Abstract
Optimal nutrition early in life is critical to ensure proper structural and functional development of infant organ systems. Although pediatric nutrition historically has emphasized research on the relation between nutrition, growth rates, and gastrointestinal maturation, efforts increasingly have focused on how nutrition influences neurodevelopment. The provision of human milk is considered the gold standard in pediatric nutrition; thus, there is interest in understanding how functional nutrients and bioactive components in milk may modulate developmental processes. The piglet has emerged as an important translational model for studying neurodevelopmental outcomes influenced by pediatric nutrition. Given the comparable nutritional requirements and strikingly similar brain developmental patterns between young pigs and humans, the piglet is being used increasingly in developmental nutritional neuroscience studies. The piglet primarily has been used to assess the effects of dietary fatty acids and their accretion in the brain throughout neurodevelopment. However, recent research indicates that other dietary components, including choline, iron, cholesterol, gangliosides, and sialic acid, among other compounds, also affect neurodevelopment in the pig model. Moreover, novel analytical techniques, including but not limited to MRI, behavioral assessments, and molecular quantification, allow for a more holistic understanding of how nutrition affects neurodevelopmental patterns. By combining early-life nutritional interventions with innovative analytical approaches, opportunities abound to quantify factors affecting neurodevelopmental trajectories in the neonate. This review discusses research using the translational pig model with primary emphasis on early-life nutrition interventions assessing neurodevelopment outcomes, while also discussing nutritionally-sensitive methods to characterize brain maturation.
Keywords: piglet, pediatric nutrition, neurodevelopment, magnetic resonance imaging, animal model, early-life nutrition
Introduction
Optimal nutrient provision early in life is essential for proper development of the neonate (1). Pediatric nutrition research commonly focuses on maturation of the gastrointestinal tract, because this system is crucial to ensuring continued growth. Although alterations in nutrition may lead to immediately evident pathophysiologies of gastrointestinal development (2), the developing brain also is highly sensitive to early-life nutrient status (3). However, the sensitivities of the brain that lead to altered structure and function may not be evident until later in life (4). Thus, it is important to identify critical aspects of early-life nutrition that modulate brain development during periods in which brain regions are rapidly developing and therefore susceptible to nutrition.
Throughout the perinatal period, the brain is rapidly developing, and its plasticity makes it highly susceptible to alterations in nutrition (5). It is well known that maternal dietary status greatly influences fetal brain development, but this is not limited to just the prenatal period (6, 7). Human milk is generally regarded as the gold standard for early-life nutrition, and known compositional differences between infant formula and human milk exist (8). Accordingly, research suggests that there are differences in brain development and cognitive function between breastfed and formula-fed infants (9–11). Throughout the first year of life, most infants receive a combination of both human milk and formula (12). Thus, there is interest in characterizing the components of human milk that support optimal brain development and can be incorporated into standard infant diets.
Although epidemiologic data and randomized controlled trials can be informative in assessing neurodevelopmental outcomes in human populations, these studies are limited in their mechanistic assessments of nutrition’s modulatory effects on the developing brain. The piglet is a commonly accepted animal model in the field of pediatric nutrition research because the gastrointestinal development and nutrient requirements of the pig are similar to those of the human infant (13). Because of the piglet’s utility in this line of research, it also has gained popularity in the field of developmental neuroscience, commonly being used to assess the interactions of nutrition and neurodevelopment. It is through these studies that researchers have been able to elucidate how specific dietary compounds influence neurodevelopment.
This review will highlight studies of pediatric nutrition that used the piglet model in which brain development outcomes were of primary interest. It will also highlight technologies used to assess brain development and seeks to offer novel insights into future areas of nutritional neurodevelopment research in which the pig model will be of great use. It should be noted that use of the pig in neuroscience is not exclusively amendable to nutritional neuroscience; rather, it has gained traction in other areas of brain-related research. Although this review focuses solely on nutrition, the reader is referred to other reviews that focus on topics such as cognition and behavior (14, 15), early-life neurodevelopmental insults (16), brain disorders (17), and anesthetic effects on the developing brain (18) in the young pig.
Piglet Neurodevelopment
The piglet is an ideal model for neurodevelopmental research because it exhibits perinatal brain development much like human infants (19). Analysis of fatty acid fractions from piglet brains ranging from 14 d preterm to 40 d postnatal indicates that the maturing piglet brain experiences 2 peaks of myelination (20). The first peak of brain growth occurs between ∼100 and 110 d gestation (mean gestation length is 114 d in the pig), depending on the specified brain region. This early myelinating event is characterized by an accumulation of DNA involved in the myelination process, followed by cerebroside accumulation in developing regions. It was noted that there is an apparent slowing of brain growth in the immediate prenatal period, with development ramping up again ∼10 d after birth. The second peak of myelination in the developing pig brain occurs ∼30–40 d after birth, and is characterized by a simultaneous appearance of myelinating DNA and cerebrosides in developing regions. Notably, cholesterol esters appear in brain regions before myelinating events, presumably functioning as carriers of fatty acids to be incorporated into the myelin sheath (20). Purvis et al. (21) also noted that perinatal changes in fatty acid accretion in the piglet brain parallel that of human brain development. Particularly, fatty acids derived from 18:2n–6 and 18:3n–3 tend to increase during gestation, but concentrations of these precursors remain low, and a rapid accumulation of saturated fatty acids (i.e., 16:0 and 18:0) also suggests active myelin synthesis (21).
Although rodents are commonly used animal models in nutritional neuroscience research, the lissencephalic cortical structure and predominantly postnatal brain development are quite different from that of humans and pigs. In contrast, the pig brain exhibits gyral patterning, vascularization, and distribution of gray and white matter that is similar to that of the human, and the pig brain is large enough for live neuroimaging procedures (17, 22). MRI methods have allowed for longitudinal characterization of volumetric changes from 2 to 24 wk of age in the pig, further establishing similarities between pig and human brain development (23). When comparing data obtained from longitudinal MRI assessment in pigs and humans, it is apparent that both species follow a similar growth trajectory, with 1 mo of human total brain volume growth being equivalent to 1 wk of piglet total brain volume growth (24). In general, the pig brain is one-tenth the total volume of the human brain throughout the lifespan, thus lending itself to easy collection and characterization of specific brain subregions (23, 24). This is in contrast to rodent brains, which are much smaller and often require pooling of tissue across the rodents in order to analyze tissue components.
The use of the pig as a neurodevelopmental model is unique in that it allows for assessment of learning and memory during critical periods of brain growth, which then may be translated directly to humans. Because of the precocial nature of the young pig, behavioral assessments can be performed immediately after birth, whereas this is not possible in human infants for several months. Because rodents’ brains develop predominantly during the postnatal period, assessments of learning and memory are not possible immediately after birth. Although behavioral analysis techniques will be discussed briefly below, the reader is referred to a more extensive review pertaining to piglet cognitive assessment (14). By using nutritional interventions during the periods of rapid brain development, researchers are able to define critical windows during which the brain is sensitive to dietary interventions.
Nutritional Studies
Research focused on optimal piglet nutrition for agricultural purposes has been a priority for many decades. Accordingly, nutritional effects on growth and development of the piglet are well characterized. Studies directly assessing the brain, however, have only recently gained popularity, with many studies focusing only on crude measures, such as brain weights or total fatty acid composition (20, 21, 25, 26). However, from these foundational studies, nutritionists and neuroscientists alike have gained an appreciation for the sensitivity of this model in the context of nutritional neurodevelopment. The piglet is unique in that it experiences perinatal brain development, thus making it a premiere model for studies of perinatal nutrition. This is unlike rodents, which develop postnatally and are not as amendable to studies of maternal nutrition. Moreover, advancements in nutritional technologies have allowed for development and characterization of novel compounds that may serve to support or enhance brain development when incorporated into pediatric products (27, 28). The following sections seek to highlight novel findings in which the modulation of specific dietary compounds resulted in alterations of brain development when using the pig as a model.
Fatty acids
Given the analysis techniques available and an intense interest in fatty acids in pediatric nutrition in the early 1990s, the piglet was identified as an optimal model for assessing brain fatty acid accretion. Decades of research with the use of the piglet have helped to establish DHA (22:6n–3) and arachidonic acid (20:4n–6) as 2 fatty acids that serve critical roles in brain maturation (29–34). Research in this area has highlighted the need to understand ways in which altered ratios and different sources of fatty acids might affect bioavailability in the developing neonate (26, 31, 32, 34–39). Although the majority of piglet neurodevelopmental research has focused on dietary fatty acid supplementation, extensive detail pertaining to such studies is beyond the scope of this review. For a more in-depth understanding of dietary fatty acids and their impact on brain development, the reader is referred to reviews by Innis (40–42).
Cholesterol
Cholesterol has many physiological functions in the body, most notably as an integral compound in cell membranes and key component of the myelin sheath (43). Although most tissues can use dietary cholesterol, it is unclear whether or not absorbed dietary cholesterol can cross the blood–brain barrier or if cholesterol must be exclusively synthesized de novo in the brain.
