants) group (P = 0.048).
The authors concluded that the use of both HMFs achieved weight gain goals. Feeding intolerance and morbidities were minimal in the 2 groups; therefore, both HMFs were deemed safe. LE-HMF may have the potential to optimize growth without increasing the risk of morbidities, as evidenced by the significantly higher mean weight of infants by the study endpoint and low incidence of NEC and sepsis. A larger equivalence trial or one powered to detect a significant difference for the incidence of NEC is needed to support this hypothesis.
Conclusions
In summary, HM has been acknowledged as the best source of nutrition for preterm infants and those at risk for NEC (8, 13, 16, 18, 26, 75, 76, 94–96). Two RCTs on preterm infants weighing between 500 and 1250 g at birth compared the effect of bovine milk–based preterm infant formula to MOM or DHM on the incidence of NEC (18, 76). Both trials found that an exclusive HM diet results in a lower incidence of NEC. A Cochrane systematic review that evaluated the effect of DHM or bovine milk–based formula on health outcomes for preterm infants also determined that formula significantly increases the risk of NEC (75). The review authors cautioned, however, that potential sources of bias, particularly the lack of blinding and unclear allocation concealment, may have influenced the results. These pivotal studies have prompted the ongoing research and development of HM-based products such as pasteurized DHM and Prolacta fortifiers. Indeed, several questions remain. Based on previous trials and a Cochrane systematic review, ∼1–3% of infants fed an exclusively human milk diet develop NEC (18, 75, 76). These studies have not explained why HM is superior or why some infants, albeit a small percentage, fed an exclusively HM diet still develop NEC.
Semielemental or elemental formulas may be an effective nutritional intervention to reduce the risk of NEC in preterm infants. The nutrients in semielemental or elemental formulas are easy to absorb, which is expected to reduce stress on the gut and potentially avoid the proinflammatory processes that lead to NEC. Although semielemental or elemental formulas do not contain immunologic factors such as MOM, the benefit of readily absorbed nutrients may outweigh this deficit. Limited research on semielemental or elemental formulas and NEC was found for this review; hence, more research evaluating the effect of these specialty formulas on the incidence of NEC is warranted. This is an area of study our group is pursuing.
Acknowledgments
We thank Khalid Aziz and Manoj Kumar for their assistance in collecting data relating to NEC. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BSA, bovine serum albumin; DHM, donor human milk; FFA, free FA; GLP-2, glucagon-like peptide 2; HM, human milk; HMF, HM fortifier; HM40, exclusive HM diet fortifier added when feeds reached 40 mL/kg; HM100, exclusive HM diet fortifier added when feeds reached 100 mL/kg; LBW, low birth weight; LE-HMF, liquid HMF with extensively hydrolyzed proteins; LOS, length of hospital stay; MOM, mother’s own milk; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; PI-HMF, powdered HMF with intact proteins; PN, parenteral nutrition; RCT, randomized control trial; TLR, toll-like receptor; VLBW, very low birth weight.
References
- 1.Thanh NX, Toye J, Savu A, Kumar M, Kaul P. Health service use and costs associated with low birth weight: a population level analysis. J Pediatr 2015;167:551–6.e1–3. [DOI] [PubMed] [Google Scholar]
- 2.Bartholomew S, Deb-Rinker P, Dzakpasu S, Gilbert NL, Nelson C, Liu S. Perinatal health indicators for Canada 2013 [Internet]. [cited 2016 Sep 26]. Available from: http://publications.gc.ca/site/eng/411563/publication.html.
