Abstract
Fructose is associated with the biochemical alterations that promote the development of metabolic syndrome (MetS), nonalcoholic fatty liver disease, and type 2 diabetes. Its consumption has increased in parallel with MetS. It is metabolized by the liver, where it stimulates de novo lipogenesis. The triglycerides synthesized lead to hepatic insulin resistance and dyslipidemia. Fructose-derived advanced glycation end products (AGEs) may be involved via the Maillard reaction. Fructose has not been a main focus of glycation research because of the difficulty in measuring its adducts, and, more importantly, because although it is 10 times more reactive than glucose, its plasma concentration is only 1% of that of glucose. In this focused review, I summarize exogenous and endogenous fructose metabolism, fructose glycation, and in vitro, animal, and human data. Fructose is elevated in several tissues of diabetic patients where the polyol pathway is active, reaching the same order of magnitude as glucose. It is plausible that the high reactivity of fructose, directly or via its metabolites, may contribute to the formation of intracellular AGEs and to vascular complications. The evidence, however, is still unconvincing. Two areas that have been overlooked so far and should be actively explored include the following: 1) enteral formation of fructose AGEs, generating an inflammatory response to the receptor for AGEs (which may explain the strong association between fructose consumption and asthma, chronic bronchitis, and arthritis); and 2) inactivation of hepatic AMP-activated protein kinase by a fructose-mediated increase in methylglyoxal flux (perpetuating lipogenesis, fatty liver, and insulin resistance). If proven correct, these mechanisms would put the fructose-mediated Maillard reaction in the limelight again as a contributing factor in chronic inflammatory diseases and MetS.
Keywords: fructose, Maillard reaction, advanced glycation, metabolic syndrome, AMPK, inflammation, RAGE, asthma, arthritis, diabetes
Introduction
The Western diet may be inducing the biochemical alterations that promote metabolic syndrome (MetS)3, type 2 diabetes, and nonalcoholic fatty liver disease (NAFLD). Fructose is a chief candidate for the following reasons: 1) the intake of fructose (especially in beverages) has greatly increased along with the incidence of MetS; 2) >90% of ingested fructose is metabolized by the liver at first pass, where it stimulates de novo lipogenesis to drive hepatic TG synthesis; and 3) this contributes to NAFLD, hepatic insulin resistance, and dyslipidemia (1–3).
Is there an as-yet undiscovered role for fructose-mediated advanced glycation end product (AGE) formation via the Maillard reaction in these processes? The Maillard reaction (adduct formation between reactive carbonyls in glucose, fructose, and their metabolites, such as methylglyoxal or deoxyglucosone, with amino groups in protein, DNA, and lipids) has been recognized as an important pathway at the root of diabetes complications (4–11). Fructose is 8–10 times more reactive than glucose in Maillard reaction product formation because of the higher stability of its open chain form and its keto group (12–17). It does not form the Amadori product, but, rather, the Heyns product. The popular clinical methods used for glucose glycation do not detect the Heyns product or other fructose-mediated adducts (18). This has compromised research on the potential role of fructose glycation in the pathogenesis of chronic disease in humans. Endogenous fructose formed in the sorbitol pathway was proposed early on as a source of AGEs in tissues implicated in diabetes complications (19–21). However, after much research on drugs targeting aldose reductase, the evidence for a critical role of endogenous fructose AGE formation in diabetic complications at the target tissue (endothelium, glomerulus, or neural) level is scant (22, 23). What about exogenous fructose? Given the role of hepatic insulin resistance in MetS, I believe that the recently proposed hypotheses for fructose AGE formation in the intestines before absorption (which can be considered to be premetabolism) and in the liver after portal flux should be given more attention. The putative mechanisms are summarized in this review, including a focused review of exogenous and endogenous fructose metabolism; fructose glycation; and in vitro, animal, and human data so far in order to frame the 2 new hypotheses in the appropriate context.
Exogenous Fructose Metabolism Skips Regulated Steps in Glycolysis and Enhances Lipogenesis
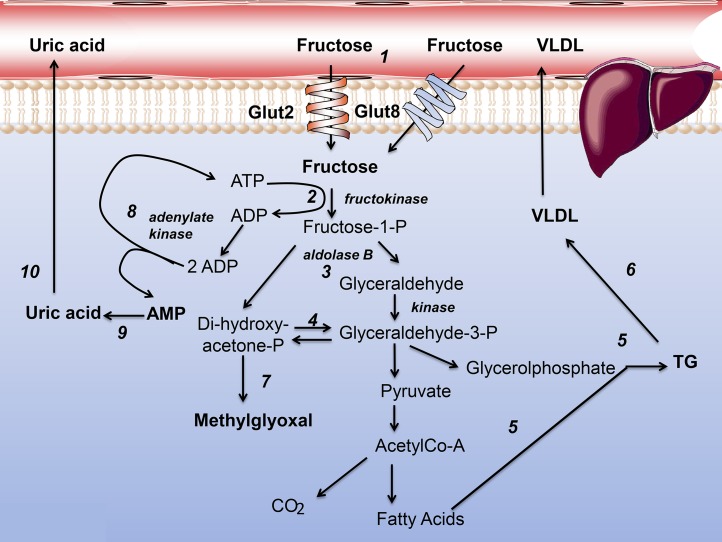
Exogenous fructose metabolism is >90% hepatic. Fructose is taken up by hepatocytes via glucose transporter (Glut) 2 and Glut8 (Figure 1, reaction 1). In our diets, fructose is usually accompanied by glucose [50%:50% in sucrose, ≤60%:40% in high-fructose corn syrup (HFCS), and 66%:33% in apple juice] (24, 25). Glucose will foster glycogenesis and stimulate insulin secretion, which fructose does not do. The crucial difference between glucose and fructose metabolism is that fructose leaps regulated steps in glycolysis-glucokinase and phosphofructokinase (24, 25). Phosphorylation by fructokinase (Figure 1, reaction 2), followed by an aldolase B splitting (Figure 1, reaction 3), leads swiftly and directly to trioses (Figure 1, reaction 4). When there is a simultaneous glucose flux, much of the triose pool becomes the backbone of TGs in de novo lipogenesis (Figure 1, reaction 5). These TGs are secreted with apoB100 as VLDL (Figure 1, reaction 6), or may initiate NAFLD. What has not attracted enough attention is the fact that an unregulated triose flux leads to excess methylglyoxal production (Figure 1, reaction 7) (26, 27). Methylglyoxal damages proteins by glycation. A notable exception is a murine study on hepatosteatosis and glycation by fructose or glucose in hepatocytes on which I will focus later in this review (28).
