Abstract
Panax notoginseng, a traditional Chinese herbal medicine, has been used for the treatment of cardiovascular diseases. The main bioactive components of this species are Panax notoginseng saponins (PNS). The present study aimed to investigate the effects of PNS and five of its main components (ginsenosides Rg1, Re, Rb1 and Rd, and notoginsenoside R1) on rat aorta rings pre-contracted with norepinephrine (NE) and to determine the underlying mechanism of action. Isolated aorta rings (with or without intact endothelium) from adult male Wistar rats were stimulated with NE to induce vasoconstriction, and subsequently treated with different concentrations of PNS and its five main components (Rg1, Re, Rb1, R1 and Rd) separately. This procedure was repeated after pre-incubation with the nitric oxide (NO) synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME), the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and the cyclooxygenase (COX) inhibitor indomethacin (INDO), in order to elucidate the mechanism of action of PNS and its components. The results demonstrated that PNS and the components Rg1, Re, Rb1 and R1, but not Rd, induced vessel relaxation in a concentration-dependent manner when the endothelium lining was intact. NO synthase inhibitor L-NAME and guanylate cyclase inhibitor ODQ attenuated the diastolic effects of PNS, Rg1, Re, Rb1 and R1 in aortic rings with intact endothelium. By contrast, INDO, a known COX inhibitor weakened the vasodilation effects of PNS, Re and Rb1 but demonstrated no effect on Rg1 and R1. In conclusion, PNS and two of its main components (Re and Rb1) exert vasodilating effects through the NO and COX pathways.
Keywords: Panax notoginseng saponins, ginsenoside Rg1, ginsenoside Rb1, ginsenoside Re, ginsenoside Rd, notoginsenoside R1, aortic ring, nitric oxide, cyclooxygenase
Introduction
Hypertension is one of the major risk factors for cardiovascular accidents (1). Its main complications include stroke, myocardial infarction, heart failure and chronic kidney disease (2–4). Hypertension is a serious threat to human health, and is one of the most actively researched areas in the biomedical field. Blood pressure is maintained by the regulation of vascular tone, which can be affected by many factors. For example, nitric oxide (NO) has been shown to be an effective vasodilator (5). Furthermore, the NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME) is known to induce sustained blood pressure elevation and left ventricular hypertrophy (6). Soluble guanylyl cyclase (sGC) is an important effector of NO (7). It acts by increasing intracellular cyclic GMP (cGMP) levels to mediate numerous biological functions (8). The compound 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ) has been identified as a selective inhibitor of this enzyme; ODQ treatment is able to increase contractile tone and inhibit relaxation in response to authentic NO (8). Indomethacin (INDO), a known cyclooxygenase (COX) inhibitor has been reported to significantly increase mean arterial pressure without altering other hemodynamic parameters through the inhibition of vasodilation (9).
Antihypertensive drugs exert their actions through a variety of pathways that regulate blood pressure. The major effects of these drugs include: Modulation of the sympathetic branch of the peripheral nervous system and of the renin-angiotensin system (RAS); blockade of calcium channels; improvement of endothelial function; regulation of cardiac blood flow; and inhibition of vascular remodeling and increased urination (10). Antihypertensive drugs include: Diuretics, calcium channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II (ATII) receptor antagonists (ARBs), α1 receptor blockers, β-blockers, renin inhibitors, central hypotensive agents, ganglion blockers and vasodilators (11). Despite their important therapeutic effects, these drugs all have potential side effects. For example, the use of diuretic antihypertensive drugs can lead to hypokalemia, hyperglycemia, hypercholesterolemia, hypertriglyceridemia, and accumulation of uric acid in the blood; β-blockers can cause bronchospasm, peripheral circulation disorders, and insulin insensitivity; and ACEIs can give rise to a dry persistent cough, for example (12).
Panax notoginseng is a species of the genus Panax which is a traditional Chinese herbal medicine (13). The main bioactive ingredient of this species is Panax notoginseng saponins (PNS), which is a phytoestrogenic composition (14). It is known that PNS exerts extensive effects on the cardiovascular system, including inhibition of platelet aggregation, augmentation of the coronary blood flow, improvement of left ventricular diastolic function in hypertensive patients, and myocardial ischemia remodeling protection (15–18). PNS also reduces myocardial oxygen consumption and is endowed with antiarrhythmic effects (19–23).
PNS is a chemical mixture containing >50 different saponins, and the five major components of PNS are ginsenosides Rg1, Rb1, Re and Rd, and notoginsenoside R1 (24–29). PNS saponins are classified into two main groups: Namely the 20(S)-protopanaxatriol saponins (PTS) such as ginsenoside Rg1 and ginsenoside Rd; and the 20(S)-protopanaxadiol saponins (PDS) such as ginsenoside Rb1 and Re, and notoginsenoside R1 (30,31).