- 3.Hamilton BE, Martin JA, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2014. Natl Vital Stat Rep 2015;64:1–64. [PubMed] [Google Scholar]
- 4.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2162–72. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine. Preterm birth: causes, consequences, and prevention. Washington (DC): National Academy of Sciences; 2007. [Google Scholar]
- 6.Yamakawa T, Itabashi K, Kusuda S. Mortality and morbidity risks vary with birth weight standard deviation score in growth restricted extremely preterm infants. Early Hum Dev 2016;92:7–11. [DOI] [PubMed] [Google Scholar]
- 7.Verd S, Ginovart G, Gutierrez A, Botet F, Barbero AH, Porta R. Hospital outcomes of extremely low birth weight infants after introduction of donor milk to supplement mother’s milk. Breastfeed Med 2015;10:150–5. [DOI] [PubMed] [Google Scholar]
- 8.Chowning R, Radmacher P, Lewis S, Serke L, Pettit N, Adamkin DH. A retrospective analysis of the effect of human milk on prevention of necrotizing enterocolitis and postnatal growth. J Perinatol 2016;36:221–4. [DOI] [PubMed] [Google Scholar]
- 9.Mizrahi A, Barlow O, Berdon W, Blanc WA, Silverman WA. Necrotizing enterocolitis in premature infants. J Pediatr 1965;66:697–705. [DOI] [PubMed] [Google Scholar]
- 10.Yajamanyam PK, Rasiah SV, Ewer AK. Necrotizing enterocolitis: current perspectives. Res Rep Neonatol 2014;4:31–42. [Google Scholar]
- 11.Good M, Sodhi CP, Hackam DJ. Evidence-based feeding strategies before and after the development of necrotizing enterocolitis. Expert Rev Clin Immunol 2014;10:875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregory KE, DeForge CE, Natale KM, Phillips M, Van Marter LJ. Necrotizing enterocolitis in the premature infant. Adv Neonatal Care 2011;11:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson TJ, Patel AL, Bigger HR, Engstrom JL, Meier PP. Cost savings of human milk as a strategy to reduce the incidence of necrotizing enterocolitis in very low birth weight infants. Neonatology 2015;107:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghoneim N, Bauchart-Thevret C, Oosterloo B, Stoll B, Kulkarni M, de Pipaon MS, Zamora IJ, Olutoye OO, Berg B, Wittke A, et al. Delayed initiation but not gradual advancement of enteral formula feeding reduces the incidence of necrotizing enterocolitis (NEC) in preterm pigs. PLoS ONE 2014;9:e106888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sho S, Neal MD, Sperry J, Hackam DJ. A novel scoring system to predict the development of necrotizing enterocolitis totalis in premature infants. J Pediatr Surg 2014;49:1053–6. [DOI] [PubMed] [Google Scholar]
- 16.Ganapathy V, Hay JW, Kim JH. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed Med 2012;7:29–37. [DOI] [PubMed] [Google Scholar]
- 17.Ganapathy V, Hay JW, Kim JH, Lee ML, Rechtman DJ. Long term healthcare costs of infants who survived neonatal necrotizing enterocolitis: a retrospective longitudinal study among infants enrolled in Texas Medicaid. BMC Pediatrics 2013;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl-Kohlendorfer U, Chan GM, Blanco CL, Abrams S, Cotten CM, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 2010;156:562–7.e1. [DOI] [PubMed] [Google Scholar]
- 19.Downard CD, Renaud E, St. Peter SD, Abdullah F, Islam S, Saito JM, Blakely ML, Huang EY, Arca MJ, Cassidy L, et al. Treatment of necrotizing enterocolitis: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg 2012;47:2111–22. [DOI] [PubMed] [Google Scholar]
- 20.Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol 2013;40:27–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markel TA, Engelstad H, Poindexter BB. Predicting disease severity of necrotizing enterocolitis: how to identify infants for future novel therapies. J Clin Neonatol 2014;3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostlie DJ, Spilde TL, St Peter SD, Sexton N, Miller KA, Sharp RJ, Gittes GK, Snyder CL. Necrotizing enterocolitis in full-term infants. J Pediatr Surg 2003;38:1039–42. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, Lillehei C, Valim C, Horbar JD, Jaksic T. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg 2009;44:1072–5. [DOI] [PubMed] [Google Scholar]
- 24.Sinha SK, Gupta S, Donn SM. Immediate respiratory management of the preterm infant. Semin Fetal Neonatal Med 2008;13:24–9. [DOI] [PubMed] [Google Scholar]
- 25.Capozzi G, Santoro G. Patent ductus arteriosus: patho-physiology, hemodynamic effects and clinical complications. J Matern Fetal Neonatal Med 2011;24 Suppl 1:15–6. [DOI] [PubMed] [Google Scholar]
- 26.Siggers RH, Siggers J, Thymann T, Boye M, Sangild PT. Nutritional modulation of the gut microbiota and immune system in preterm neonates susceptible to necrotizing enterocolitis. J Nutr Biochem 2011;22:511–21. [DOI] [PubMed] [Google Scholar]
- 27.Neu J. Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr 2007;85:629S–34S. [DOI] [PubMed] [Google Scholar]
- 28.Barcellini W, Imperiali FG, Zaninoni A, Reda G, Consonni D, Fattizzo B, Lonati S, Nobili L, Zanella A, Cortelezzi A. Toll-like receptor 4 and 9 expression in B-chronic lymphocytic leukemia: relationship with infections, autoimmunity and disease progression. Leuk Lymphoma 2014;55:1768–73. [DOI] [PubMed] [Google Scholar]