FIGURE 1.
Fructose metabolism in hepatocytes. Numbers indicate key reactions and are explained in text. Glut, glucose transporter; P, phosphate.
Exogenous Fructose Metabolism Depletes ATP and Increases Uric Acid Production
Because of the unregulated activity of fructokinase, a large fructose load depletes cytosolic ATP, especially when catalyzed by fructokinase C (Figure 1, reaction 2) (29, 30). This is relieved in part by adenylate kinase (Figure 1, reaction 8) with the production of AMP (Figure 1, reaction 9). AMP is turned into uric acid (Figure 1, reaction 10). This pathway has been demonstrated in animals, and data from humans coincide: fructose consumption correlates with hyperuricemia and hypertension (29–32). There is another effect of raising AMP concentrations or lowering ATP:AMP ratios: the activation of AMP-activated protein kinase (AMPK). AMPK is the master allosteric energy regulator of cells, and it senses low energy and promotes catabolic processes while arresting anabolic ones.
Endogenous Fructose Production: The Sorbitol Pathway
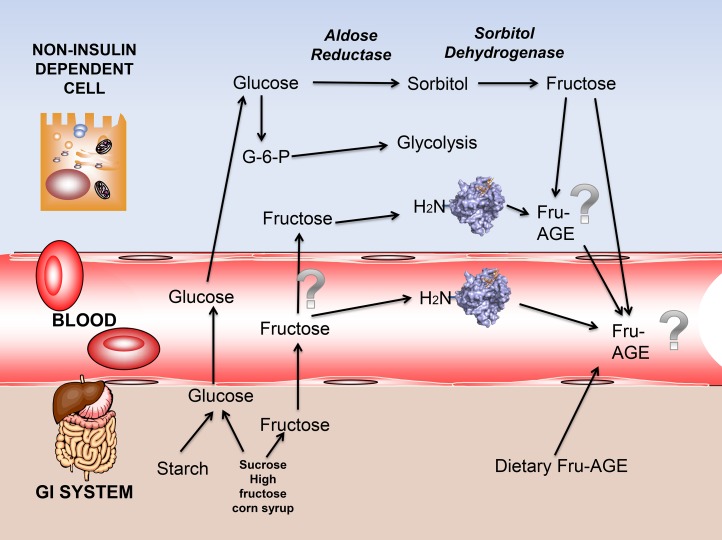
The contribution of fructose as an intracellular glycating agent was first suggested as a result of the polyol pathway. The intracellular concentration of fructose is high in the kidney, lens, and peripheral nerves of diabetic patients in whom the polyol pathway is active (19–21, 33). Aldose reductase has a high Michaelis constant for glucose, as depicted in Figure 2. The polyol pathway is inactive with normal glycemia. Conversely, in hyperglycemic conditions, glucose concentrations in insulin-independent tissues, such as neural tissue, glomerulus, lens, and erythrocytes, increase. Accordingly, the polyol pathway is activated. Sorbitol is acted upon by sorbitol dehydrogenase, which leads to fructose and potentially affects redox balance in the cell (19–21, 33). The newly synthesized fructose can be transported or leak passively across the plasma membrane. Acceleration of the polyol pathway is implicated in the pathogenesis of diabetic vascular complications (21–23, 34–36). Although fructose-derived AGEs may be formed intracellularly as a consequence of the polyol pathway, only a few studies have measured this directly. Increasing fructose concentrations in cultured pericytes necessitated >30 mM glucose and sorbitol dehydrogenase overexpression (37). This dampens our enthusiasm with regard to the actual pathogenic role of the process in humans. Moreover, after multiple clinical trials targeting aldose reductase, the evidence of a critical role of endogenous fructose AGE formation in diabetic complications at the target tissue (endothelium, glomerulus, or neural) level is meager, if there is any at all (22, 23).
FIGURE 2.
The sorbitol pathway as an intracellular source, and dietary sources of fructose and AGEs. Question marks indicate areas in which our knowledge or evidence is scant. Dietary fructose is mainly kept at the liver at first pass; therefore, the role of circulating fructose in AGE formation is questionable. AGE, advanced glycation end product; Fru-AGE, fructose-advanced glycation end product; GI, gastrointestinal; G-6-P, glucose-6-phosphate.
Fructose Glycation and AGE Formation
The simplified sequence for glucose and fructose mediated glycation of proteins is shown in Figure 3.
FIGURE 3.
The Maillard reaction by glucose (A) and fructose (B) produce different early and late glycation products. Fru-AGE, fructose-advanced glycation end product; Glc-AGE, glucose-derived advanced glycation end product.
The reaction between D-glucose or D-fructose and the N-terminal amino-acid and/or ε-amino groups of a protein forms Schiff base adducts. In the case of glucose, the Schiff base then undergoes Amadori rearrangement to yield a more stable adduct. With fructose, the reaction is similar, but the reaction is termed a Heyns rearrangement product (with carbon 2 instead of carbon 1 of the hexose), and results in the formation of 2 products (13–17). These early glycation products undergo further rearrangement, dehydration, and condensation reactions to produce irreversibly crosslinked fluorescent derivatives, or AGEs. N-carboxymethyllysine stems from degradation of the Amadori products. Several glucose-derived AGEs have been characterized, such as N-carboxymethyllysine, pentosidine, crosslines, and glucosepane. There is overlap between glucose and fructose AGEs (N-carboxymethyllysine, carboxyethyl-lysine, and pentosidine), as shown in Figure 3. Fructose-specific AGEs, however, have not been well characterized (13–17).
In Vitro Studies of Fructose Glycation
Although well known by food chemists for decades, the Maillard reaction by fructose at physiologic temperatures and pressures started to be studied only in the 1980s. Early studies helped establish the fact that the potential harmful effects of fructose on proteins was far more potent than those of glucose. A wide array of in vitro model proteins was used, as well as tissue culture conditions (13–15, 38–48). Several endogenous compounds, as well as those from food, were shown to inhibit this glycation (49–54). Although providing a chemical framework to explain why fructose may be harmful via the Maillard reaction, all these studies were conducted with concentrations far beyond the ranges found in vivo, and with proteins that may not encounter fructose at all. Indeed, fructose concentrations in plasma are in the lower micromolar range, whereas glucose is 5 mM, 2–3 orders of magnitude higher. The lack of a good circulating marker of fructose AGE adducts or the Heyns product continues to prevent the effective translation of the in vitro information to actual data of clinical relevance.