In the present study, the aim was to assess the role of PNS and its main components in vascular tone, and thereby explain the mechanism by which they benefit cardiovascular function. The study was conducted using in vitro aortic vascular rings. The endothelium-derived relaxing factors and pathways were examined to elucidate the vasodilation effects of PNS and its major components. This should provide an experimental basis for and improve the clinical application of PNS and its major components.
Materials and methods
Drugs and reagents
PNS, ginsenoside Rg1, ginsenoside Rb1, notoginsenoside R1, ginsenoside Re and ginsenoside Rd were provided by Zhongxin Pharmaceutical Group Corporation, Ltd. (Tianjin, China). Norepinephrine (NE), acetylcholine chloride (ACh), dimethyl sulfoxide (DMSO), L-NAME, INDO, sodium chloride (NaCl), ODQ, potassium chloride (KCl), potassium dihydrogen phosphate (KH2PO4), magnesium sulfate heptahydrate (MgSO4.7H2O), sodium bicarbonate (NaHCO3), glucose and calcium chloride (CaCl2) were purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Animals
Adult male Wistar rats (weight, 250–300 g), were purchased from the Experimental Animal Center, Institute of Radiation Medicine, Chinese Academy of Medical Sciences, Tianjin, China [permit: SCXK200F (JING) 0004]. Rats (8–10 weeks) were housed at 22 ± 2°C and a relative humidity of 40 ± 10% under a 12-h light/dark cycle and given standard laboratory diet and water. Rats were fasted for 12 h before experiments but allowed to access water freely. All experimental procedures that involved animals were submitted to and approved by the Animal Ethics Committee of Tianjin University of Traditional Chinese Medicine Medical Center (permit: LAEC2013005).
Preparation of aortic rings
Male Wistar rats were sacrificed by cervical dislocation and their thoracic aortas were carefully dissected and removed. Fat and other non-vascular tissues were dissected, and the thoracic aortas were sliced into 3-4-mm ring segments after placing in Krebs-Henseleit (K-H) solution at 4°C (in mM: NaCl, 118; KCl, 4.7; NaHCO3, 25; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 1.3; glucose, 10) (32). The vessels were not stretched and the endothelium was protected during handling. For some of the rings, the endothelium was removed gently by rubbing the ring with a glass rod.
The aorta rings were mounted onto two stainless-steel stirrups immersed in a 10-ml organ chamber, containing K-H buffer that was continuously bubbled with 95% O2 and 5% CO2, and maintained at 37°C. Isometric tension change was measured with the force-displacement transducer and recorded using a PowerLab 8/30 bio-signal recording system (AD Instruments Pty Ltd., Bella Vista, Australia). The aorta rings were stretched progressively to a basal tension of 2.0 g and equilibrated for 90 min; during this period the bath solution was replaced with K-H buffer every 15 min. After stabilization the rings were repeatedly contracted with KCl (60 mmol/l) three times until the muscle tension returned to the basal level.
The aortic rings were pre-treated with NE (1×10−6 mol/l) to achieve the plateau phase, and ACh (1×10−5 mol/l) was then added to induce vasodilation. Compared with the maximum contraction extent induced by NE, if the relaxation extent achieved was >60%, the endothelium was regarded as intact and functional, while if it was <10%, the aorta rings would be regarded as completely denuded endothelium.
Measurement of vascular relaxation
Once a sustained contraction plateau in response to NE (1×10−6 mol/l) was achieved, 10 µl H2O or DMSO; PNS (0.2, 0.4, 0.6 or 0.8 mg/ml); or Rg1, Rb1, Re, R1 and Rd (1×10−8, 1×10−7, 1×10−6 or 1×10−5 mol/l) was cumulatively added with an interval of 8 min to the organ bath containing the aortic rings with or without endothelium. H2O was used for PNS. DMSO was used for Rg1, Rb1, R1, Re and Rd.
In order to investigate the involvement of the endothelial NO pathway and cyclooxygenase (COX) pathway in vasorelaxation to PNS and its main five components (ginsenoside Rg1, Re, Rb1 and Rd, and notoginsenoside R1), the rings were exposed to 0.1 mmol/l L-NAME, 0.01 mmol/l ODQ and 0.01 mmol/l INDO for 20 min prior to application of NE to blunt the endothelial function by inhibiting NO and COX synthesis following repeated washout and subsequent equilibration for 45 min.
Statistical analysis
Results are shown as mean ± standard deviation values. Statistical comparisons were carried out by analysis of variance followed by a Dunnett's multiple comparison test. P<0.05 was considered to indicate a statistically significant difference. Each data point represents the mean of a minimum of 10 aortic rings from different animals unless otherwise noted.