The piglet has proven to be a useful model for assessing genetic factors leading to high or low serum cholesterol concentrations and the impact of dietary cholesterol on the developing brain. Piglets that were selected for high or low circulating cholesterol concentrations were provided either a diet supplemented with cholesterol or an unsupplemented control diet, and brain cholesterol accretion and behavioral outcomes were measured (44). In an open field behavioral assessment, piglets that were selected for low serum cholesterol and provided the control diet exhibited reduced exploratory behavior compared with all other groups. Analysis of brain cholesterol content indicated that regardless of genetic predisposition, piglets that were provided cholesterol-supplemented diets had higher brain cholesterol concentrations. Therefore, these results tend to support the notion that cholesterol is transported into the brain; however, this is contradictory to the generally accepted idea of brain de novo cholesterol synthesis (45). Given the reduced brain cholesterol content and altered behavior in the unsupplemented piglets, this study suggests that provision of dietary cholesterol may have functional implications in the developing brain.
Supporting this work, Boleman et al. (46) showed that regardless of genetic predisposition for serum cholesterol concentrations, piglets fed cholesterol-deficient diets early in life exhibited decreased brain weight and diminished brain cholesterol content, even after receiving a cholesterol-replete diet later in life. Although contrary to the previously described studies, piglet genetics also appear to have an influence on brain weight, with piglets of high serum cholesterol status exhibiting higher brain weights (47). Open field behavioral assessment in this study indicated that there were differences in exploratory behavior from genetics at 2 wk of age. Low serum cholesterol piglets did not explore the arena, and piglets selected for high serum cholesterol that were provided a cholesterol-supplemented diet explored more than piglets selected for high serum cholesterol but provided an unsupplemented diet. However, these genetically influenced exploratory behaviors were not different between any groups at 4 wk of age. Pond et al. (47) also observed increased vocalization in low serum cholesterol piglets that were provided the cholesterol-supplemented diet, which they ascribed to increased emotionality, although it is unclear how increased emotionality in piglets might manifest in structural and functional differences later in life.
Studies involving supplementation of piglet milk replacer with cholesterol or DHA indicate that both compounds influence amino acid metabolism in the developing brain (48). Dietary addition of the 2 compounds appeared to have interactive effects on glutamate, asparagine, serine, histidine, alanine, tryptophan, tyrosine, and γ-aminobutyric acid in the developing piglet brain. Compared with values in unsupplemented piglets, dietary cholesterol alone increased brain serine, glycine, lysine, and γ-animobutyric acid, whereas glutamate, glutamine, threonine, β-alanine, methionine, isoleucine, and leucine were all decreased. Similarly, supplementation with DHA alone in the diet enhanced brain concentrations of glycine, taurine, alanine, and γ-aminobutryic acid, but reduced concentrations of glutamate, glutamine, threonine, methionine, and leucine compared with those in control piglets. Although the effects of altering concentrations of these amino acids remains to be elucidated, it is important to note that several of these amino acids are involved in neurotransmitter synthesis and could have functional implications, thereby warranting further research.
From the limited studies involving supplementation with cholesterol in piglets, it appears that this nutrient differentially affects the developing brain. Results from these studies indicate that cholesterol may cross the blood-brain barrier and could possibly have functional implications, although more research is needed to better understand the mechanisms in which dietary cholesterol influences brain development.
Milk bioactives
As the field of pediatric nutrition research advances, greater emphasis has been placed on understanding the impact of bioactive compounds such as sialic acid, lactoferrin, milk fat globule membrane, prebiotics, and gangliosides present in milk (49). To date, much of this research has focused on the impact of these compounds in the developing gut, because they exert beneficial effects in disease prevention and gut maturation. Recently, however, there has been interest in understanding how these compounds might affect the developing brain.
Sialic acid compounds.
Sialic acid is a monosaccharide that can be attached to glycoproteins and glycolipids in biological tissues to elicit physiological effects during neurodevelopment (50). Dietary supplementation with sialic acid in young pigs has been shown to increase learning and memory, affect expression of learning-related and neural cell adhesion molecule genes, and elevate concentrations of protein-bound cortical sialic acid in a dose-dependent manner (51–53).
Lactoferrin is a sialylated, iron-binding, functional glycoprotein commonly found in human milk (54). Provision of bovine lactoferrin, as well as naturally present lactoferrin, in porcine colostrum increases circulating cerebrospinal fluid lactoferrin concentrations within hours of ingestion, possibly affecting iron metabolism in the brain (55). Recent findings from piglets suggests that supplementation with bovine lactoferrin results in increased hippocampal mRNA and increased protein concentrations of brain-derived neurotrophic factor, phosphorylated cAMP response element–binding protein, and polysialic acid, all of which also are upregulated after learning events and during memory consolidation (56). Moreover, provision of this functional protein enhanced 8-arm radial maze performance in supplemented piglets compared with control piglets (56). Supplementation with lactoferrin also facilitates enteric nervous system development by upregulating duodenal mRNA expression of brain-derived neurotrophic factor and glial-cell line-derived neurotrophic factor (54). From its functional roles in both the central and enteric nervous systems, it is possible that early-life ingestion of lactoferrin might modulate development of the gut-brain axis; however, specific studies are needed to elucidate these modulatory effects.
Sialyllactose, a human milk oligosaccharide consisting of sialic acid conjugated to lactose (57), was shown to influence ganglioside-bound sialic acid concentrations in the corpus callosum of piglets. Interestingly, supplementation with 3′- or 6′-sialyllactose at 2 g/L increased the corpus callosum sialic acid concentration by 15%, whereas supplementation with 3′-sialyllactose at 4 g/L only increased corpus callosum sialic acid concentrations by 10% compared with control pigs (58). This differential response suggests an apparent sensitivity of the developing brain to increased dietary intake of this compound.
Gangliosides and phospholipids.
Human milk is a highly concentrated source of phospholipids and gangliosides, both of which are rapidly accreted in the developing brain (59, 60). A study that supplemented a milk protein concentrate enriched in phospholipids, sphingolipids, gangliosides, and phosphatidylserine in developing piglets resulted in fewer errors in a spatial T-maze assessment, suggesting enhancement of cognitive development compared with that of control piglets (61). MRI of the same pigs indicated that supplementation with this compound increased the volumes of the internal capsule, putamen, and thalamus relative to the control treatment. Furthermore, metabolomic analysis indicated that supplementation with this milk fat enriched protein concentrate at a rate of 2.5% (wt:wt) increased hippocampal metabolites involved in the glutathione cycle, acetylcholine, cytidine 5′-diphosphocholine, and lactate compared with in control piglets.
A separate study assessed the impact of deuterium-labeled, milk-derived ganglioside GD3 supplementation on the biosynthesis of frontal lobe gangliosides in piglets (62). This study concluded that supplementation with GD3 in milk at lower amounts (18.2 μg/mL) compared with higher amounts (25 μg/mL) resulted in greater incorporation of deuterium in steric acid bound to brain gangliosides GM1 and GD1a, as well as increased concentrations of GDB1, within the frontal lobe. Reis et al. (62) also noted a greater incorporation of deuterium in b-series gangliosides than in a-series gangliosides, regardless of dietary treatment, highlighting the potential importance of such molecules early in development. Furthermore, the presence of specific ganglioside moieties in human milk changes throughout lactation, again suggesting differential impacts of gangliosides at various stages of development. From the studies by Lui et al. (61) and Reis et al. (62), it is increasingly evident that supplementation with particular phospholipids and gangliosides during specific developmental time periods may play an important role in defining brain structure.
α-Lipoic acid.
Although little research has focused on specific antioxidants present in human milk, there is evidence to suggest higher antioxidant capacity in human milk than in infant formula (63, 64). α-Lipoic acid is considered to be a universal antioxidant because of its multitude of physiological functions, including transition metal chelation, free-radical quenching, antioxidant regeneration, and regulation of gene and protein expression (65). Recent work from our laboratory assessed dietary α-lipoic acid supplementation on piglet neurodevelopment (66). Supplementation with this compound did not result in any differences in piglet cognition; however, MRI analysis indicated structural differences within the internal capsule (66). Piglets that were provided α-lipoic acid at a higher supplementation rate (240 mg α-lipoic acid/100 g milk replacer powder) exhibited lower diffusion tensor imaging (DTI) fractional anisotropy and axial diffusivity measures, indicative of altered myelination or fiber coherence, within the internal capsule compared with pigs fed either a control or lower dietary supplementation rate (120 mg α-lipoic acid/100 g milk replacer powder). Importantly, piglets that received the lower dietary α-lipoic acid concentration were not different from the control piglets, suggesting that this supplementation rate supported normal neurodevelopment. Analysis of white matter tracts with the use of tract-based spatial statistics confirmed the DTI outcomes by highlighting specific differences in white matter diffusion properties within the internal capsule of piglets fed the higher concentration of α-lipoic acid compared with control piglets. Thus, these data suggest a threshold effect whereby α-lipoic acid supplementation supports neurodevelopment up to a certain dietary concentration, without continued benefits once this limit is exceeded.
Novel dietary combinations.