Animal Studies
AGEs accumulate in the tissues of fructose-fed rats.
In the past 2 decades, a plethora of studies have been conducted in animal models, chiefly rats. The studies in lenses may be the most physiologic, because the sorbitol pathway has a high level of activity in this tissue, and relevant concentrations of fructose may be produced (44). The role of fructose in cataracts has been observed (11, 55). The majority of the rest of the studies used the fructose-fed insulin-resistant rat model. The accumulation of pentosidine and other AGEs in tissues was documented, and its possible association with tendon and vascular rigidity was reported (6–8, 56).
Drugs and natural substances decrease AGEs in the tissues of fructose-fed rats.
The effects of taurine, genistein, aminoguanidine, glycyrrhizin, ursodeoxycolic acid, soy protein, eucalyptus, caffeine, and betaine, ferulic, and lipoic acid on outcomes related to AGE formation or their surrogates were explored in the same model in several studies (56–81). Although valuable in providing proof of concept and underscoring the effects of several antiglycation agents in this model, these studies have several pitfalls that prevent them from translation into human disease. First and foremost, they used very large amounts of free fructose (300 g/L) ad libitum, even though humans do not consume free fructose, but rather consume it usually accompanied by glucose at a ratio of between 50:50 and 66:33 (and sometimes more in pure honey, which is not, however, used by the majority of people to sweeten foods and beverages). Second, these studies did not address modifications in the key organ that may indeed be affected by fructose: the liver. Third, they did not take into account the pleiotropic effects of the compounds studied, which largely may have been acting as antioxidants and not inhibitors of fructose AGE formation. Recent studies showing neural metabolism of fructose, although interesting, were artificial, with fructose being injected directly into cells (59).
Animal studies focusing on the main target of fructose: the liver.
The first hard evidence for a role of hepatic fructose glycation in major clinically relevant outcomes comes from a recent study in mice and hepatosteatosis (28). After 30 wk of an intervention feeding experiment with fructose or glucose, mice that consumed both fructose and glucose ad libitum in drinking water that contained 100 g either fructose or glucose/L had higher fasting glycemia, glucose intolerance, hyperlipidemia, and hepatosteatosis than did controls that drank water alone. Mice that consumed fructose had higher liver TG concentrations than did those that consumed glucose, which was associated with higher expression and activity of sterol regulatory element–binding protein 1, the transcription factor responsible for de novo lipogenesis. LC-MS analysis uncovered a different profile of AGE production in hepatocytes between mice that consumed fructose and those that consumed glucose. Fructose generated more AGEs derived from glyoxal, such as glyoxal-lysine dimer and N-carboxymethyllysine, whereas glucose generated more AGEs derived from methylglyoxal and methylglyoxal-lysine dimer. The high concentrations of N-carboxymethyllysine and activation of sterol regulatory element–binding protein pathway induced by fructose support an important role of this signaling pathway in mediating fructose-induced lipogenesis. To my knowledge, this is the first relevant study linking a major pathway in a key tissue responsible for insulin resistance with fructose and the Maillard reaction. The same authors found similar results for skeletal muscle, but again, this tissue only receives micromolar concentrations of fructose (82).
Human Studies
Fructose consumption is associated with MetS, diabetes, and obesity
The association of high fructose diets with MetS, diabetes, and obesity is undeniable (3, 83), which large epidemiologic studies have demonstrated, notably with soda and fruit juice consumption. Nevertheless, correlation does not necessarily mean causation, and some researchers maintain that this correlation is just due to extra calories, not to specific mechanistic action (3, 83–86). The role of fructose as a lipogenic substrate is well known (3, 83–92). Increased de novo lipogenesis may lead to hepatic steatosis or increased VLDL production associated with small-dense LDL formation and what is called the atherogenic dyslipidemia complex (2). One intervention that increased dietary fructose consumption in adults documented worsening lipid profiles (88). This and many other mechanistic studies were flawed by the fact that they substituted glucose for fructose, when, as stated above, we rarely sweeten food and drinks with pure fructose. Studies that add fructose to the diet also lead to and are confounded by weight change.
Isocaloric fructose restriction decreases insulin resistance, lipogenesis, and the atherogenic dyslipidemia complex
We thought that isocaloric fructose restriction was a better model to mechanistically prove that fructose overconsumption leads to MetS (1, 2). To better demonstrate a primary effect of fructose restriction unrelated to energy intake or weight change, we substituted dietary added fructose calorie-for-calorie with glucose (in starch) in order to retain equivalence for calories, total carbohydrate content, and body weight. We recently reported the effects of a controlled dietary intervention study of isocaloric substitution of starch for sugar on metabolic markers in children with obesity and metabolic comorbidities. We studied 50 children and explored the effects of such a diet, maintained for just 9 d (1, 2). Significant changes in glucose, insulin resistance, the atherogenic dyslipoproteinemia complex (TGs, LDL cholesterol, apoB, apoC-III, apoC-II, and small-dense LDL), and blood pressure were found (1, 2). Notably, another study pointed to opposite effects of fructose overconsumption in adults: serum concentrations of non-HDL cholesterol, LDL cholesterol, apoB, and apoC-III increased in a dose-dependent manner in young adults who consumed beverages that provided ≤25% of calories as HFCS for 2 wk (88). The crossdirectionality of the effects found by us and this group strengthen the validity of the data, which clearly show the mechanisms involved.
Although this is very interesting, is there a role for AGEs in these processes?
As stated earlier, because of the paucity of appropriate practical methods to measure fructose-derived early and late glycation adducts, there is scant information in regard to their presence or their role in humans. With the use of a fructose AGE antibody, researchers found that serum fructose AGEs were 4-fold higher in diabetic subjects than in control subjects (37). Nevertheless, these concentrations did not correlate to glycated hemoglobin, fasting blood glucose, TGs, or total cholesterol concentrations in type 1 diabetic patients, which complicates interpretation. The data, however, if confirmed, lend support to the contention that fructose AGEs are formed in humans especially under hyperglycemic conditions. Are these AGEs formed in serum, or do they leak from high sorbitol pathway tissues? As stated earlier, serum fructose concentrations are very low (10–35 μM), making the in situ production of fructose AGEs in plasma less likely. Would AGEs leak from hepatocytes, given the fact that they get most of the load? Would they come from food?
Indeed, fructose AGE content was measured in >100 commercially available products. The highest concentrations of fructose AGEs were observed in yogurt beverages. Fructose glycation adducts can then be absorbed (≤10% of dietary AGEs are absorbed) and detected in serum (37).