Results
Effects of solvents (H2O and DMSO) on aortic rings
It was first verified that the solvents H2O and DMSO each had no effect on the NE-induced vasoconstriction of the rat aortic rings. Mean values of the contraction at four different time points for each solvent are presented in Table I. As detailed in Materials and methods, the test substances (H2O or DMSO) were delivered every 8 min and no wash-out was performed during the experiment. Statistical comparison demonstrated that the curves for the two solvents were not significantly different (P>0.05).
Table I.
Effects of solvent on the vasoconstriction of aortic rings (%; mean ± standard deviation).
| Time (min) | H2O (n=15) | DMSO (n=15) |
|---|---|---|
| 8 | 104.13±3.83 | 104.04±2.75 |
| 16 | 105.22±6.09 | 107.39±4.93 |
| 24 | 102.68±4.95 | 106.82±5.97 |
| 32 | 95.31±5.06 | 99.98±7.69 |
DMSO, dimethylsulfoxide.
Effects of PNS and its main components on aortic rings with or without endothelium
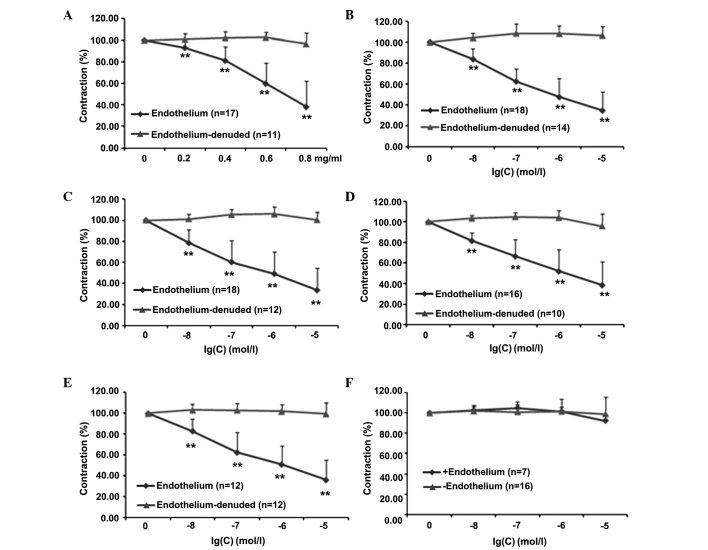
Whether the endothelium plays a major role in mediating the effect of PNS and its five main components (Rg1, Re, Rb1, R1 and Rd) was then investigated. In Fig. 1, dose-response curves for these compounds are shown; each curve represents the ability of the compound to reduce the tonic contraction induced by NE in the presence and absence of the endothelium. Results clearly indicate that when the endothelium lining was intact, PNS, Rg1, Re, Rb1 and R1 (Fig. 1A-E, respectively) significantly reduced the tonic contraction (% of NE) at all doses investigated. The effect was completely lost when the endothelium was absent. Notably, the administration of Rd was not able to induce any response in either the presence or the absence of the endothelium. Detailed statistical comparisons of the data are presented in Table II.
Figure 1.
Norepinephrine-induced vasoconstriction of aortic rings with or without endothelium. After being progressively stretched to a basal tension of 2.0 g and equilibrated for ≥90 min, different concentrations of (A) Panax notoginseng saponins, (B) ginsenoside Rg1, (C) ginsenoside Re, (D) ginsenoside Rb1, (E) notoginsenoside R1 and (F) ginsenoside Rd were added cumulatively. **P<0.01 vs. time 0.
Table II.
Effects of PNS and five main components on aortic rings with or without endothelium.