Single-nutrient interventions are necessary for elucidating specific mechanisms by which neurodevelopment outcomes are altered. However, it is equally important to assess novel combinations of dietary compounds, because this is more representative of how bioactive components are presented to the infant in human milk. Accordingly, a recent study in our laboratory studied combined supplementation with milk fat globule membrane, lactoferrin, and prebiotics on brain development in the piglet model (67). No differences in cognition were observed between control and supplemented piglets; however, MRI analysis again highlighted the sensitivity of the internal capsule to early-life dietary intervention. Piglets that received the supplemented diet exhibited decreased mean and radial diffusivity measures generated from DTI, which suggested increased myelination or fiber coherence within this specific brain region. Using voxel-based morphometry, a method to sensitively characterize changes in gray and white matter on a voxel-by-voxel basis, we observed differences in gray and white matter tissue concentrations within motor and sensory regions of the brain. These observed differences suggested advanced development in supplemented compared with control piglets. Interestingly, the internal capsule is an early-developing fiber tract that serves to connect motor and sensory cortices to the rest of the body, meaning the gray and white matter tissue observations served to corroborate diffusion findings in this study (67).
From the studies described above, it is important to highlight the novelty of the bioactive compounds being tested, while also focusing on the sensitivity of the brain to their presence. It is increasingly apparent that higher amounts of one compound may not always confer better neurodevelopment, thereby suggesting the importance of defining optimal amounts at which specific compounds should be incorporated into the diets used in future studies. As analysis methods become more refined and optimal supplementation amounts are empirically determined, researchers will be able to better understand the mechanisms by which supplemented compounds influence the trajectory of brain development.
Micronutrients
A deficiency of individual micronutrients, including folate, iron, copper, and zinc, alters the neurodevelopmental trajectory of infants (5). Accordingly, the piglet has proven to be a sensitive model for characterizing developmental aberrations caused by such nutrient deficiencies.
Iron.
Piglets provided severely iron-deficient diets (10 mg Fe/kg milk solids) for the first 4 wk of life exhibited decreased learning in the spatial T-maze assessment, whereas mildly iron-deficient (25 mg Fe/kg milk solids) piglets exhibited learning similar to that of control piglets (100 mg Fe/kg milk solids) (68). Further analyses indicated that both amounts of iron deficiency resulted in more movement and longer latency to choice, suggesting that there were alterations in the dopaminergic pathways of these piglets. Quantification of brain iron content indicated reduced iron in the hippocampus of iron-deficient piglets, possibly contributing to observed cognitive deficits in severely deficient piglets. Interestingly, the expression of transferrin receptor mRNA was reduced in the prefrontal cortex, but not the hippocampus, of pigs receiving iron-deficient diets. MRI indicated no differences in whole-brain or hippocampal volumes due to dietary iron status.
In a follow-up study, Leyshon et al. (69) used more sensitive MRI measures to characterize the effect of early-life iron deficiency on brain development. Results from this study corroborated the lack of effect of iron deficiency on brain volume observed by Rytych et al. (68); however, a global reduction in white matter volumes was observed in pigs receiving the iron-deficient diet (10 mg Fe/kg milk solids). Voxel-based morphometric analysis revealed decreased white matter volumes in iron-deficient piglets, whereas gray matter was increased or decreased on a regional basis compared with that of control (100 mg Fe/kg milk solids) piglets. DTI indicated decreased fractional anisotropy of the whole brain and corpus callosum in iron-deficient piglets. Fractional anisotropy generally is used as an indicator of myelination within the brain; thus, observed decreases in iron-deficient piglets may suggest decreased myelination. Further analysis with the use of histologic staining indicated decreased corpus callosum width in iron-deficient piglets, thereby suggesting that the micronutrient deficiency altered development of this region; this outcome may have cognitive implications later in life. Hippocampal development also appeared to be altered where iron-deficient piglets exhibited decreased diffusion tensor–measured radial and mean diffusivities, suggesting decreased cellularity or myelination in this particular region. From this study, magnetic resonance spectroscopy revealed altered hippocampal concentrations, with myo-inositol decreasing and phosphocholine increasing in iron-deficient piglets, which may indicate decreased astrocytic activity and altered myelination, respectively. Together, these sensitive MRI observations by Leyshon et al. (69) serve to support the cognitive deficits previously quantified through behavioral assessments.
Iron deficiency not only alters cognitive function early in life, but also appears to have lasting implications even when an iron-replete diet is provided later in life. In one such longitudinal assessment, piglets were provided an iron-deficient (21 mg Fe/kg milk solids) or control (88 mg Fe/kg milk solids) milk replacer from birth until 4 wk of life, and then all pigs were switched to an iron-replete diet (190–240 mg Fe/kg diet) from 4 to 12 wk of age (70). With the use of the spatial cognitive hole-board task to assess long-term memory, it was observed that early-life iron-deficient piglets exhibited impaired long-term memory compared with control piglets, despite exhibiting no clinical signs of iron deficiency at the time of behavioral testing. Hippocampal analysis at 12 wk of age revealed that early-life iron-deficient piglets had fewer iron-containing cells in the hippocampal CA1 and dentate gyrus subregions than did control piglets. This study reveals the lifelong implications of early-life nutrient deficiencies in the developing brain, despite dietary rehabilitation to correct for known deficiencies.
Porcine milk is known to have low iron concentrations; as such, it is common agricultural practice to supplement piglets with an iron dextran injection to deliver ∼250 mg Fe early in life. As a follow-up study to their artificially reared iron-deficient piglets, Antonides et al. (71) assessed iron deficiency in piglets that were maternally reared and then provided iron-replete feed from 4 to 16 wk of life. Sibling pairs were divided into 2 groups, iron deficient (i.e., saline injection on days 3 and 10 of life) or iron sufficient (i.e., iron dextran shot on days 3 and 10 of life), and allowed to ingest maternal milk throughout the preweaning period. Hematocrit and hemoglobin concentrations did not change throughout the preweaning period and were lower in the iron-deficient piglets than in the controls. Serum iron also was lower than in control pigs, and increased throughout the preweaning period. Despite the lower concentrations of blood hematocrit, hemoglobin, and iron, the iron-deficient piglets did not exhibit clinical signs of anemia, and piglets at all levels recovered to normal during the postweaning period. Analysis of piglet spatial memory at 10 wk of age indicated that early-life nonanemic iron deficiency did not alter piglet performance in the spatial hole-board task. Therefore, this study suggests that despite the low concentrations of iron in sow milk, it was not enough to induce iron-deficiency anemia; thus, the maternally reared piglet may not be an ideal model of iron deficiency. Together with their previous findings, Antonides et al. (71) concluded that early-life iron-deficiency anemia may be a sensitive indicator of long-term cognitive and physiologic deficits.
Although characterization of iron deficiency and its impact on neurodevelopment is of critical importance, it is worth noting that excess iron might also cause adverse effects. It has been discussed recently that many infant formulas contain bioavailable iron concentrations that are 10–60 times greater than that of human milk (72). Because iron metabolism is known to influence brain development, future studies should use the piglet to identify possible adverse effects of iron oversupplementation. Studies of this nature could serve to identify an upper limit of iron ingestion, whereby too much of this micronutrient negatively influences brain development in the young pig.
One-carbon metabolites.
Choline is a critical micronutrient that plays a pivotal role in neurotransmitter synthesis, myelin development, and cell membrane integrity (73). Our work with the use of the piglet as a model for perinatal choline deficiency indicates that exposure to prenatal choline deficiency greatly alters brain development (74). We observed smaller whole-brain volumes in piglets from sows that were provided choline-deficient diets compared with piglets from choline-sufficient sows. When expressing individual regions as a proportion of total brain volume, prenatally choline-deficient piglets exhibited proportionally larger midbrains, pons, right hippocampi, thalamus, and corpus callosum. Interestingly, these structures are largely subcortical, which suggests a preferential development of these regions when choline availability is limited. Using DTI methods, we observed indications of delayed development in the cerebellum of the prenatally choline-deficient piglets. We also observed indications of altered hippocampal and thalamic development, which was dependent on the timing of the induced choline deficiency (i.e., prenatal, postnatal, or both). We conclude that, as a methyl donor, choline may play a role in metabolic imprinting, depending on when it is provided. Therefore, the interactive nature of our results suggests that brain regions are sensitive to such imprinting when choline status is changed between the pre- and postnatal period. Future work should seek to quantify epigenetic changes in the brain from choline deficiency and its effects on myelination and cognitive development in the piglet model.
Betaine is a direct metabolite of choline oxidation and is known to be important throughout perinatal development (75). A study in which sows were supplemented with betaine indicated that there was altered hippocampal development in offspring compared with development in offspring from unsupplemented sows (76). In this study, hippocampi were collected at birth, and piglets from betaine-supplemented sows exhibited increased glucocorticoid receptor mRNA, but not glucocorticoid protein, compared with piglets from control sows. Betaine also plays an important role in epigenetic modifications; as such, hypermethylation of the glucocorticoid promoter region was observed in betaine-supplemented offspring. In addition, betaine supplementation influenced specific micro-RNA expression, which may have resulted in a lack of differences observed in the expression of glucocorticoid protein in piglets from betaine-supplemented sows.