Time to focus on putative roles of AGE formation by exogenous fructose in tissues in which its concentration is high: the intestines and liver
Intestinal fate of fructose: are fructose AGEs formed in the lumen of the small intestine?
It is well known that the absorption of fructose via Glut5 in the enterocyte is slower when unaccompanied by glucose. Fructose malabsorption from a high intake of HFCS has been shown (93–96). It is not rare to achieve a bolus dose of 40 g fructose in liquid form, as is found in 600 mL soda or juice, in many cases along with a large intake of protein and fat to delay gastric motility. As proposed by DeChristopher (97), given fructose high reactivity, would that not lead to luminal AGE formation? Would this not be another source of dietary AGEs formed in situ? Dietary AGEs are believed to be proinflammatory via engagement of the receptor for advanced glycation end products (RAGE). They may participate in chronic inflammation enhancing insulin resistance in MetS and diabetes, as well as in renal failure (97). Studies have been conducted to provide epidemiologic evidence for the luminal fructose AGE formation hypothesis. These studies led to the discovery of previously unsuspected associations of fructose consumption with nonmetabolic inflammatory diseases (98–100). Associations between childhood asthma and consumption of beverages that contain excess fructose (vis-a-vis glucose) have been unveiled. With the use of data from 2801 adults aged 20–55 y from the 2003–2006 NHANES, researchers showed that, independent of all covariates, intake of nondiet soda ≥5 times/wk (compared with the consumption of diet soda) was associated with nearly twice the likelihood of having chronic bronchitis (OR: 1.80; 95% CI: 1.01, 3.20). Results support the hypothesis that fructose malabsorption and fructose reactivity in the intestines could contribute to asthma and chronic bronchitis. Notably, the association is not found when sucrose is involved. The same authors also found that young adults consuming any combination of high-fructose beverages (sodas or fruit juices) 5 times/wk (but not diet soda) were 3 times as likely to have arthritis as nonconsumers or low consumers (OR: 3.01; 95% CI: 1.20, 7.59), independent of all covariates, including physical activity, other dietary factors, blood glucose, and smoking.
The authors suggest that these associations may be caused by ingesting beverages or foods with a fructose-to-glucose ratio that is >1:1, leading to fructose malabsorption and prolonged life of fructose in the gut (97–100). Fructose may then lead to in situ formation of fructose AGEs, which may contribute to lung or joint disease via engagement of RAGE, as depicted on the left in in Figure 4. Are fructose AGEs free adducts forming in the intestinal lumen? Research on the Maillard reaction between fructose and amino acids was conducted by food chemists >50 y ago. But, to my knowledge, no research seems to exist that explores the reaction at millimolar concentrations, with a physiologic pH and ion composition at 37°C. If fructose AGEs are formed in a period of minutes to a few hours, then they can be a source of dietary AGEs, which we have not considered so far. Given the huge consumption of HFCS at least in the United States, the likelihood that AGEs formed in the intestinal lumen may contribute to the circulating AGE load may be of pathogenic significance. With regard to the above-described association, longitudinal and biochemical research is needed to confirm and clarify the mechanisms involved. I believe this Maillard biochemistry should be revisited.
FIGURE 4.
Two areas of fructose-mediated Maillard reaction that have been overlooked. Enteral formation of fructose AGEs, generating an RAGE inflammatory response (A); inactivation of hepatic AMPK by a fructose-mediated increase in methylglyoxal flux (B). AcCoA, acetyl coenzyme A; AGE, advanced glycation end product; AMPK, AMP-activated protein kinase; MG, methylglyoxal; RAGE, receptor for advanced glycation end products.
Hepatic fate of fructose: are fructose-derived AGE precursors involved in hepatic damage?
As stated earlier, fructose is essentially metabolized by the liver to the exclusion of the rest of the body. Fructose is taken up by hepatocytes via Glut2 and Glut8 (100, 101) (Figure 1). What has been overlooked is that an augmented, unregulated triose flux would raise methylglyoxal production (100, 101). Concomitantly, unregulated phosphorylation of large fructose loads depletes cytosolic ATP. Changes in the AMP:ADP ratio greatly increase the activity of AMPK. Fructose flux increases uric acid production, ergo its precursor AMP (3). When following this sequence of events, a high liver flux of fructose would activate AMPK. AMPK induces a cascade of events within cells in response to the fluxes and availability of metabolites. The role of AMPK in regulating cellular energy status [by sensing low energy–using (AMP) as its signal] and activating catabolic pathways while inhibiting anabolic routes places this enzyme at a central control point in maintaining energy homeostasis (102). When activated, AMPK-mediated downstream phosphorylation events switch cells from active ATP consumption to active ATP production. Consequently, an active AMPK raises glucose transport, glycolysis, and β oxidation, and lowers lipogenesis and cholesterol biosynthesis (101). The activation of AMPK also has long-term effects on gene expression and protein synthesis, inhibits mechanistic target of rapamycin pathways (and protein synthesis), and activates mitochondrial biogenesis and autophagy. The latter is an important pathway that prevents liver steatosis (102).
However, essentially the opposite occurs in chronic fructose overconsumption. Actually, reduced glucose transport (insulin resistance) and increased lipogenesis and cholesterol biosynthesis are found both in animal models and in humans when fructose consumption is excessive, which leads to fatty liver and dyslipoproteinemia (89).
Fructose-induced increased methylglyoxal flux may silence AMPK.
To explain this apparent paradox, I recently proffered the hypothesis that surges of fructose in the portal vein lead to increased unregulated flux to trioses accompanied by unavoidable methylglyoxal production (100) (Figure 4). The new sudden flux exerts carbonyl stress on proteins, including the 3 arginines on the γ subunits AMP binding site of AMPK, irreversibly blocking some of the enzyme molecules to allosteric modulation. The above explains why, even when fructose increases AMP and should therefore activate AMPK, the effects of fructose are compatible with the inactivation of AMPK
During evolution, liver AMPK was certainly not exposed to fluxes in fructose such as those effortlessly achieved with 600 mL of juices or soda (in many cases several times per day). The increased flux of trioses produces fragmentation of glyceraldehyde-3-phosphate to methylglyoxal. Methylglyoxal forms stable imidazolone adducts with arginine residues. There are precisely 3 of them in the AMP allosteric site of the enzyme. Therefore, if the above happens, a methylglyoxal-modified AMPK would not be activated by AMP. Although methylglyoxal is detoxified by the glyoxalase system, this pathway could be overwhelmed by the current large fructose loads. De novo lipogenesis, (which should be inhibited by AMPK activation by high AMP) will be not inhibited during fructose surges because of defective allosteric activation of methylglyoxal-modified AMPK, as depicted on the right in Figure 4. The pathways inhibited by AMPK will be there for activated, i.e., gluconeogenesis, glucose output, and cholesterol biosynthesis, all of which are relevant to MetS. Autophagy, which is involved in the control of lipid accumulation in the liver, will now be inhibited.