| Contraction (% of NE) | |||
|---|---|---|---|
| Drugs | Concentration | Endothelium (n=10–18) | Endothelium-denuded (n=10–18) |
| PNS (mg/ml) | 0.2 | 93.02±9.11a | 100.91±5.29 |
| 0.4 | 80.37±13.44a | 102.43±5.37 | |
| 0.6 | 60.62±20.71a | 102.73±4.96 | |
| 0.8 | 37.19±25.23a | 96.69±10.37 | |
| Rg1 (mol/l) | 1×10−8 | 83.67±10.41a | 104.28±4.50 |
| 1×10−7 | 62.47±12.07a | 108.68±8.96 | |
| 1×10−6 | 47.61±17.61a | 108.68±7.67 | |
| 1×10−5 | 34.75±17.88a | 106.68±8.61 | |
| Re (mol/l) | 1×10−8 | 78.50±12.82a | 101.30±4.79 |
| 1×10−7 | 60.44±20.44a | 105.36±5.20 | |
| 1×10−6 | 49.26±20.63a | 105.97±7.28 | |
| 1×10−5 | 33.63±20.74a | 100.39±7.35 | |
| Rb1 (mol/l) | 1×10−8 | 81.77±7.78a | 103.24±3.06 |
| 1×10−7 | 66.69±16.36a | 104.67±4.12 | |
| 1×10−6 | 52.13±20.94a | 103.83±7.21 | |
| 1×10−5 | 38.69±22.79a | 95.90±11.76 | |
| R1 (mol/l) | 1×10−8 | 83.06±11.58a | 103.21±5.73 |
| 1×10−7 | 62.55±19.06a | 103.13±6.19 | |
| 1×10−6 | 51.01±17.96a | 101.90±6.66 | |
| 1×10−5 | 36.04±19.23a | 99.57±10.87 | |
| Rd (mol/l) | 1×10−8 | 101.87±5.57 | 102.78±3.29 |
| 1×10−7 | 101.06±10.06 | 104.93±3.55 | |
| 1×10−6 | 101.52±12.46 | 101.73±3.95 | |
| 1×10−5 | 99.06±16.80 | 92.43±5.90 | |
P<0.01 vs. time 0. PNS, Panox notoginseng saponins; NE, norepinephrine.
Effects of PNS and its main components on the endothelial NO pathway
Effects of PNS and its main components are partially conserved by L-NAME
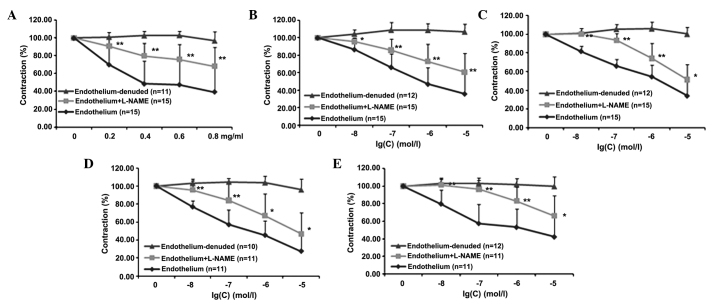
In order to evaluate whether endothelial NO plays a significant role in the mediation of the previously observed effects of PNS and four of its main components (Rg1, Re, Rb1 and R1), experiments were carried out with L-NAME, which is a compound known to selectively block NO synthase and therefore the pathways downstream of NO production. The results shown in Fig. 2 reveal that pre-incubation of the aortic rings with intact endothelium using L-NAME significantly reduced the effects of the drugs at all concentrations tested (P<0.01). Detailed statistical comparisons of the data are presented in Table III.
Figure 2.
Vasoconstriction of rat aortic rings in the presence and absence of L-NAME. Inhibition of norepinephrine-pre-contracted rat thoracic aorta rings with intact endothelium in response to the cumulative addition of (A) PNS, (B) Rg1, (C) Re, (D) Rb1 and (E) R1 in the presence and absence of L-NAME. PNS, Panax notoginsenoside saponins; L-NAME, NG-nitro-L-arginine methyl ester. *P<0.05, **P<0.01 vs. the group with endothelium.
Table III.
Effects of PNS and four main components on aortic rings in the presence of L-NAME.
| Contraction (% of NE) | ||||
|---|---|---|---|---|
| Drugs | Concentration | Endothelium (n=11–15) | Endothelium + L-NAME (n=11–15) | Endothelium-denuded (n=10–12) |
| PNS (mg/ml) | 0.2 | 70.03±21.71 | 90.59±7.80a | 100.91±5.29 |
| 0.4 | 48.39±25.60 | 79.81±14.41a | 102.43±5.37 | |
| 0.6 | 47.41±26.50 | 75.94±16.68a | 102.73±4.96 | |
| 0.8 | 39.30±27.01 | 68.23±21.04a | 96.69±10.37 | |
| Rg1 (mol/l) | 1×10−8 | 86.58±13.21 | 95.48±4.42b | 104.28±4.50 |
| 1×10−7 | 66.23±15.37 | 85.71±12.67a | 108.68±8.96 | |
| 1×10−6 | 46.96±18.98 | 72.85±19.64a | 108.68±7.67 | |
| 1×10−5 | 35.72±22.03 | 60.54±21.56a | 106.68±8.61 | |
| Re (mol/l) | 1×10−8 | 81.72±5.53 | 100.40±5.53a | 101.30±4.79 |
| 1×10−7 | 65.98±7.06 | 93.49±7.46a | 105.36±5.20 | |
| 1×10−6 | 54.59±12.43 | 74.19±16.16a | 105.97±7.28 | |
| 1×10−5 | 34.14±19.26 | 51.36±16.36b | 100.39±7.35 | |
| Rb1 (mol/l) | 1×10−8 | 76.76±6.63 | 95.61±12.15a | 103.24±3.06 |
| 1×10−7 | 56.99±16.34 | 83.97±19.92a | 104.67±4.12 | |
| 1×10−6 | 45.25±16.09 | 66.92±24.55b | 103.83±7.21 | |
| 1×10−5 | 27.40±16.61 | 46.92±23.21b | 95.90±11.76 | |
| R1 (mol/l) | 1×10−8 | 79.83±15.76 | 101.40±6.26a | 103.21±5.73 |
| 1×10−7 | 57.67±21.30 | 96.26±10.68a | 103.13±6.19 | |
| 1×10−6 | 53.34±20.68 | 83.03±15.84a | 101.90±6.66 | |
| 1×10−5 | 42.40±23.16 | 66.02±23.26b | 99.57±10.87 | |
P<0.01
P<0.05 vs. the group with endothelium. PNS, Panox notoginseng saponins; NE, norepinephrine; L-NAME, NG-nitro-L-arginine methyl ester.