- 29.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward JB, Keely SJ, Keely SJ. Oxygen in the regulation of intestinal epithelial transport. J Physiol 2014;592:2473–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammers AL, Sanchez-Ramos L, Kaunitz AM. Antenatal exposure to indomethacin increases the risk of severe intraventricular hemorrhage, necrotizing enterocolitis, and periventricular leukomalacia: a systematic review with meta-analysis. Am J Obstet Gynecol 2015;212:505.e1–13. [DOI] [PubMed] [Google Scholar]
- 32.Tappenden KA. Provision of phosphorylatable substrate during hypoxia decreases jejunal barrier function. Nutrition 2002;18:168–72. [DOI] [PubMed] [Google Scholar]
- 33.Neu J. Preterm infant nutrition, gut bacteria, and necrotizing enterocolitis. Curr Opin Clin Nutr Metab Care 2015;18:285–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neu J, Douglas-Escobar M, Lopez M. Microbes and the developing gastrointestinal tract. Nutr Clin Pract 2007;22:174–82. [DOI] [PubMed] [Google Scholar]
- 35.Rezaie A, Pimentel M, Rao SS. How to test and treat small intestinal bacterial overgrowth: an evidence-based approach. Curr Gastroenterol Rep 2016;18:8. [DOI] [PubMed] [Google Scholar]
- 36.Freedberg DE, Lebwohl B, Abrams JA. The impact of proton pump inhibitors on the human gastrointestinal microbiome. Clin Lab Med 2014;34:771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 2006;368:1271–83. [DOI] [PubMed] [Google Scholar]
- 38.Sankaran K, Puckett B, Lee DSC, Seshia M, Boulton J, Qiu Z, Lee SK. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr 2004;39:366–72. [DOI] [PubMed] [Google Scholar]
- 39.Stey A, Barnert ES, Tseng C-H, Keeler E, Needleman J, Leng M, Kelley-Quon LI, Shew SB. Outcomes and costs of surgical treatments of necrotizing enterocolitis. Pediatrics 2015;135:e1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soraisham AS, Amin HJ, Al-Hindi MY, Singhal N, Sauve RS. Does necrotising enterocolitis impact the neurodevelopmental and growth outcomes in preterm infants with birthweight ≤1250 g? J Paediatr Child Health 2006;42:499–504. [DOI] [PubMed] [Google Scholar]
- 41.Merhar SL, Ramos Y, Meinzen-Derr J, Kline-Fath BM. Brain magnetic resonance imaging in infants with surgical necrotizing enterocolitis or spontaneous intestinal perforation versus medical necrotizing enterocolitis. J Pediatr 2014;164:410–2.e1. [DOI] [PubMed] [Google Scholar]
- 42.Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed 2007;92:F193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prolacta Bioscience. Prolact+ H2MF® human milk-based liquid human milk fortifier [Internet]. [cited 2016 Feb 1]. Available from: http://www.prolacta.com/human-milk-fortifier-1.
- 44.drugstore.com. Enfamil human milk fortifier, powder, 71g foil sachets [Internet]. [cited 2016 Apr 5]. Available from: http://www.drugstore.com/enfamil-human-milk-fortifier-powder-71g-foil-sachets/qxp308429.
- 45.Assad M, Elliott MJ, Abraham JH. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J Perinatol 2016;36:216–20. [DOI] [PubMed] [Google Scholar]
- 46.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol 2008;32:70–82. [DOI] [PubMed] [Google Scholar]
- 47.Fallon EM, Nehra D, Potemkin AK, Gura KM, Simpser E, Compher C, Puder M. A.S.P.E. N. clinical guidelines: nutrition support of neonatal patients at risk for necrotizing enterocolitis. JPEN J Parenter Enteral Nutr 2012;36:506–23. [DOI] [PubMed] [Google Scholar]
- 48.Robinson DT, Shah S, Murthy K. Parenteral nutrition use and associated outcomes in a select cohort of low birth weight neonates. Am J Perinatol 2014;31:933–8. [DOI] [PubMed] [Google Scholar]
- 49.Blackmer A, Luisa PM. Three-in-one parenteral nutrition in neonates and pediatric patients: risks and benefits. Nutr Clin Pract 2015;30:337–43. [DOI] [PubMed] [Google Scholar]
- 50.Senterre T. Practice of enteral nutrition in very low birth weight and extremely low birth weight infants. In: Koletzko B, Poindexter B, Uauy R, editors. Nutritional care of preterm infants scientific basis and practical guidelines. Basel (Switzerland): Karger; 2014. p. 201–14. [DOI] [PubMed] [Google Scholar]
- 51.Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database Syst Rev 2013;3: CD000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah P, Nathan E, Doherty D, Patole S. Optimising enteral nutrition in growth restricted extremely preterm neonates—a difficult proposition. J Matern Fetal Neonatal Med 2015;28:1981–4. [DOI] [PubMed] [Google Scholar]
- 53.Kim JH, Chan G, Schanler R, Groh-Wargo S, Bloom B, Dimmit R, Williams L, Baggs G, Barrett-Reis B. Growth and tolerance of preterm infants fed a new extensively hydrolyzed liquid human milk fortifier. J Pediatr Gastroenterol Nutr 2015;61:665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adamkin DH, Radmacher PG. Fortification of human milk in very low birth weight infants (VLBW <1500 g birth weight). Clin Perinatol 2014;41:405–21. [DOI] [PubMed] [Google Scholar]
- 55.Prince A, Groh-Wargo S. Nutrition management for the promotion of growth in very low birth weight premature infants. Nutr Clin Pract 2013;28:659–68. [DOI] [PubMed] [Google Scholar]
- 56.Mead Johnson Nutritionals. Estimated nutrient content of preterm human milk and Enfamil human milk fortifier [Internet]. [cited 2016 Apr 25]. Available from: https://www.meadjohnson.com/pediatrics/us-en/product-information/products/premature/enfamil-human-milk-fortifier-powder#nutrients-sup-sup.