The accumulated epidemiologic evidence with respect to fructose consumption and metabolic health—together with animal studies and recent mechanistic studies in humans, including those from our group (1–3, 83, 87, 100)—indicates that fructose overconsumption may well be an initiating factor in the development of fatty liver, liver insulin resistance, MetS, and finally diabetes. One of the multiple mechanisms by which fructose may be causative is by abrupt excessive carbonyl pressure on AMPK, which in part loses its allosteric regulation, thereby leading to unrestrained lipogenesis. The Maillard reaction, which is so important in the pathogenesis of long-term diabetic complications, may also participate as an initial causative event via fructose glycation. Research is warranted to prove this mechanism.
Conclusions
Fructose has not been a main focus of glycation research, mainly because of the difficulty of measuring its adducts in a practical way, and, more importantly, because even though fructose is 10 times more reactive than glucose, its concentration in plasma is 1% that of glucose. Intracellularly, fructose is elevated in a number of tissues of diabetic patients in which the polyol pathway is active (101), reaching the same order of magnitude as glucose. It is plausible that the high reactivity of fructose and its metabolites may contribute to the formation of intracellular AGEs and to vascular complications. The evidence is, however, still unconvincing. I believe that there are 2 areas that have been overlooked so far: 1) enteral formation of fructose AGEs (when unpaired excess free fructose is ingested), generating an RAGE inflammatory response (which may explain the strong association between fructose consumption and asthma, chronic bronchitis, and arthritis); and 2) inactivation of hepatic AMPK by a fructose-mediated increase in methylglyoxal flux (perpetuating lipogenesis, fatty liver, and insulin resistance) should be actively explored. If proven correct, these mechanisms would put the fructose-mediated Maillard reaction in the limelight again, as a contributing factor in chronic inflammatory diseases and MetS.
Acknowledgments
I thank Mallory Davis for expert editorial assistance and A Garramela for helpful support. The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviations used: AGE, advanced glycation end product; AMPK, AMP-activated protein kinase; Glut, glucose transporter; HFCS, high-fructose corn syrup; MetS, metabolic syndrome; NAFLD, nonalcoholic fatty liver disease; RAGE, receptor for advanced glycation end products.
References
- 1.Lustig RH, Mulligan K, Noworolski SM, Tai VW, Wen MJ, Erkin-Cakmak A, Gugliucci A, Schwarz JM. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity (Silver Spring) 2016;24:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gugliucci A, Lustig RH, Caccavello R, Erkin-Cakmak A, Noworolski SM, Tai VW, Wen MJ, Mulligan K, Schwarz JM. Short-term isocaloric fructose restriction lowers apoC-III levels and yields less atherogenic lipoprotein profiles in children with obesity and metabolic syndrome. Atherosclerosis 2016;253:171–7. [DOI] [PubMed] [Google Scholar]
- 3.Malik VS, Hu FB. Fructose and cardiometabolic health: what the evidence from sugar-sweetened beverages tells us. J Am Coll Cardiol 2015;66:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–25. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl 2000;77:S26–30. [DOI] [PubMed] [Google Scholar]
- 6.Monnier VM, Taniguchi N. Advanced glycation in diabetes, aging and age-related diseases: editorial and dedication. Glycoconj J 2016;33:483–6. [DOI] [PubMed] [Google Scholar]
- 7.Monnier VM, Sun W, Sell DR, Fan X, Nemet I, Genuth S. Glucosepane: a poorly understood advanced glycation end product of growing importance for diabetes and its complications. Clin Chem Lab Med 2014;52:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation—a mini-review. Gerontology 2012;58:227–37. [DOI] [PubMed] [Google Scholar]
- 9.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 2000;275:39027–31. [DOI] [PubMed] [Google Scholar]
- 10.Wells-Knecht MC, Thorpe SR, Baynes JW. Pathways of formation of glycoxidation products during glycation of collagen. Biochemistry 1995;34:15134–41. [DOI] [PubMed] [Google Scholar]
- 11.Lal S, Szwergold BS, Taylor AH, Randall WC, Kappler F, Wells-Knecht K, Baynes JW, Brown TR. Metabolism of fructose-3-phosphate in the diabetic rat lens. Arch Biochem Biophys 1995;318:191–9. [DOI] [PubMed] [Google Scholar]
- 12.Suárez G, Maturana J, Oronsky AL, Raventos-Suarez C. Fructose-induced fluorescence generation of reductively methylated glycated bovine serum albumin: evidence for nonenzymatic glycation of Amadori adducts. Biochim Biophys Acta 1991;1075:12–9. [DOI] [PubMed] [Google Scholar]
- 13.Suárez G, Rajaram R, Oronsky AL, Gawinowicz MA. Nonenzymatic glycation of bovine serum albumin by fructose (fructation). Comparison with the Maillard reaction initiated by glucose. J Biol Chem 1989;264:3674–9. [PubMed] [Google Scholar]
- 14.Oimomi M, Sakai M, Ohara T, Igaki N, Nakamichi T, Nishimoto S, Hata F, Baba S. The effect of fructose on collagen glycation. Kobe J Med Sci 1989;35:195–200. [PubMed] [Google Scholar]
- 15.Oimomi M, Sakai M, Ohara T, Igaki N, Nakamichi T, Hata F, Baba S. Acceleration of fructose mediated collagen glycation. J Int Med Res 1989;17:249–53. [DOI] [PubMed] [Google Scholar]
- 16.Oimomi M, Nakamichi T, Ohara T, Sakai M, Igaki N, Hata F, Baba S. Fructose-related glycation. Diabetes Res Clin Pract 1989;7:137–9. [DOI] [PubMed] [Google Scholar]
- 17.McPherson JD, Shilton BH, Walton DJ. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988;27:1901–7. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed N, Furth AJ. Failure of common glycation assays to detect glycation by fructose. Clin Chem 1992;38:1301–3. [PubMed] [Google Scholar]