These studies highlight the importance of adequate micronutrient status throughout perinatal development. Provided the meaningful findings from prenatal choline and betaine deficiency, it is clear that proper maternal nutrition is equally as important as adequate postnatal nutrition for ensuring optimal neurodevelopment. Interestingly, a human study assessed child cognition at 7 y of age and related these measures to maternal gestational choline intake. By longitudinally assessing piglet brain development in the context of micronutrient deficiencies, the maternal choline deficiency model may help explain the mechanisms that underlie the decreased childhood cognition observed in human studies of low gestational choline (77) and corroborate previous findings of altered structural brain development.
Nutrition for preterm infants
Infants born preterm exhibit altered neurodevelopmental patterns compared with term infants, and it is well known that these infants have specific nutritional needs (78). The piglet is being used increasingly as a model for preterm infancy to establish the utility of different feeding paradigms (i.e., parenteral or enteral) and their impact on gut maturation and neurodevelopment (79). An assessment of neurodevelopment in preterm and term piglets indicated that there were delays in arousal, physical activity, coordination, exploration, and learning in preterm piglets relative to term piglets (80). Results from this study indicated that the provision of parenteral nutrition in addition to enteral nutrition for the first 5 d did not affect later neurodevelopmental outcomes.
In a separate study, piglets delivered at 92% of complete gestation (114 d in the pig) and provided parenteral nutrition had smaller brain weights, reduced motor activity, and indications of underdeveloped myelination at 10 d of age compared with piglets that received enteral nutrition (81). Late in gestation, the fetus begins to swallow amniotic fluid. This is thought to help stimulate the gastrointestinal tract (82). However, preterm infants often miss this critical time period in utero and are regularly provided parenteral feedings, thereby circumventing gut stimulation. A preterm piglet model has shown that provision of an oral fluid supplement, formulated to mimic the nutrient and electrolyte composition of amniotic fluid, results in increased brain weights compared with that of piglets that receive lactated Ringer solution either intravenously or enterally (83). Furthermore, Cao et al. (84) demonstrated that development of physical activity in preterm piglets was dependent on the route of supplement provision in the early postnatal period. This study showed that enteral provision of bovine colostrum or human milk resulted in increased motor activity in preterm piglets compared with that of preterm piglets that were provided infant formula. Although the direct link between the gut and brain was not assessed in this study, these authors concluded that early enteral feedings stimulated physical activity, which may be due to increased neural maturation. Thus, it is possible that early stimulation of the enteric nervous system may influence central nervous system development as well.
Malnutrition
Undernutrition is a common cause of neurodevelopmental delays in infants worldwide (85–87). Protein malnutrition in piglets from 3 to 10 wk of age resulted in decreased brain weight, total protein concentrations, and total DNA content, thus highlighting the vulnerability of the developing brain to suboptimal nutrition (88). Piglets that were subjected to protein malnutrition early in life but then provided nutritionally adequate diets later in life exhibited brain weights similar to those of control piglets at 18 wk of age. However, protein and DNA content tended to be lower in the early-life malnourished piglets, suggesting that early-life undernutrition has life-long implications in the brain. Barnes et al. (89) also noted behavioral and learning differences in adult pigs that had been subjected to early-life malnutrition, even after a period of nutritional rehabilitation.
Undernutrition also is commonly observed in utero, with some infants being born small for gestational age. Piglets that were born small for their gestational age took longer to learn a spatial T-maze than did litter-matched siblings that were born average for gestational age (90). Moreover, MRI analysis indicated reduced gray matter volumes and smaller internal capsules, along with regional alterations in gray and white matter tissue concentrations, in small-for-gestational-age piglets. This research suggests that nutritional status in utero may set the foundation for later brain development when an otherwise nutritionally adequate diet is provided.
Often, babies who are considered to have a very low birth weight require parenteral nutrition to ensure optimal performance (78). Amusquivar et al. (91) used the full-term piglet to assess the impact of parenteral solutions compared with enteral solutions on brain fatty acids. This study indicated the resilience of the brain to differences in provision of nutrients, with neither enteral nor parenteral provision, nor the type of parenteral solution, affecting the fatty acid composition of the developing brain at 7 d of age. This suggests that metabolism in the brain at this time point is sufficient to ensure proper fatty acid accretion, regardless of the route of nutrient provision.
Techniques Used to Assess Brain Development
The field of pediatric nutrition continues to identify and develop novel compounds that may exert beneficial effects on the developing neonate. As these technologies become available, there is a need to better understand the ways in which they influence brain development. Previously, the most common methods of assessing alterations in brain development were largely focused on brain weight and global fatty acid profiling. However, inconsistencies in how the brain is extracted and overall fluid content of the brain can lead to large variability in brain weights, thereby making it difficult to compare findings across studies. Many studies also have assessed the fatty acid profiles of the whole cerebrum, although it is now clear that this practice dilutes the impact of dietary interventions on specific regions, because brain regions develop at different rates (92, 93). Below, we seek to highlight advancements in neurodevelopmental analytic techniques (Table 1), all of which have proven to be sensitive to early-life dietary intervention.
TABLE 1.
Analytical techniques used in piglet developmental neuroscience
| Technique | Definition | Dietary sensitivity (reference) |
| MRI | ||
| Voxel-based morphometry | Comparison of gray or white matter tissue volumes on a voxel-by-voxel basis between treatment groups. Reported as edge-connected voxel clusters, which may span multiple brain regions. | Milk protein concentrate (enriched with phospholipids and gangliosides) (61) |
| Milk fat globule membrane and lactoferrin (67) | ||
| Iron deficiency (69) | ||
| Small for gestational age (90) | ||
| Volumetric analysis | Defines absolute (cubic millimeter) or relative (percentage total brain volume) volumes for distinct brain regions, which are then compared between treatment groups. Includes both gray and white matter in individual brain regions. | Milk protein concentrate (enriched with phospholipids and gangliosides) (61) |
| Milk fat globule membrane and lactoferrin (67) | ||
| Perinatal choline deficiency (74) | ||
| Small for gestational age (90) | ||
| Diffusion tensor imaging | Characterizes microscopic water movement within the brain, which can help to infer structural changes, such as myelination, fiber coherence, and changes in neuron size. Reported as axial diffusivity (movement parallel to magnetization gradient), radial diffusivity (movement perpendicular to magnetization gradient), mean diffusivity (overall water movement), and fractional anisotropy (measure of rate and direction of water movement) for distinct brain regions. | α-Lipoic acid (66) |
| Milk fat globule membrane and lactoferrin (67) | ||
| Iron deficiency (69) | ||
| Perinatal choline deficiency (74) | ||
| Small for gestational age (90) | ||
| Tract-based spatial statistics | Characterizes diffusion tensor measures along predefined white matter tracts. | α-Lipoic acid (66) |
| Magnetic resonance spectroscopy | Quantifies neurometabolites (parts per million) within a specified voxel. | Iron deficiency (69) |
| Perinatal choline deficiency (74) | ||
| Behavioral techniques | ||
| Home cage | Used to assess daily bouts of activity and rest. May be useful in characterizing repetitive behaviors. | Preterm piglet (80) |
| Open field | Assessment for anxiety-like behavior in which measures exploration and vocalization are observed. | Cholesterol (47) |
| Spatial T-maze | Assessment of discrimination and spatial learning and memory in which piglets use visual cues to learn the location of a food reward. | Milk protein concentrate (enriched with phospholipids and gangliosides) (61) |
| Iron deficiency (68) | ||
| Small for gestational age (90) | ||
| 8-arm radial maze | Assessment of discrimination and spatial learning and memory in which piglets use visual cues to learn the location of a food reward. | Sialic acid (51) |
| Cognitive hole-board | Assessment of spatial learning and memory in which habituation, reference memory, search strategies, exploration, and anxiety-related behaviors can be assessed. | Iron deficiency (70) |
| Molecular assessments | ||
| Gene, mRNA, and protein expression | Allows for characterizations in transcription and translation from dietary treatment | Sialic acid (51, 52) |
| Lactoferrin (54, 56) | ||
| Iron deficiency (68) | ||
| Betaine (76) | ||
| Protein deficiency (88) | ||
| Fatty acid profiling | Used to quantify changes in fatty acid composition within the brain. | Various fatty acid supplementations (29–39) |
| Histologic staining | Allows for visual characterization of structural changes in distinct brain regions. | Lactoferrin (56) |
| Iron deficiency (69, 70) | ||
| Stable-isotope labeling | Characterizes accretion of labeled dietary compounds in brain tissue. | Gangliosides and phospholipids (62) |
| DHA (94) |
MRI.