- 57.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med 2008;2:e99–110. [PMC free article] [PubMed] [Google Scholar]
- 59.Armand M, Hamosh M, Mehta NR, Angelus PA, Philpott JR, Henderson TR, Dwyer NK, Lairon D, Hamosh P. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr Res 1996;40:429–37. [DOI] [PubMed] [Google Scholar]
- 60.Butte NF, Lopez-Alarcon MG, Garza C. Nutrient adequacy of exclusive breastfeeding for the term infant during the first six months of life. Geneva (Switzerland): WHO; 2002. [Google Scholar]
- 61.Infant Feeding Joing Working Group. Nutrition for healthy term infants: recommendations from birth to six months [Internet]. [cited 2016 Apr 25]. Available from: http://www.hc-sc.gc.ca/fn-an/nutrition/infant-nourisson/recom/index-eng.php#a4.
- 62.Andreas NJ, Kampmann B, Le-Doare KM. Human breast milk: a review on its composition and bioactivity. Early Hum Dev 2015;91:629–35. [DOI] [PubMed] [Google Scholar]
- 63.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McInnes RJ, Shepherd AJ, Cheyne H, Niven C. Infant feeding in the neonatal unit. Matern Child Nutr 2010;6:306–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caplan MS, Amer M, Jilling T. The role of human milk in necrotizing enterocolitis. Adv Exp Med Biol 2002;503:83–90. [DOI] [PubMed] [Google Scholar]
- 66.WHO. Donor human milk for low-birth-weight infants [Internet]. [cited 2016 Apr 4]. Available from: http://www.who.int/elena/titles/donormilk_infants/en.
- 67.Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr 2014;14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meier PP. Breastfeeding in the special care nursery. Pediatr Clin North Am 2001;48:425–42. [DOI] [PubMed] [Google Scholar]
- 69.Groh-Wargo S, Sapsford A. Enteral nutrition support of the preterm infant in the neonatal intensive care unit. Nutr Clin Pract 2009;24:363–76. [DOI] [PubMed] [Google Scholar]
- 70.Human Milk Banking Association of North America. Donor human milk processing [Internet]. [cited 2016 Feb 3]. Available from: https://www.hmbana.org/milk-processing.
- 71.Aceti A, Corvaglia L, Faldella G. Human milk banks: lights and shadows. JNIM 2014;3: e030225. [Google Scholar]
- 72.Stoltz Sjöström E, Ohlund I, Tornevi A, Domellof M. Intake and macronutrient content of human milk given to extremely preterm infants. J Hum Lact 2014;30:442–9. [DOI] [PubMed] [Google Scholar]
- 73.de Halleux V, Rigo J. Variability in human milk composition: benefit of individualized fortification in very-low-birth-weight infants. Am J Clin Nutr 2013;98:529S–35S. [DOI] [PubMed] [Google Scholar]
- 74.Underwood MA. Human milk for the premature infant. Pediatr Clin North Am 2013;60:189–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 2014;4:CD002971. [DOI] [PubMed] [Google Scholar]
- 76.Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, Dudell G, Rechtman DJ, Lee ML, Lucas A, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr 2013;163:1592–5.e1. [DOI] [PubMed] [Google Scholar]
- 77.Di Lorenzo M, Bass J, Krantis A. An intraluminal model of necrotizing enterocolitis in the developing neonatal piglet. J Pediatr Surg 1995;30:1138–42. [DOI] [PubMed] [Google Scholar]
- 78.Thymann T, Støy CAF, Bering SB, Mølbak L, Sangild PT. Casein addition to a whey-based formula has limited effects on gut function in preterm pigs. J Anim Sci 2012;90(Suppl 4):378–80. [DOI] [PubMed] [Google Scholar]
- 79.Timby N, Hernell O, Vaarala O, Melin M, Lönnerdal B, Domellöf M. Infections in infants fed formula supplemented with bovine milk fat globule membranes. J Pediatr Gastroenterol Nutr 2015;60:384–9. [DOI] [PubMed] [Google Scholar]
- 80.International Society for the Study of Fatty Acids and Lipids. ISSFAL statement on dietary fats in infant nutrition [Internet]. [cited 2016 Sep 26]. Available from: http://www.issfal.org/statements/pufa-recommendations/statement-2.