- 19.Gabbay KH. Role of sorbitol pathway in neuropathy. Adv Metab Disord 1973;2 Suppl 2:417–32. [DOI] [PubMed] [Google Scholar]
- 20.Gabbay KH. The sorbitol pathway and the complications of diabetes. N Engl J Med 1973;288:831–6. [DOI] [PubMed] [Google Scholar]
- 21.Gabbay KH, Merola LO, Field RA. Sorbitol pathway: presence in nerve and cord with substrate accumulation in diabetes. Science 1966;151:209–10. [DOI] [PubMed] [Google Scholar]
- 22.Obrosova IG. Increased sorbitol pathway activity generates oxidative stress in tissue sites for diabetic complications. Antioxid Redox Signal 2005;7:1543–52. [DOI] [PubMed] [Google Scholar]
- 23.Hamada Y, Odagaki Y, Sakakibara F, Naruse K, Koh N, Hotta N. Effects of an aldose reductase inhibitor on erythrocyte fructose 3-phosphate and sorbitol 3-phosphate levels in diabetic patients. Life Sci 1995;57:23–9. [DOI] [PubMed] [Google Scholar]
- 24.Tappy L, Le KA, Tran C, Paquot N. Fructose and metabolic diseases: new findings, new questions. Nutrition 2010;26:1044–9. [DOI] [PubMed] [Google Scholar]
- 25.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 2010;90:23–46. [DOI] [PubMed] [Google Scholar]
- 26.Rabbani N, Thornalley PJ. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem Biophys Res Commun 2015;458:221–6. [DOI] [PubMed] [Google Scholar]
- 27.Rabbani N, Thornalley PJ. Glyoxalase in diabetes, obesity and related disorders. Semin Cell Dev Biol 2011;22:309–17. [DOI] [PubMed] [Google Scholar]
- 28.Mastrocola R, Collino M, Rogazzo M, Medana C, Nigro D, Boccuzzi G, Aragno M. Advanced glycation end products promote hepatosteatosis by interfering with SCAP-SREBP pathway in fructose-drinking mice. Am J Physiol Gastrointest Liver Physiol 2013;305:G398–407. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, Ishimoto T, Sautin YY, Lanaspa MA. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013;62:3307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson RJ, Lanaspa MA, Roncal-Jimenez C, Sanchez-Lozada LG. Effects of excessive fructose intake on health. Ann Intern Med 2012;156:905, author reply 905–6. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan JS, Le MT, Pan Z, Rivard C, Love-Osborne K, Robbins K, Johnson RJ, Sokol RJ, Sundaram SS. Oral fructose absorption in obese children with non-alcoholic fatty liver disease. Pediatr Obes 2015;10:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ, Cicerchi C, McMahan RH, Abdelmalek MF, Rosen HR, Jackman MR, et al. High-fat and high-sucrose (Western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 2013;58:1632–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jedziniak JA, Chylack LT Jr, Cheng HM, Gillis MK, Kalustian AA, Tung WH. The sorbitol pathway in the human lens: aldose reductase and polyol dehydrogenase. Invest Ophthalmol Vis Sci 1981;20:314–26. [PubMed] [Google Scholar]
- 34.Ido Y, Kilo C, Williamson JR. Interactions between the sorbitol pathway, non-enzymatic glycation, and diabetic vascular dysfunction. Nephrol Dial Transplant 1996;11 Suppl 5:72–5. [DOI] [PubMed] [Google Scholar]
- 35.Van den Enden MK, Nyengaard JR, Ostrow E, Burgan JH, Williamson JR. Elevated glucose levels increase retinal glycolysis and sorbitol pathway metabolism. Implications for diabetic retinopathy. Invest Ophthalmol Vis Sci 1995;36:1675–85. [PubMed] [Google Scholar]
- 36.Van Heyningen RE. Sorbitol pathway–reminiscences. Exp Eye Res 1990;50:583–8. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi M, Iwaki M, Takino J, Shirai H, Kawakami M, Bucala R, Yamagishi S. Immunological detection of fructose-derived advanced glycation end-products. Lab Invest 2010;90:1117–27. [DOI] [PubMed] [Google Scholar]
- 38.Levi B, Werman MJ. Fructose and related phosphate derivatives impose DNA damage and apoptosis in L5178Y mouse lymphoma cells. J Nutr Biochem 2003;14:49–60. [DOI] [PubMed] [Google Scholar]
- 39.Kańska U, Boratynski J. Thermal glycation of proteins by D-glucose and D-fructose. Arch Immunol Ther Exp (Warsz) 2002;50:61–6. [PubMed] [Google Scholar]
- 40.Levi B, Werman MJ. Fructose triggers DNA modification and damage in an Escherichia coli plasmid. J Nutr Biochem 2001;12:235–41. [DOI] [PubMed] [Google Scholar]
- 41.Zhao W, Devamanoharan PS, Varma SD. Fructose-mediated damage to lens alpha-crystallin: prevention by pyruvate. Biochim Biophys Acta 2000;1500:161–8. [DOI] [PubMed] [Google Scholar]
- 42.Zhao W, Devamanoharan PS, Varma SD. Fructose induced deactivation of antioxidant enzymes: preventive effect of pyruvate. Free Radic Res 2000;33:23–30. [DOI] [PubMed] [Google Scholar]
- 43.Zhao W, Devamanoharan PS, Varma SD. Fructose induced deactivation of glucose-6-phosphate dehydrogenase activity and its prevention by pyruvate: implications in cataract prevention. Free Radic Res 1998;29:315–20. [DOI] [PubMed] [Google Scholar]
- 44.Zhao HR, Smith JB, Jiang XY, Abraham EC. Sites of glycation of beta B2-crystallin by glucose and fructose. Biochem Biophys Res Commun 1996;229:128–33. [DOI] [PubMed] [Google Scholar]
- 45.Takagi Y, Kashiwagi A, Tanaka Y, Asahina T, Kikkawa R, Shigeta Y. Significance of fructose-induced protein oxidation and formation of advanced glycation end product. J Diabetes Complications 1995;9:87–91. [DOI] [PubMed] [Google Scholar]
- 46.Syrový I. Glycation of albumin: reaction with glucose, fructose, galactose, ribose or glyceraldehyde measured using four methods. J Biochem Biophys Methods 1994;28:115–21. [DOI] [PubMed] [Google Scholar]
- 47.Pennington J, Harding JJ. Identification of the site of glycation of gamma-II-crystallin by (14C)-fructose. Biochim Biophys Acta 1994;1226:163–7. [DOI] [PubMed] [Google Scholar]
- 48.Gugliucci A. Advanced glycation of rat liver histone octamers: an in vitro study. Biochem Biophys Res Commun 1994;203:588–93. [DOI] [PubMed] [Google Scholar]