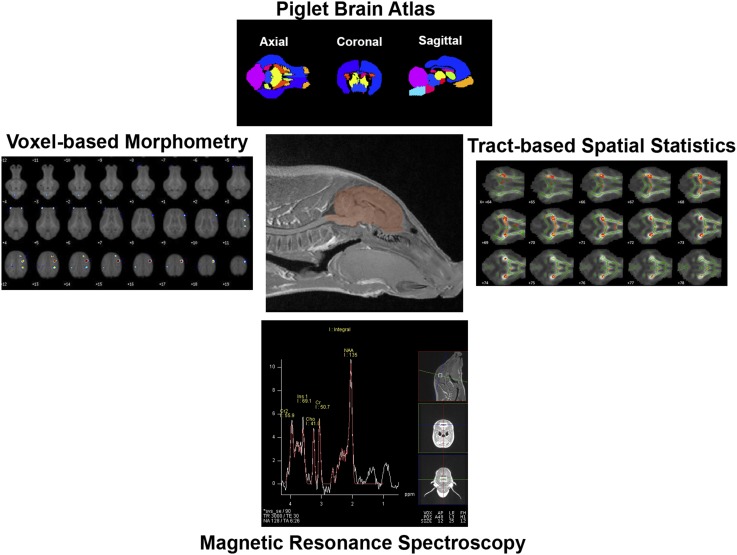
Currently, there is a large initiative in the United States to characterize normal brain development from birth through childhood (95, 96). In doing so, researchers are regularly using MRI in nonsedated children to elucidate brain growth trajectories. Although this method is becoming increasingly popular in pediatric studies, piglet researchers have been developing similar translational methods in parallel (Figure 1). MRI can sensitively assess changes in white and gray matter, microstructural diffusion properties, blood flow, and even neurometabolites. Neuroimaging of piglet brains can occur in sedated piglets or in postmortem brains. Although postmortem scanning allows for longer scanning sessions and therefore higher resolution sequences, tissue shrinkage and lack of blood flow negatively affect observed outcomes, hence, the need for longer scans. Pig-specific brain atlases have been developed to parcellate the brain into individual brain regions (97, 98), which has allowed for the characterization of regions that are sensitive to dietary intervention, as evidenced by recent work that consistently shows the internal capsule to be altered by early-life nutrition (61, 66, 67, 90).
FIGURE 1.
Piglet MRI. The center shows a representative image acquired during MRI procedures, with the piglet brain shaded in red. At the top, the piglet brain atlas is applied to each individual piglet’s MRI data and is used to assess the volumes and diffusion properties of 19 distinct regions. On the left, a representative example of voxel-based morphometry analysis in which differences in white matter between 2 groups (visualized as a statistical heat map) are being displayed on a population-averaged brain image. On the right, a representative example of tract-based spatial statistics. This image displays a population-averaged piglet brain with green marking areas of white matter common to all piglets being compared. Red and yellow indicate areas in which one treatment group has higher fractional anisotropy measures than does the other treatment group. At the bottom, a representative magnetic resonance spectrum that characterizes neurometabolites within a specified voxel. Individual peaks represent the quantification of the following metabolites: Cr, Cho, Ins, and NAA. Cho, choline; Cr, creatine; Ins, myo-inositol; NAA, N-acetylaspartate.
Within neuroimaging, there are many different analytical techniques, several of which we will briefly describe here. Voxel-based morphometry is used to compare tissue volumes between subjects’ gray matter and white matter on a voxel-by-voxel basis. Comparisons of this nature can then be used to show localized differences in individual brain tissue types, which can indicate how dietary components influence brain development. This method has proven sensitive in showing alterations to iron deficiency (69) and supplementation with milk fat globule membrane and lactoferrin (67), and helps researchers understand how gray matter and white matter can be differentially sensitive to dietary treatment.
Another volumetric assessment of brain regions uses the piglet brain atlas and is able to define either absolute (i.e., cubic millimeters) or relative (i.e., percentage of total brain volume) volumes for each of the regions defined by the atlas. This method is powerful because it quantifies brain regions that are maturing at a greater rate relative to other regions, as hypothesized in recent nutrition studies involving perinatal choline deficiency (74) and supplementation with milk fat globule membrane and lactoferrin (67). This method is different from voxel-based morphometry, however, in that the observed volume differences do not differentiate between white and gray matter, but rather quantify the physical volume of discernable brain structures in the live subject.
Another commonly used method in neurodevelopmental research is DTI. Very broadly, this method characterizes water movement within the brain and can help to infer microstructural changes. These observed diffusion changes might be due to several factors, including myelination, fiber coherence, and changes in neuron size. This method provides measures of axial diffusivity, radial diffusivity, mean diffusivity, and fractional anisotropy, each of which can be used to infer specific differences within the tissue and are described in detail in a review by Le Bihan et al. (100). DTI has proven to be sensitive to detecting regional differences from iron and choline deficiency, as well as dietary supplementation with compounds including α-lipoic acid, milk fat globule membrane, and lactoferrin (66, 67, 69, 74). With the use of DTI data, researchers also are able to apply a method known as tract-based spatial statistics, which allows for the comparison of diffusion properties along white matter fiber tracts (100, 101). Our laboratory previously used this method to further corroborate evidence that supplementation with α-lipoic acid influences internal capsule development (66).
Magnetic resonance spectroscopy measures also have been applied to developmental nutritional neuroscience studies, and have proven sensitive to dietary interventions of iron deficiency (69) and perinatal choline deficiency (74). The use of this technique allows for the quantification of important metabolites, including N-acetylaspartate, choline, inositol, γ-aminobutyric acid, glutamate, glutamine, and creatine, among others. Through the quantification of these metabolites, researchers are better able to understand the mechanisms whereby early-life nutrition might influence brain metabolism.
The development of fiber tracts that connect different parts of the brain is critical to neurodevelopment and may be influenced by diet. Future work in this field should seek to expand upon diffusion data by applying fiber tractography methods (102). In doing so, researchers will better understand the mechanisms whereby specific nutrients direct brain development, which may have functional implications later in life. Myelination is a pivotal event that occurs throughout neurodevelopment, and MRI methods have been developed to specifically assess this process. Work by Deoni et al. (103) uses the differences in water diffusion between myelin water and nonmyelin water, thus allowing for a general understanding of the amount of myelin within a region. This work has been performed in human infants and has helped characterize the developmental trajectory of different brain regions, highlighting the various time points at which regions are most rapidly maturing (92, 93). By applying this method to the piglet model, researchers can assess the impact of specific nutrients on myelination.
Behavioral assessment paradigms.
The use of behavioral analysis techniques is one of the easiest and least invasive methods to grossly assess brain development in infants and pigs. Importantly, these measures are commonly used as biomarkers of developmental milestones, all of which can inform doctors of aberrations in this process. Piglet researchers also use measures of cognitive assessment to uncover possible developmental alterations from diet. One common method of assessment is to observe daily piglet behavior in the home cage, as is done in the preterm piglet model (80). Methods such as this seek to assess bouts of activity, which might be indicative of neuromuscular development. This method of home cage analysis could also prove useful in assessing social and repetitive behaviors in the absence of a human observer; however, when using these methods, researchers should have standardized and objective criteria free of observer bias. Assessment of open field behavior is commonly used to assess anxiety in rodent models, and has also proven useful in piglets (47). Researchers found that piglets bred to have lower serum cholesterol and provided a high-cholesterol diet exhibited more vocalizations in an open field test (47). The researchers ascribed this to increased emotionality in the piglets, although some might argue that this is anthropomorphizing of the piglet.
Although behavioral observations have their utility, cognitive assessments such as the 8-arm radial maze, spatial T-maze (104), and cognitive hole-board task (105) have also proven sensitive to dietary intervention (51, 68, 70, 71). Each of these assessments may introduce possible bias through human interaction, although consistent performance between handlers helps to alleviate this potential confounder. A review by Gieling et al. (14) highlights many classic and operant conditioning assessments that are known to be effective in the piglet, yet many of these techniques have not been used in the context of pediatric nutrition. Although it is difficult to know which brain regions might be affected by dietary treatment, researchers should carefully choose a behavioral assessment that is sensitive to the specific brain region expected to be altered by the dietary intervention. In addition, high variability in behavioral outcomes necessitates greater replication per treatment, which is not needed for more sensitive measures, such as neuroimaging and molecular techniques. Moreover, if outcomes do not yield cognitive differences, it may be due to the timing of the intervention or an inappropriate test, or it truly may be a lack of impact due to diet, which highlights the complexity associated with the use of behavioral assessment as a pivotal outcome in a nutrition study.
It is common to pair analytical methods such as electroencephalography and functional near-infrared spectrometry with cognitive assessments in human infants. By using these methods, researchers are better able to understand underlying functional differences in the brain that manifest in cognitive outcomes. Near-infrared spectroscopy and electroencephalography methods currently are being used in piglet models of hypoxic ischemia, and may prove useful in nutrition intervention studies as well (106). By combining these functional measures with cognitive performance outcomes, researchers stand to gain a greater understanding of how diet affects brain activity.
Molecular techniques.
The noninvasive nature of MRI and cognitive assessments are beneficial in allowing for immediately translatable methods between young pigs and humans. However, the resolution with which researchers are able to understand alterations in neurodevelopment are limited when these methods are used. Thus, the ability to analyze gene, mRNA, and protein expression along with cellular structure in the piglet brain can serve to better elucidate dietary mechanisms that influence brain development; these methods have proven sensitive to dietary interventions of protein deficiency (88), betaine (76), sialic acid (51, 52), lactoferrin (54, 56) and iron (68). Combined analyses of gene, mRNA, and protein expression may help researchers to understand whether dietary compounds have a greater impact on transcriptional or translational events, as Sun et al. (76) observed with maternal betaine supplementation.