Effects of PNS and its main components are partially conserved by ODQ
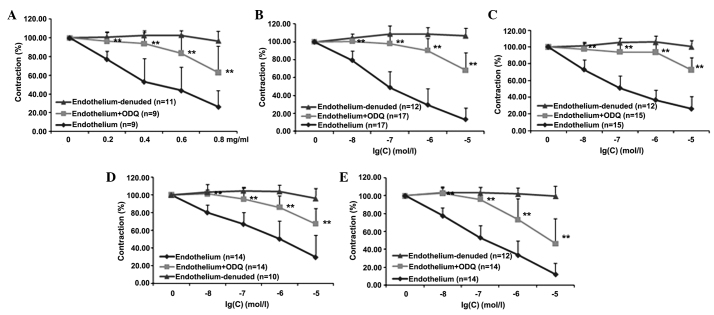
It was then evaluated whether the endothelial NO-mediated pathway requires the functional integrity of guanylyl cyclase and, therefore, cGMP production. Thus, whether pre-treatment of aortic rings with intact endothelium with ODQ, a selective, irreversible, heme-site inhibitor of soluble guanylyl cyclase and competitive inhibitor of NO, was able to inhibit the effects of PNS, Rg1, Re, Rb1 and R1 was tested. As shown in Fig. 3, pre-incubation of the aortic rings with intact endothelium using ODQ significantly reduced the effects of PNS and four of its main components (Rg1, Re, Rb1 and R1) at all concentrations tested (P<0.05). Detailed statistical comparisons of the data are presented in Table IV.
Figure 3.
Vasoconstriction of rat aortic rings in the presence and absence of ODQ. Inhibition of norepinephrine-pre-contracted rat thoracic aorta rings with intact endothelium in response to cumulative addition of (A) PNS, (B) Rg1, (C) Re, (D) Rb1 and (E) R1 in the presence and absence of ODQ. PNS, Panax notoginsenoside saponins; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.**P<0.01 vs, the group with endothelium.
Table IV.
Effects of PNS and four main components on aortic rings in the presence of ODQ.
| Contraction (% of NE) | ||||
|---|---|---|---|---|
| Drug | Concentration | Endothelium (n=9–17) | Endothelium + ODQ (n=9–17) | Endothelium-denuded (n=11–14) |
| PNS (mg/ml) | 0.2 | 77.25±8.74 | 96.249±10.18a | 100.91±5.29 |
| 0.4 | 53.15±24.64 | 93.82±12.36a | 102.43±5.37 | |
| 0.6 | 43.75±25.06 | 83.74±19.50a | 102.73±4.96 | |
| 0.8 | 26.31±17.15 | 62.98±28.98a | 96.69±10.37 | |
| Rg1 (mol/l) | 1×10−8 | 79.48±10.38 | 100.59±4.64a | 104.28±4.50 |
| 1×10−7 | 49.00±17.82 | 98.15±8.38a | 108.68±8.96 | |
| 1×10−6 | 29.48±18.62 | 90.36±13.49a | 108.68±7.67 | |
| 1×10−5 | 13.23±13.16 | 68.28±19.82a | 106.68±8.61 | |
| Re (mol/l) | 1×10−8 | 73.04±11.74 | 97.52±6.79a | 101.30±4.79 |
| 1×10−7 | 51.18±14.45 | 94.06±11.81a | 105.36±5.20 | |
| 1×10−6 | 36.87±11.64 | 93.87±9.34a | 105.97±7.28 | |
| 1×10−5 | 26.38±14.52 | 72.73±14.95a | 100.39±7.35 | |
| Rb1 (mol/l) | 1×10−8 | 80.01±8.93 | 101.39±10.77a | 103.24±3.06 |
| 1×10−7 | 66.97±13.66 | 95.26±12.94a | 104.67±4.12 | |
| 1×10−6 | 50.44±20.56 | 86.12±12.67a | 103.83±7.21 | |
| 1×10−5 | 29.81±24.59 | 67.31±17.27a | 95.90±11.76 | |
| R1 (mol/l) | 1×10−8 | 77.64±9.05 | 102.81±6.96a | 103.21±5.73 |
| 1×10−7 | 53.26±13.45 | 95.89±13.65a | 103.13±6.19 | |
| 1×10−6 | 33.62±15.63 | 73.17±23.19a | 101.90±6.66 | |
| 1×10−5 | 12.14±12.43 | 46.44±28.21a | 99.57±10.87 | |