- 81.Innis S. Lipids for neonates In: Polin R, Fox WW, editors. Fetal and neonatal physiology. 2nd ed Amsterdam (Netherlands): Elsevier; 2012, p. 190. [Google Scholar]
- 82.Perrella SL, Hepworth AR, Simmer KN, Geddes DT. Influences of breast milk composition on gastric emptying in preterm infants. J Pediatr Gastroenterol Nutr 2015;60:264–71. [DOI] [PubMed] [Google Scholar]
- 83.Granot E, Ishay-Gigi K, Malaach L, Flidel-Rimon O. Is there a difference in breast milk fatty acid composition of mothers of preterm and term infants? J Matern Fetal Neonatal Med 2016;29:832–5. [DOI] [PubMed] [Google Scholar]
- 84.Picaud JC, Rigo J, Normand S, Lapillonne A, Reygrobellet B, Claris O, Salle BL. Nutritional efficacy of preterm formula with a partially hydrolyzed protein source: a randomized pilot study. J Pediatr Gastroenterol Nutr 2001;32:555–61. [DOI] [PubMed] [Google Scholar]
- 85.Penn AH, Altshuler AE, Small JW, Taylor SF, Dobkins KR, Schmid-Schonbein GW. Digested formula but not digested fresh human milk causes death of intestinal cells in vitro: implications for necrotizing enterocolitis. Pediatr Res 2012;72:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hua Z, Turner JM, Mager DR, Sigalet DL, Wizzard PR, Nation PN, Ball RO, Pencharz PB, Wales PW. Effects of polymeric formula vs elemental formula in neonatal piglets with short bowel syndrome. JPEN J Parenter Enteral Nutr 2014;38:498–506. [DOI] [PubMed] [Google Scholar]
- 87.Connor EE, Evock-Clover CM, Wall EH, Baldwin RL, Santin-Duran M, Elsasser TH, Bravo DM. Glucagon-like peptide 2 and its beneficial effects on gut function and health in production animals. Domest Anim Endocrinol 2016;56(Suppl):S56–65. [DOI] [PubMed] [Google Scholar]
- 88.Stoll B, Price P, Reeds P, Chang X, Henry J, van Goudoever J, Holst J, Burrin D. Feeding an elemental diet vs a milk-based formula does not decrease intestinal mucosal growth in infant pigs. JPEN J Parenter Enteral Nutr 2006;30:32–9. [DOI] [PubMed] [Google Scholar]
- 89.Ghandehari H, Lee ML, Rechtman DJ. An exclusive human milk-based diet in extremely premature infants reduces the probability of remaining on total parenteral nutrition: a reanalysis of the data. BMC Res Notes 2012;5:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci 2011;4:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Gross SJ. Growth and biochemical response of preterm infants fed human milk or modified infant formula. N Engl J Med 1983;308:237–41. [DOI] [PubMed] [Google Scholar]
- 92.Lucas A, Gore S, Cole T, Bamford M, Dossetor J, Barr I, Dicarlo L, Cork S, Lucas P. Multicentre trial on feeding low birthweight infants: effects of diet on early growth. Arch Dis Child 1984;59:722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tyson JE, Lasky R, Mize C, Richards C, Blair-Smith N, Whyte R, Beer A. Growth, metabolic response, and development in very-low-birth-weight infants fed banked human milk or enriched formula. I. Neonatal findings. J Pediatr 1983;103:95–104. [DOI] [PubMed] [Google Scholar]
- 94.Herrmann K, Carroll K. An exclusively human milk diet reduces necrotizing enterocolitis. Breastfeed Med 2014;9:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 1990;336:1519–23. [DOI] [PubMed] [Google Scholar]
- 96.Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O’Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol 2007;27:428–33. [DOI] [PubMed] [Google Scholar]