The use of histologic staining in the piglet brain also allows for a better characterization of dietary interventions in the context of brain development. By using this technique, Leyshon et al. (69) observed decreased corpus callosum widths in iron-deficient piglets, thus allowing for better interpretation of observed neuroimaging results. Antinodes et al. (70) also used histologic methods to stain for iron-containing cells in the hippocampus, and observed fewer of these cells in piglets that were provided iron-deficient diets. The reduction of iron-containing cells in the deficient piglets was consistent with observed reductions in long-term memory, suggesting the importance of this compound in memory formation.
Stable isotope–labeling of dietary compounds also has proven useful in understanding the mechanisms of tissue accretion within the piglet brain. Deuterium-labeled gangliosides provided in piglet milk replacer were shown to differentially accumulate in specific ganglioside moieties of the developing piglet brain (62). A study that used 13C-DHA bound to either triglycerides or phosphatidylcholine showed greater gray matter accretion of the labeled fatty acid when provided in the phospholipid form (94). Human studies of choline metabolism have used these labeling techniques to better characterize the physiologic mechanisms of choline metabolism (107). Given the proven sensitivity of the developing piglet brain to perinatal choline intervention, it would be of great interest to provide gestating sows with labeled choline and trace how the deficiency alters the neurodevelopmental trajectory in offspring. Through the use of labeled compounds, researchers may better understand how ingested compounds are altered in the body and transferred across the blood-brain barrier.
Conclusions and Future Directions
Substantial advances in pediatric nutrition have been made through sustained use of the piglet model. With the continued application of the novel analytical technologies described herein, the piglet is well poised to answer foundational questions pertaining to early-life nutrition and neurodevelopment. As the field of developmental nutritional neuroscience matures, researchers should seek to include longitudinal study designs to further characterize specific windows of sensitivity to dietary intervention. Moreover, the use of novel nutritional targets, both individually and combined, will permit optimization of nutritional profiles for the developing brain. Furthermore, as our understanding of the gut-brain axis matures, the piglet may prove beneficial in elucidating how nutrition can modulate this connection. Continued research that uses the piglet to better understand neurodevelopment will serve to optimize early-life nutrition and consequently brain development for future generations.
Acknowledgments
We thank Sharon Donovan for careful review and thoughtful comments on this manuscript. Both authors read and approved the final manuscript.
References
- 1.World Health Organization. Infant nutrition. Infant and young child feeding: model chapter for textbooks for medical students and allied health professionals. Geneva (Switzerland): World Health Organization; 2009:1–99. [PubMed] [Google Scholar]
- 2.Jacobi SK, Odle J. Nutritional factors influencing intestinal health of the neonate. Adv Nutr 2012;3:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cusick SE, Georgieff M. Nutrient supplementation and neurodevelopment. Arch Pediatr Adolesc Med 2012;166:481–2. [DOI] [PubMed] [Google Scholar]
- 4.Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev 2014;72:267–84. [DOI] [PubMed] [Google Scholar]
- 5.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 2007;85:614S–20S. [DOI] [PubMed] [Google Scholar]
- 6.Morgane PJ, Austin-LaFrance R, Bronzino J, Tonkiss J, Díaz-Cintra S, Cintra L, Kemper T, Galler JR. Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev 1993;17:91–128. [DOI] [PubMed] [Google Scholar]
- 7.Dobbing J. Vulnerable periods in developing brain. Brain, behavior, and iron in the infant diet. London: Springer-Verlag;1990:1–17. [Google Scholar]
- 8.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ou X, Andres XA, Pivik XRT, Cleves XMA, Snow XJH, Ding Z, Badger TM. Voxel-based morphometry and fMRI revealed differences in brain gray matter in breastfed and milk formula – fed children. Am J Neuroradiol 2016;37:713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deoni SCL, Dean DC 3rd, Piryatinsky I, O’Muircheartaigh J, Waskiewicz N, Lehman K, Han M, Dirks H. Breastfeeding and early white matter development: a cross-sectional study. Neuroimage 2013;82:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr 1999;70:525–35. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia J, Greer F. Use of soy protein-based formulas in infant feeding. Pediatrics 2008;121:1062–8. [DOI] [PubMed] [Google Scholar]
- 13.Odle J, Lin X, Jacobi SK, Kim SW, Stahl CH. The suckling piglet as an agrimedical model for the study of pediatric nutrition and metabolism. Annu Rev Anim Biosci 2014;2:419–44. [DOI] [PubMed] [Google Scholar]
- 14.Gieling ET, Schuurman T, Nordquist RE, van der Staay FJ. The pig as a model animal for studying cognition and neurobehavioral disorders. Curr Top Behav Neurosci 2011;7:359–83. [DOI] [PubMed] [Google Scholar]
- 15.Held S, Mendl M, Laughlin K, Byrne RW. Cognition studies with pigs: livestock cognition and its implication for production. J Anim Sci 2002;80:e10–7. [Google Scholar]
- 16.Conrad MS, Johnson R. The domestic piglet: an important model for investigating the neurodevelopmental consequences of early life insults. Annu Rev Anim Biosci 2015;3:245–64. [DOI] [PubMed] [Google Scholar]
- 17.Lind NM, Moustgaard A, Jelsing J, Vajta G, Cumming P, Hansen AK. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev 2007;31:728–51. [DOI] [PubMed] [Google Scholar]
- 18.Whitaker EE, Bissonnette B, Miller AD, Koppert TL, Tobias JD, Pierson CR, Christofi FL. A novel, clinically relevant use of a piglet model to study the effects of anesthetics on the developing brain. Clin Transl Med 2016;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev 1979;3:79–83. [DOI] [PubMed] [Google Scholar]
- 20.Sweasey D, Patterson DSP, Glancy EM. Biphasic myelination and the fatty acid composition of cerebrosides and cholesterol esters in the developing central nervous system of the domestic pig. J Neurochem 1976;27:375–80. [DOI] [PubMed] [Google Scholar]
- 21.Purvis JM, Clandinin MT, Hacker RR. Fatty acid accretion during perinatal brain growth in the pig. A model for fatty acid accretion in human brain. Comp Biochem Physiol B 1982;72:195–9. [DOI] [PubMed] [Google Scholar]
- 22.Sauleau P, Lapouble E, Val-Laillet D, Malbert C-H. The pig model in brain imaging and neurosurgery. Animal 2009;3:1138–51. [DOI] [PubMed] [Google Scholar]
- 23.Conrad MS, Dilger RN, Nickolls A, Johnson RW. Magnetic resonance imaging of the neonatal piglet brain. Pediatr Res 2012;71:179–84. [DOI] [PubMed] [Google Scholar]
- 24.Knickmeyer RC, Gouttard S. A structural MRI study of human brain development from birth to 2 years. J Neurosci 2008;28:12176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hrboticky N, MacKinnon MJ, Innis SM. Effect of a vegetable oil formula rich in linoleic acid on tissue fatty acid accretion in the brain, liver, plasma, and erythrocytes of infant piglets. Am J Clin Nutr 1990;51:173–82. [DOI] [PubMed] [Google Scholar]
- 26.Arbuckle LD, Rioux FM, Mackinnon MJ, Hrboticky N, Innis SM. Response of (n-3) and (n-6) fatty acids in piglet brain, liver and plasma to increasing, but low, fish oil supplementation of formula. J Nutr 1991;121:1536–47. [DOI] [PubMed] [Google Scholar]
- 27.Lönnerdal B. Biological effects of novel bovine milk fractions. Nestle Nutr Workshop Ser Pediatr Program 2011;67:41–54. [DOI] [PubMed] [Google Scholar]
- 28.Lönnerdal B. Infant formula and infant nutrition: bioactive proteins of human milk and implications for composition of infant formulas. Am J Clin Nutr 2014;99:712S–7S. [DOI] [PubMed] [Google Scholar]
- 29.Arbuckle LD, Innis SM. Docosahexaenoic acid is transferred through maternal diet to milk and to tissues of natural milk-fed piglets. J Nutr 1993;123:1668–75. [DOI] [PubMed] [Google Scholar]
- 30.Craig-Schmidt MC, Stieh KE, Lien EL. Retinal fatty acids of piglets fed docosahexaenoic and arachidonic acids from microbial sources. Lipids 1996;31:53–9. [DOI] [PubMed] [Google Scholar]