P<0.01 vs. the group with endothelium. PNS, Panox notoginseng saponins; NE, norepinephrine; ODQ, ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
Effects of PNS and its main components on the COX pathway
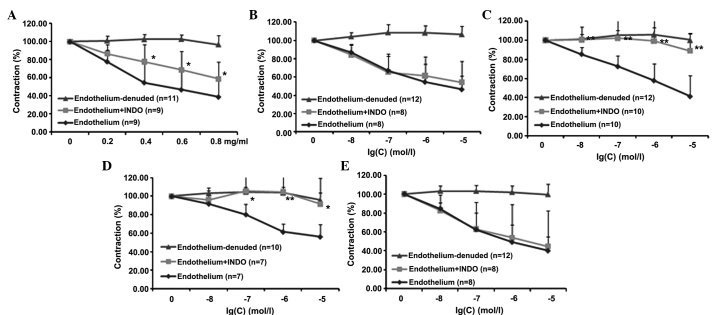
In this set of experiments, the involvement of the COX pathway in mediating the effects of PNS, Rg1, Re, Rb1 and R1 was investigated. As shown in Fig. 4, pre-incubation of the rat aortic rings with intact endothelium using INDO, a known COX inhibitor, significantly reduced the effects of PNS, Re and Rb1 (P<0.05); by contrast, Rg1 and R1 did not elicit any effects. Detailed statistical comparisons of the data are provided in Table V.
Figure 4.
Vasoconstriction of rat aortic rings in the presence and absence of INDO. Inhibition of norepinephrine-pre-contracted rat thoracic aorta rings with intact endothelium in response to cumulative addition of (A) PNS, (B) Rg1, (C) Re, (D) Rb1 and (E) R1 in the presence and absence of INDO. PNS, Panax notoginsenoside saponins; INDO, indomethacin. *P<0.05, **P<0.01 vs. the group with endothelium.
Table V.
Effects of PNS and four main components on the cyclooxygenase pathway in aortic rings.
| Contraction (% of NE) | ||||
|---|---|---|---|---|
| Drugs | Concentration | Endothelium (n=7–10) | Endothelium + INDO (n=7–10) | Endothelium-denuded (n=10–12) |
| PNS (mg/ml) | 0.2 | 77.96±13.39 | 86.70±10.23 | 100.91±5.29 |
| 0.4 | 54.72±21.15 | 77.67±19.08a | 102.43±5.37 | |
| 0.6 | 46.86±21.48 | 68.71±20.56a | 102.73±4.96 | |
| 0.8 | 38.79±18.11 | 58.69±18.68a | 96.69±10.37 | |
| Rg1 (mol/l) | 1×10−8 | 87.06±7.99 | 84.24±11.55 | 104.28±4.50 |
| 1×10−7 | 67.11±18.11 | 65.32±17.48 | 108.68±8.96 | |
| 1×10−6 | 54.66±19.60 | 61.45±120.90 | 108.68±7.67 | |
| 1×10−5 | 46.46±14.63 | 54.25±22.84 | 106.68±8.61 | |
| Re (mol/l) | 1×10−8 | 85.41±7.41 | 100.67±13.15b | 101.30±4.79 |
| 1×10−7 | 72.67±11.16 | 102.29±21.72b | 105.36±5.20 | |
| 1×10−6 | 57.98±17.28 | 99.28±21.33b | 105.97±7.28 | |
| 1×10−5 | 41.47±21.54 | 89.04±17.89b | 100.39±7.35 | |
| Rb1 (mol/l) | 1×10−8 | 91.80±5.28 | 95.88±13.18 | 103.24±3.06 |
| 1×10−7 | 80.37±10.72 | 105.62±24.91a | 104.67±4.12 | |
| 1×10−6 | 61.59±8.39 | 104.70±25.69b | 103.83±7.21 | |
| 1×10−5 | 56.32±13.22 | 91.48±28.07a | 95.90±11.76 | |
| R1 (mol/l) | 1×10−8 | 84.62±6.04 | 82.64±16.32 | 103.21±5.73 |
| 1×10−7 | 62.56±17.43 | 62.95±28.20 | 103.13±6.19 | |
| 1×10−6 | 49.19±18.10 | 53.80±35.32 | 101.90±6.66 | |
| 1×10−5 | 40.13±14.70 | 44.55±37.98 | 99.57±10.87 | |
P<0.05
P<0.01 vs. the group with endothelium. PNS, Panox notoginseng saponins; NE, norepinephrine; INDO, indomethacin.