- 49.Jakas A, Katic A, Bionda N, Horvat S. Glycation of a lysine-containing tetrapeptide by D-glucose and D-fructose–influence of different reaction conditions on the formation of Amadori/Heyns products. Carbohydr Res 2008;343:2475–80. [DOI] [PubMed] [Google Scholar]
- 50.Jairajpuri DS, Fatima S, Saleemuddin M. Immunoglobulin glycation with fructose: a comparative study. Clin Chim Acta 2007;378:86–92. [DOI] [PubMed] [Google Scholar]
- 51.Bakhti M, Habibi-Rezaei M, Moosavi-Movahedi AA, Khazaei MR. Consequential alterations in haemoglobin structure upon glycation with fructose: prevention by acetylsalicylic acid. J Biochem 2007;141:827–33. [DOI] [PubMed] [Google Scholar]
- 52.Ardestani A, Yazdanparast R. Cyperus rotundus suppresses AGE formation and protein oxidation in a model of fructose-mediated protein glycoxidation. Int J Biol Macromol 2007;41:572–8. [DOI] [PubMed] [Google Scholar]
- 53.Yan H, Harding JJ. Carnosine inhibits modifications and decreased molecular chaperone activity of lens alpha-crystallin induced by ribose and fructose 6-phosphate. Mol Vis 2006;12:205–14. [PubMed] [Google Scholar]
- 54.Hinton DJ, Ames JM. Site specificity of glycation and carboxymethylation of bovine serum albumin by fructose. Amino Acids 2006;30:425–34. [DOI] [PubMed] [Google Scholar]
- 55.Lal S, Szwergold BS, Taylor AH, Randall WC, Kappler F, Brown TR. Production of fructose and fructose-3-phosphate in maturing rat lenses. Invest Ophthalmol Vis Sci 1995;36:969–73. [PubMed] [Google Scholar]
- 56.Mikulíková K, Eckhardt A, Kunes J, Zicha J, Miksik I. Advanced glycation end-product pentosidine accumulates in various tissues of rats with high fructose intake. Physiol Res 2008;57:89–94. [DOI] [PubMed] [Google Scholar]
- 57.El-Bassossy H, Badawy D, Neamatallah T, Fahmy A. Ferulic acid, a natural polyphenol, alleviates insulin resistance and hypertension in fructose fed rats: Effect on endothelial-dependent relaxation. Chem Biol Interact 2016;254:191–7. [DOI] [PubMed] [Google Scholar]
- 58.Ojima A, Matsui T, Nakamura N, Higashimoto Y, Ueda S, Fukami K, Okuda S, Yamagishi S. DNA aptamer raised against advanced glycation end products (AGEs) improves glycemic control and decreases adipocyte size in fructose-fed rats by suppressing AGE-RAGE axis. Horm Metab Res 2015;47:253–8. [DOI] [PubMed] [Google Scholar]
- 59.Hassel B, Elsais A, Froland AS, Tauboll E, Gjerstad L, Quan Y, Dingledine R, Rise F. Uptake and metabolism of fructose by rat neocortical cells in vivo and by isolated nerve terminals in vitro. J Neurochem 2015;133:572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han J, Tan C, Wang Y, Yang S, Tan D. Betanin reduces the accumulation and cross-links of collagen in high-fructose-fed rat heart through inhibiting non-enzymatic glycation. Chem Biol Interact 2015;227:37–44. [DOI] [PubMed] [Google Scholar]
- 61.Yeh TC, Liu CP, Cheng WH, Chen BR, Lu PJ, Cheng PW, Ho WY, Sun GC, Liou JC, Tseng CJ. Caffeine intake improves fructose-induced hypertension and insulin resistance by enhancing central insulin signaling. Hypertension 2014;63:535–41. [DOI] [PubMed] [Google Scholar]
- 62.Nayak Y, Venkatachalam H, Daroji VK, Mathew G, Jayashree BS, Unnikrishnan MK. Antidiabetic activity of 3-hydroxyflavone analogues in high fructose fed insulin resistant rats. EXCLI J 2014;13:1055–74. [PMC free article] [PubMed] [Google Scholar]
- 63.Mahmoud AA, Elshazly SM. Ursodeoxycholic acid ameliorates fructose-induced metabolic syndrome in rats. PLoS One 2014;9:e106993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Bassossy HM, Dsokey N, Fahmy A. Characterization of vascular complications in experimental model of fructose-induced metabolic syndrome. Toxicol Mech Methods 2014;24:536–43. [DOI] [PubMed] [Google Scholar]
- 65.Sil R, Ray D, Chakraborti AS. Glycyrrhizin ameliorates insulin resistance, hyperglycemia, dyslipidemia and oxidative stress in fructose-induced metabolic syndrome-X in rat model. Indian J Exp Biol 2013;51:129–38. [PubMed] [Google Scholar]
- 66.Masterjohn C, Park Y, Lee J, Noh SK, Koo SI, Bruno RS. Dietary fructose feeding increases adipose methylglyoxal accumulation in rats in association with low expression and activity of glyoxalase-2. Nutrients 2013;5:3311–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhar I, Dhar A, Wu L, Desai KM. Increased methylglyoxal formation with upregulation of renin angiotensin system in fructose fed Sprague Dawley rats. PLoS One 2013;8:e74212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palanisamy N, Venkataraman Anuradha C. Soy protein prevents renal damage in a fructose-induced model of metabolic syndrome via inhibition of NF-kB in male rats. Pediatr Nephrol 2011;26:1809–21. [DOI] [PubMed] [Google Scholar]
- 69.Palanisamy N, Kannappan S, Anuradha CV. Genistein modulates NF-kappaB-associated renal inflammation, fibrosis and podocyte abnormalities in fructose-fed rats. Eur J Pharmacol 2011;667:355–64. [DOI] [PubMed] [Google Scholar]
- 70.Vasdev S, Gill VD, Randell E, Han Y, Gadag V. Fructose and moderately high dietary salt-induced hypertension: prevention by a combination of N-acetylcysteine and L-arginine. Mol Cell Biochem 2010;337:9–16. [DOI] [PubMed] [Google Scholar]
- 71.Sugimoto K, Hosotani T, Kawasaki T, Nakagawa K, Hayashi S, Nakano Y, Inui H, Yamanouchi T. Eucalyptus leaf extract suppresses the postprandial elevation of portal, cardiac and peripheral fructose concentrations after sucrose ingestion in rats. J Clin Biochem Nutr 2010;46:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Jia X, Chang T, Desai K, Wu L. Attenuation of hypertension development by scavenging methylglyoxal in fructose-treated rats. J Hypertens 2008;26:765–72. [DOI] [PubMed] [Google Scholar]
- 73.Balasaraswathi K, Rajasekar P, Anuradha CV. Changes in redox ratio and protein glycation in precataractous lens from fructose-fed rats: effects of exogenous L-carnitine. Clin Exp Pharmacol Physiol 2008;35:168–73. [DOI] [PubMed] [Google Scholar]