- 31.de la Presa-Owens S, Innis SM, Rioux FM. Addition of triglycerides with arachidonic acid or docosahexaenoic acid to infant formula has tissue- and lipid class-specific effects on fatty acids and hepatic desaturase activities in formula-fed piglets. J Nutr 1998;128:1376–84. [DOI] [PubMed] [Google Scholar]
- 32.de la Presa Owens S, Innis SM. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and alpha-linolenic acid deficient diet in formula-fed piglets. J Nutr 1999;129:2088–93. [DOI] [PubMed] [Google Scholar]
- 33.de la Presa Owens S, Innis SM. Diverse, region-specific effects of addition of arachidonic and docosahexaenoic acids to formula with low or adequate linoleic and alpha-linolenic acids on piglet brain monoaminergic neurotransmitters. Pediatr Res 2000;48:125–30. [DOI] [PubMed] [Google Scholar]
- 34.Tyburczy C, Brenna ME, DeMari JA, Kothapalli KS, Blank BS, Valentine H, McDonough SP, Banavara D, Diersen-Schade DA, Brenna JT. Evaluation of bioequivalency and toxicological effects of three sources of arachidonic acid (ARA) in domestic piglets. Food Chem Toxicol 2011;49:2320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foote KD, Mackinnon J, Innis M. Brain synaptosomal, liver, plasma, and red blood piglets fed exclusively on a vegetable-oil-containing formula with and without fish-oil supplements. Am J Clin Nutr 1990;51:1001–6. [DOI] [PubMed] [Google Scholar]
- 36.Alessandri JM, Goustard B, Guesnet P, Durand G. Docosahexaenoic acid concentrations in retinal phospholipids of piglets fed an infant formula enriched with long-chain polyunsaturated fatty acids: effects of egg phospholipids and fish oils with different ratios of eicosapentaenoic acid to docosahexaenoic acid. Am J Clin Nutr 1998;67:377–85. [DOI] [PubMed] [Google Scholar]
- 37.Arbuckle LD, Rioux FM, MacKinnon MJ, Innis SM. Formula alpha-linolenic (18:3(n - 3)) and linoleic (18:2(n - 6)) acid influence neonatal piglet liver and brain saturated fatty acids, as well as docosahexaenoic acid (22:6(n - 3)). Biochim Biophys Acta 1992;1125:262–7. [DOI] [PubMed] [Google Scholar]
- 38.Arbuckle LD, MacKinnon MJ, Innis SM. Formula 18:2(n-6) and 18:3(n-3) content and ratio influence long-chain polyunsaturated fatty acids in the developing piglet liver and central nervous system. J Nutr 1994;124:289–98. [DOI] [PubMed] [Google Scholar]
- 39.Huang M-C, Brenna JT, Chao AC, Tschanz C, Diersen-Schade DA, Hung H. Differential tissue dose responses of (n-3) and (n-6) PUFA in neonatal piglets fed docosahexaenoate and arachidonoate. J Nutr 2007;137:2049–55. [DOI] [PubMed] [Google Scholar]
- 40.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr 2007;137:855–9. [DOI] [PubMed] [Google Scholar]
- 41.Innis SM. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev Neurosci 2000;22:474–80. [DOI] [PubMed] [Google Scholar]
- 42.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res 2008;1237:35–43. [DOI] [PubMed] [Google Scholar]
- 43.Norton WT, Cammer W. Isolation and characterization of myelin. In: Morell P, editor. Myelin. New York: Springer Science+Business Media;1984:147–95. [Google Scholar]
- 44.Schoknecht PA, Ebner S, Pond WG, Zhang S, Mcwhinney V, Wong WW, et al. Dietary cholesterol supplementation improves growth and behavioral response of pigs selected for genetically high and low serum cholesterol. J Nutr 1994;124:305–14. [DOI] [PubMed] [Google Scholar]
- 45.Jurevics H, Morell P. Cholesterol for synthesis of myelin is made locally, not imported into brain. J Neurochem 1995;64:895–901. [DOI] [PubMed] [Google Scholar]
- 46.Boleman SL, Graf TL, Mersmann HJ, Su DR, Krook LP, Savell JW, Park YW, Pond WG. Pigs fed cholesterol neonatally have increased cerebrum cholesterol as young adults. J Nutr 1998;128:2498–504. [DOI] [PubMed] [Google Scholar]
- 47.Pond WG, Mersmann HJ, Su D, McGlone JJ, Wheeler MB, Smith EO. Neonatal dietary cholesterol and alleles of cholesterol 7-alpha hydroxylase affect piglet cerebrum weight, cholesterol concentration, and behavior. J Nutr 2008;138:282–6. [DOI] [PubMed] [Google Scholar]
- 48.Li P, Kim SW, Li X, Datta S, Pond WG, Wu G. Dietary supplementation with cholesterol and docosahexaenoic acid affects concentrations of amino acids in tissues of young pigs. Amino Acids 2009;37:709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donovan SM, Lönnerdal B. Introduction: emerging roles of bioactive components in pediatric nutrition. J Pediatr 2016;173:S1–3. [DOI] [PubMed] [Google Scholar]
- 50.Wang B, Miller JB, McNeil Y, McVeagh P. Sialic acid concentration of brain gangliosides: Variation among eight mammalian species. Comp Biochem Physiol A Mol Integr Physiol 1998;119:435–9. [DOI] [PubMed] [Google Scholar]
- 51.Wang B, Yu B, Karim M, Hu H, Sun Y, McGreevy P, Petocz P, Held S, Brand-Miller J. Dietary sialic acid supplementation improves learning and memory. Am J Clin Nutr 2007;85:561–9. [DOI] [PubMed] [Google Scholar]
- 52.Wang B, Hu H, Yu B. Molecular characterization of pig ST8Sia IV–a critical gene for the formation of neural cell adhesion molecule and its response to sialic acid supplement in piglets. Nutr Neurosci 2006;9:147–54. [DOI] [PubMed] [Google Scholar]
- 53.Wang B, Downing JA, Petocz P, Brand-Miller J, Bryden WL. Metabolic fate of intravenously administered N-acetylneuraminic acid-6–14C in newborn piglets. Asia Pac J Clin Nutr 2007;16:110–5. [PubMed] [Google Scholar]
- 54.Yang C, Zhu X, Liu N, Chen Y, Gan H, Troy FA 2nd, Wang B. Lactoferrin up-regulates intestinal gene expression of brain-derived neurotrophic factors BDNF, UCHL1 and alkaline phosphatase activity to alleviate early weaning diarrhea in postnatal piglets. J Nutr Biochem 2014;25:834–42. [DOI] [PubMed] [Google Scholar]
- 55.Harada E, Araki Y, Furumura E, Takeuchi T, Sitizyo K, Yajima T, Kuwata T. Characteristic transfer of colostrum-derived biologically active substances into cerebrospinal fluid via blood in natural suckling neonatal pigs. J Vet Med A Physiol Pathol Clin Med 2002;49:358–64. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Zheng Z, Zhu X, Shi Y, Tian D, Zhao F, Liu N, Hüppi PS, Troy FA 2nd, Wang B. Lactoferrin promotes early neurodevelopment and cognition in postnatal piglets by upregulating the BDNF signaling pathway and polysialylation. Mol Neurobiol 2015;52:256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarr AJ, Galley JD, Fisher SE, Chichlowski M, Berg BM, Bailey MT. The prebiotics 3′sialyllactose and 6′sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: evidence for effects on the gut-brain axis. Brain Behav Immun 2015;50:166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobi SK, Yatsunenko T, Li D, Dasgupta S, Yu RK, Berg BM, Chichlowski M, Odle J. Dietary isomers of sialyllactose increase ganglioside sialic acid concentrations in the corpus callosum and cerebellum and modulate the colonic microbiota of formula-fed piglets. J Nutr 2016;146:200–8. [DOI] [PubMed] [Google Scholar]
- 59.Ryan JM, Rice GE, Mitchell MD. The role of gangliosides in brain development and the potential benefits of perinatal supplementation. Nutr Res 2013;33:877–87. [DOI] [PubMed] [Google Scholar]
- 60.McJarrow P, Schnell N, Jumpsen J, Clandinin T. Influence of dietary gangliosides on neonatal brain development. Nutr Rev 2009;67:451–63. [DOI] [PubMed] [Google Scholar]
- 61.Liu H, Radlowski EC, Conrad MS, Li Y, Dilger RN, Johnson RW. Early supplementation of phospholipids and gangliosides affects brain and cognitive development in neonatal piglets. J Nutr 2014;144:1903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reis MM, Bermingham EN, Reis MG, Deb-Choudhury S, MacGibbon A, Fong B, McJarrow P, Bibiloni R, Bassett SA, Roy NC. Effect of dietary complex lipids on the biosynthesis of piglet brain gangliosides. J Agric Food Chem 2016;64:1245–55. [DOI] [PubMed] [Google Scholar]
- 63.Aycicek A, Erel O, Kocyigit A, Selek S, Demirkol MR. Breast milk provides better antioxidant power than does formula. Nutrition 2006;22:616–9. [DOI] [PubMed] [Google Scholar]
- 64.Friel JK, Martin SM, Langdon M, Herzberg GR, Buettner GR. Milk from mothers of both premature and full-term infants provides better antioxidant protection than does infant formula. Pediatr Res 2002;51:612–8. [DOI] [PubMed] [Google Scholar]
- 65.Packer L, Witt E, Tritschler H. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med 1995;19:227–50. [DOI] [PubMed] [Google Scholar]
- 66.Mudd AT, Waworuntu RV, Berg BM, Dilger RN. Dietary alpha-lipoic acid alters piglet neurodevelopment. Front Pediatr 2016;4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mudd AT, Alexander L, Berding K, Waworuntu R, Berg BM, Donovan SM, Dilger, RN. Dietary prebiotics, milk fat globule membrane and lactoferrin affects structural neurodevelopment in the young piglet. Front Pediatr 2016;4:4. [DOI] [PMC free article] [PubMed]