Discussion
PNS is a traditional Chinese herbal medicine that has protective effects on heart function; particularly, it significantly ameliorates myocardial ischemia-reperfusion injury, reduces myocardial damage, decreases the incidence of irreversible ventricular fibrillation, and protects against myocardial ischemia (33). In addition, PNS has a protective effect on blood vessels; it inhibits the proliferation of vascular smooth muscle, protects the vascular endothelium, lowers blood pressure, and exhibits anti-thrombosis, anti-atherosclerosis and anti-platelet aggregation effects (34). Due to these multiple beneficial effects, PNS is frequently used in Chinese medicine for the treatment of cardiovascular diseases.
In the present study, the effects and mechanism of action of PNS and it main five components (Rg1, Re, Rb1, R1 and Rd) on rat aorta rings re-contracted with NE were evaluated. Ginsenosides Rg1, Re, Rb1 and Rd and notoginsenoside R1 are all found in the root and rhizome of Panax notoginseng, and they are main components of PNS. Ginsenosides Rg1 and Rb1 present anti-amnestic and anti-aging effects (35). Rb1 also plays a role in neurogenesis and the cardiovascular system (36,37). Ginsenoside Re mainly functions in the cardiovascular system, where its effects include changing cardiac electrophysiological properties, which may account for its antiarrhythmic effect (38). Finally, ginsenoside Rd, a dammarane-type steroid glycoside, presents a neuroprotective effect on ischemic brain (39). R1 protected the rat heart from I/R-induced structure and function injury, suggesting R1 as a potential adjuvant therapy for patients presenting with acute myocardial infarction (40). Under the experimental conditions used in the present study, PNS, Rg1, Re, Rb1 and R1 induced a significant concentration-dependent relaxation, while Rd was not effective at any of the concentrations investigated (Fig. 1).
NE induces vasoconstriction through increasing intracellular Ca2+ concentration (41,42). It stimulates α1 adrenergic receptors located on vascular smooth muscle causing Ca2+ to move into cells through receptor-operated Ca2+ channels as well as Ca2+ release from internal stores (43,44). Increasing intracellular Ca2+ concentration is an important condition for the production of vascular endothelial relaxing factor and the regulation of vascular tone.
The endothelium in blood vessels has been identified to be a critical regulator of vascular tone (45). Endothelial dependence has since been reported for a number of other vasoactive substances (46,47). In particular, it is now known that the vascular endothelium plays a critical role in vascular tone regulation due to its ability to produce both vasoconstrictors (endothelin-1, ATII and thromboxane A2) and vasodilation (NO and prostacyclin) factors (48,49). In the present study, it was demonstrated that PNS and four of its main components (Rg1, Re, Rb1 and R1) had vasodilation effects in aortic rings with intact endothelium. By contrast, these vasodilation effects were not present when the endothelium was removed from the aortic rings.
Endothelial cells release various vasodilators to exert their diastolic activity upon being stimulated. Endothelial cells release vasodilators which include the following three main categories: NO, prostaglandin I2 (PGI2) and endothelium-derived hyperpolarizing factor (EDHF). NO and PGI2 appear to be important vasodilators as they share a redundancy interaction and they are both activated by multiple compounds and mechanical signals (50). NO is the strongest vasodilator in vascular endothelial cells, and is synthesized by L-arginine via a reaction that is catalytically synthesized by endothelial NO synthase (51). NO is able to activate sGC to produce cGMP which in turn activates protein kinase G (PKG, also known as cGMP-dependent protein kinase). PKG acts to prevent calcium influx and increase the opening of ATP channels, thus decreasing intracellular Ca2+ levels and ultimately causing vasodilation (52).