- 74.Chang KC, Liang JT, Tseng CD, Wu ET, Hsu KL, Wu MS, Lin YT, Tseng YZ. Aminoguanidine prevents fructose-induced deterioration in left ventricular-arterial coupling in Wistar rats. Br J Pharmacol 2007;151:341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thirunavukkarasu V, Nandhini AT, Anuradha CV. Lipoic acid prevents collagen abnormalities in tail tendon of high-fructose-fed rats. Diabetes Obes Metab 2005;7:294–7. [DOI] [PubMed] [Google Scholar]
- 76.Rajamani S, Suganthi R, Ravichandran MK, Anuradha CV. Food seasoning spices mixture improves glucose metabolism and lipid profile in fructose-fed hyperinsulinemic rats. J Med Food 2005;8:502–7. [DOI] [PubMed] [Google Scholar]
- 77.Nandhini TA, Thirunavukkarasu V, Ravichandran MK, Anuradha CV. Taurine prevents fructose-diet induced collagen abnormalities in rat skin. J Diabetes Complications 2005;19:305–11. [DOI] [PubMed] [Google Scholar]
- 78.Nandhini AT, Thirunavukkarasu V, Anuradha CV. Taurine prevents collagen abnormalities in high fructose-fed rats. Indian J Med Res 2005;122:171–7. [PubMed] [Google Scholar]
- 79.Thirunavukkarasu V, Nandhini AT, Anuradha CV. Fructose diet-induced skin collagen abnormalities are prevented by lipoic acid. Exp Diabesity Res 2004;5:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Lin YT, Tseng YZ, Chang KC. Aminoguanidine prevents fructose-induced arterial stiffening in Wistar rats: aortic impedance analysis. Exp Biol Med (Maywood) 2004;229:1038–45. [DOI] [PubMed] [Google Scholar]
- 81.Sakai M, Oimomi M, Kasuga M. Experimental studies on the role of fructose in the development of diabetic complications. Kobe J Med Sci 2002;48:125–36. [PubMed] [Google Scholar]
- 82.Mastrocola R, Nigro D, Chiazza F, Medana C, Dal Bello F, Boccuzzi G, Collino M, Aragno M. Fructose-derived advanced glycation end-products drive lipogenesis and skeletal muscle reprogramming via SREBP-1c dysregulation in mice. Free Radic Biol Med 2016;91:224–35. [DOI] [PubMed] [Google Scholar]
- 83.Rodríguez LA, Madsen KA, Cotterman C, Lustig RH. Added sugar intake and metabolic syndrome in US adolescents: cross-sectional analysis of the National Health and Nutrition Examination Survey 2005–2012. Public Health Nutr 2016;19:2424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imamura F, O’Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yilmaz Y. Review article: fructose in non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2012;35:1135–44. [DOI] [PubMed] [Google Scholar]
- 86.Seneff S, Wainwright G, Mascitelli L. Is the metabolic syndrome caused by a high fructose, and relatively low fat, low cholesterol diet? Arch Med Sci 2011;7:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwarz JM, Noworolski SM, Wen MJ, Dyachenko A, Prior JL, Weinberg ME, Herraiz LA, Tai VW, Bergeron N, Bersot TP, et al. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J Clin Endocrinol Metab 2015;100:2434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stanhope KL, Schwarz JM, Havel PJ. Adverse metabolic effects of dietary fructose: results from the recent epidemiological, clinical, and mechanistic studies. Curr Opin Lipidol 2013;24:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stanhope KL, Griffen SC, Bremer AA, Vink RG, Schaefer EJ, Nakajima K, Schwarz JM, Beysen C, Berglund L, Keim NL, et al. Metabolic responses to prolonged consumption of glucose- and fructose-sweetened beverages are not associated with postprandial or 24-h glucose and insulin excursions. Am J Clin Nutr 2011;94:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 2010;7:251–64. [DOI] [PubMed] [Google Scholar]
- 91.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005;54:1907–13. Erratum in: Diabetes 2006;55:563. [DOI] [PubMed] [Google Scholar]
- 93.Ebert K, Witt H. Fructose malabsorption. Mol Cell Pediatr 2016;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DiNicolantonio JJ, Lucan SC. Is fructose malabsorption a cause of irritable bowel syndrome? Med Hypotheses 2015;85:295–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Biesiekierski JR. Fructose-induced symptoms beyond malabsorption in FGID. United European Gastroenterol J 2014;2:10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Putkonen L, Yao CK, Gibson PR. Fructose malabsorption syndrome. Curr Opin Clin Nutr Metab Care 2013;16:473–7. [DOI] [PubMed] [Google Scholar]
- 97.DeChristopher LR. Consumption of fructose and high fructose corn syrup: is fructositis triggered bronchitis, asthma and autoimmune reactivity merely a side bar in the etiology of metabolic syndrome II (to be defined)? Evidence and hypothesis [master thesis]. New York: Medical College BIOBlab LLC; 2012.
- 98.DeChristopher LR, Uribarri J, Tucker KL. Intakes of apple juice, fruit drinks and soda are associated with prevalent asthma in US children aged 2–9 years. Public Health Nutr 2016;19:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DeChristopher LR, Uribarri J, Tucker KL. Intake of high fructose corn syrup sweetened soft drinks is associated with prevalent chronic bronchitis in U.S. adults, ages 20–55 y. Nutr J 2015;14:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DeChristopher LR, Uribarri J, Tucker KL. Intake of high-fructose corn syrup sweetened soft drinks, fruit drinks and apple juice is associated with prevalent arthritis in US adults, aged 20–30 years. Nutr Diabetes 2016;6:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gugliucci A. Fructose surges damage hepatic adenosyl-monophosphate-dependent kinase and lead to increased lipogenesis and hepatic insulin resistance. Med Hypotheses 2016;93:87–92. [DOI] [PubMed] [Google Scholar]
- 102.Ross FA, MacKintosh C, Hardie DG. AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J 2016;283:2987–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]