- 68.Rytych JL, Elmore MRP, Burton MD, Conrad MS, Donovan SM, Dilger RN, Johnson RW. Early life iron deficiency impairs spatial cognition in neonatal piglets. J Nutr 2012;142:2050–6. [DOI] [PubMed] [Google Scholar]
- 69.Leyshon BJ, Radlowski EC, Mudd AT, Steelman AJ, Johnson RW. Postnatal iron deficiency impairs brain development in piglets. J Nutr 2016;146:1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antonides A, Schoonderwoerd AC, Scholz G, Berg BM, Nordquist RE, van der Staay FJ. Pre-weaning dietary iron deficiency impairs spatial learning and memory in the cognitive holeboard task in piglets. Front Behav Neurosci 2015;9:291–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Antonides A, van Laarhoven S, van der Staay FJ, Nordquist RE. Non-anemic iron deficiency from birth to weaning does not impair growth or memory in piglets. Front Behav Neurosci 2016;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lönnerdal B, Georgieff MK, Hernell O. Developmental physiology of iron absorption, homeostasis, and metabolism in the healthy term infant. J Pediatr 2015;167:S8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeisel SH. Nutritional importance of choline for brain development. J Am Coll Nutr 2004;23:621S–6S. [DOI] [PubMed] [Google Scholar]
- 74.Mudd AT, Getty C, Sutton B, Dilger R. Perinatal choline deficiency delays brain development and alters metabolite concentrations in the young pig. Nutr Neurosci 2015 Jun 5 (Epub ahead of print; DOI: 10.1179/1476830515Y.0000000031). [DOI] [PubMed] [Google Scholar]
- 75.Caudill MA. Pre- and postnatal health: evidence of increased choline needs. J Am Diet Assoc 2010;110:1198–206. [DOI] [PubMed] [Google Scholar]
- 76.Sun Q, Li X, Pan S, Li R, Yang X, Zhao R. Maternal betaine supplementation during gestation modifies hippocampal expression of GR and its regulatory miRNAs in neonatal piglets. J Vet Med Sci 2016;78:921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boeke CE, Gillman MW, Hughes MD, Rifas-Shiman SL, Villamor E, Oken E. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol 2013;177:1338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan SHT, Johnson MJ, Leaf AA, Vollmer B. Nutrition and neurodevelopmental outcomes in preterm infants: a systematic review. Acta Paediatr 2016;105:587–99. [DOI] [PubMed] [Google Scholar]
- 79.Sangild PT, Thymann T, Schmidt M, Stoll B, Burring DG, Buddington RK. Invited review: the preterm pig as a model in pediatric gastroenterology. J Anim Sci 2013;91:4713–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersen AD, Sangild PT, Munch SL, van der Beek EM, Renes IB, Ginneken Cv, Greisen GO, Thymann T. Delayed growth, motor function and learning in preterm pigs during early postnatal life. Am J Physiol Regul Integr Comp Physiol 2016;310:R481–92. [DOI] [PubMed] [Google Scholar]
- 81.Choudhri AF, Sable HJ, Chizhikov VV, Buddington KK, Buddington RK. Parenteral nutrition compromises neurodevelopment of preterm pigs. J Nutr 2014;144:1920–7. [DOI] [PubMed] [Google Scholar]
- 82.Ross MG, Nijland MJ. Development of ingestive behavior. Am J Physiol 1998;274:R879–93. [DOI] [PubMed] [Google Scholar]
- 83.Berding K, Makarem P, Hance B, Axel AM, Nolan V, Buddington KK, Buddington RK Responses of preterm pigs to an oral fluid supplement during parenteral nutrition. JPEN J Parenter Enteral Nutr 2016;40:934–43. [DOI] [PubMed] [Google Scholar]
- 84.Cao M, Andersen AD, Ginneken CV, Shen RL, Petersen SO, Thymann T, Jing J, Sangild PT. Physical activity level is impaired and diet dependent in preterm newborn pigs. Pediatr Res 2015;78:137–44. [DOI] [PubMed] [Google Scholar]
- 85.Scrimshaw NS. Malnutrition, brain development, learning, and behavior. Nutr Res 1998;18:351–79. [Google Scholar]
- 86.Chase HP, Martin HP. Undernutrition and child development. N Engl J Med 1970;282:933–9. [DOI] [PubMed] [Google Scholar]
- 87.Ivanovic DM, Leiva BP, Perez HT, Inzunza NB, Almagià AF, Toro TD, Urrutia MS, Cervilla JO, Bosch EO. Long-term effects of severe undernutrition during the first year of life on brain development and learning in Chilean high-school graduates. Nutrition 2000;16:1056–63. [DOI] [PubMed] [Google Scholar]
- 88.Pond WG, Ellis KJ, Mersmann HJ, Heath JP, Krook LP, Burrin DG, Dudley MA, Sheng HP. Severe protein deficiency and repletion alter body and brain composition and organ weights in infant pigs. J Nutr 1996;126:290–302. [DOI] [PubMed] [Google Scholar]
- 89.Barnes RH, Moore AU, Pond WG. Behavioral abnormalities in young adult pigs caused by malnutrition in early life. J Nutr 1970;100:149–55. [DOI] [PubMed] [Google Scholar]
- 90.Radlowski EC, Conrad MS, Lezmi S, Dilger RN, Sutton B, Larsen R, Johnson RW. A neonatal piglet model for investigating brain and cognitive development in small for gestational age human infants. PLoS One 2014;9:e91951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amusquivar E, Sánchez M, Hyde MJ, Laws J, Clarke L, Herrera E. Influence of fatty acid profile of total parenteral nutrition emulsions on the fatty acid composition of different tissues of piglets. Lipids 2008;43:713–22. [DOI] [PubMed] [Google Scholar]
- 92.Dean DC, O’Muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, Walker L, Piryatinsky I, Deoni SC. Estimating the age of healthy infants from quantitative myelin water fraction maps. Hum Brain Mapp 2015;36:1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deoni SCL, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SC, Murphy DG. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci 2011;31:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu L, Bartke N, Van Daele H, Lawrence P, Qin X, Park HG, Kothapalli K, Windust A, Bindels J, Wang Z, et al. Higher efficacy of dietary DHA provided as a phospholipid than as a triglyceride for brain DHA accretion in neonatal piglets. J Lipid Res 2014;55:531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Evans AC. The NIH MRI study of normal brain development. Neuroimage 2006;30:184–202. [DOI] [PubMed] [Google Scholar]
- 96.Almli CR, Rivkin MJ, McKinstry RC. The NIH MRI study of normal brain development (Objective-2): newborns, infants, toddlers, and preschoolers. Neuroimage 2007;35:308–25. [DOI] [PubMed] [Google Scholar]
- 97.Conrad MS, Sutton BP, Dilger RN, Johnson R. An in vivo three-dimensional magnetic resonance imaging-based averaged brain collection of the neonatal piglet (Sus scrofa). PLoS One 2014;9:e107650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gan H, Zhang Q, Zhang H, Chen Y, Lin J, Kang T, Zhang J, Troy FA 2nd, Wang B. Development of new population-averaged standard templates for spatial normalization and segmentation of MR images for postnatal piglet brains. Magn Reson Imaging 2014;32(10):1396–402. [DOI] [PubMed] [Google Scholar]
- 99.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001;13:534–46. [DOI] [PubMed] [Google Scholar]
- 100.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–505. [DOI] [PubMed] [Google Scholar]
- 101.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23 Suppl 1:S208–19. [DOI] [PubMed] [Google Scholar]
- 102.Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol 2008;29:632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deoni SCL, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med 2008;60:1372–87. [DOI] [PubMed] [Google Scholar]
- 104.Elmore MRP, Dilger RN, Johnson RW. Place and direction learning in a spatial T-maze task by neonatal piglets. Anim Cogn 2012;15:667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Staay FJ, Gieling ET, Pinzón NE, Nordquist RE, Ohl F. The appetitively motivated “cognitive” holeboard: a family of complex spatial discrimination tasks for assessing learning and memory. Neurosci Biobehav Rev 2012;36:379–403. [DOI] [PubMed] [Google Scholar]
- 106.Zhang D, Hou X, Liu Y, Zhou C, Luo Y, Ding H. The utility of amplitude-integrated EEG and NIRS measurements as indices of hypoxic ischaemia in the newborn pig. Clin Neurophysiol 2012;123:1668–75. [DOI] [PubMed] [Google Scholar]
- 107.Davenport C, Yan J, Taesuwan S, Shields K, West AA, Jiang X, Perry CA, Malysheva OV, Stabler SP, Allen RH, et al. Choline intakes exceeding recommendations during human lactation improve breast milk choline content by increasing PEMT pathway metabolites. J Nutr Biochem 2015;26:903–11. [DOI] [PubMed] [Google Scholar]