L-NAME, an NO synthase inhibitor, reduces the formation of NO and inhibits vasodilation (53). The key enzyme of the NO-cGMP pathway is guanylate cyclase; if guanylate cyclase is inhibited, the NO-cGMP pathway is subsequently blocked and vasodilation is inhibited (54). ODQ, a guanylate cyclase inhibitor, is able to prevent the generation of cGMP and the activation of PKG, thus leading to inhibition of the vasodilation (55). When endothelial cells are stimulated, COX oxidizes arachidonic acid to generate unstable prostaglandin G2 (PGG2) and prostaglandin H2 (PGH2). Through the action of prostacyclin synthase, PGG2 and PGH2 generate PGI2 (56). PGI2 plays a role in vascular smooth muscle cells by promoting the generation of intracellular cAMP for vasodilation (57). COX is thus a key enzyme for the synthesis of PGI2. INDO is an inhibitor of COX and, through the reduction of this enzyme, reduces the generation of PGI2, thereby interfering with vasodilation.
In the present study, the NO synthase inhibitor L-NAME and the guanylate cyclase inhibitor ODQ were shown to reduce the diastolic effects of PNS and four of its main components (Rg1, Re, Rb1 and R1) in aortic rings with intact endothelium (Figs. 2 and 3). It thus may be concluded that these substances cause vasodilation by increasing the production of NO in blood vessels and, furthermore, the endothelium-dependent vasodilation effects of PNS and four of its main components (Rg1, Re, Rb1 and R1) are exerted upon the guanylate cyclase pathway. INDO, which is a COX inhibitor, is able to block the vasodilatory effects exerted by PNS and ginsenosides Re and Rb1 (Fig. 4). This indicates that PNS, Re and Rb1 may stimulate the release of PGI2 to dilate blood vessels. However, INDO was not found to block the vasodilatory effects of Rg1 and R1, which indicates that the vasodilation effects of Rg1 and R1 are not associated with the release of PGI2.
The present study has certain limitations, which are that it was only performed in normal rat aorta in vitro and so it does not best represent hypertensive circumstances or the situation in vivo. Therefore, the conclusions reached in this study require further clarification in future studies.
References
- 1.Kim SJ, Lee J, Jee SH, Nam CH, Chun KH, Park IS, Lee SY. Cardiovascular Risk Factors for Incident Hypertension in the Prehypertensive Population. Epidemiol Health. 2010;32:e2010003. doi: 10.4178/epih/e2010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2010;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Erdine S, Ari O, Zanchetti A, Cifkova R, Fagard R, Kjeldsen S, Mancia G, Poulter N, Rahn KH, Rodicio JL, et al. ESH-ESC guideline for the management of hypertension. Herz. 2006;31:331–338. doi: 10.1007/s00059-006-2829-3. [DOI] [PubMed] [Google Scholar]
- 5.Toda N, Nakanishi S, Tanabe S. Aldosterone affects blood flow and vascular tone regulated by endothelium-derived NO: Therapeutic implications. Br J Pharmacol. 2013;168:519–533. doi: 10.1111/j.1476-5381.2012.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopincova J, Puzserova A, Bernatova I. Chronic low-dose L-NAME treatment effect on cardiovascular system of borderline hypertensive rats: Feedback regulation? Neuro Endocrinol Lett. 2008;29:784–789. [PubMed] [Google Scholar]
- 7.Tsai AL, Berka V, Sharina I, Martin E. Dynamic ligand exchange in soluble guanylyl cyclase (sGC): Implications for sGC regulation and desensitization. J Biol Chem. 2011;286:43182–43192. doi: 10.1074/jbc.M111.290304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feelisch M, Kotsonis P, Siebe J, Clement B, Schmidt HH. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation. Mol Pharmacol. 1999;56:243–253. doi: 10.1124/mol.56.2.243. [DOI] [PubMed] [Google Scholar]
- 9.Kasznicki J, Wiktorowsha-Owczarek A. Effects of indomethacin on hymodynamic parameters after intravenous administration of propranolol and enalaprilat in rabbits. Pol J Pharmacol. 2001;53:487–493. [PubMed] [Google Scholar]
- 10.Zhang XY, Guo SL, Lv GY. Research progress of the mechanism of antihypertensive Traditional Chinese Medicine. Zhejiang Zhong Yi Yao Da Xue Xue Bao. 2005;29:84–86. (In Chinese) [Google Scholar]
- 11.Wang HQ, Yang HJ, Li ZH. Research progress of antihypertension drug. Jibing Jiance Yu Kongzhi. 2013;1:35–38. (In Chinese) [Google Scholar]
- 12.Bai Y. Evaluation and rational application of antihypertensive drugs. Chifeng Xue Yuan Xue Bao. 2007:88–89. (In Chinese) [Google Scholar]
- 13.Guo HB, Cui XM, An N, Cai GP. Sanchi ginseng (Panax notoginseng (Burkill) F. H. Chen) in China: Distribution, cultivation and variations. Genet Resour Crop Evol. 2010;57:453–460. doi: 10.1007/s10722-010-9531-2. [DOI] [Google Scholar]
- 14.Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:1007–1719. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 15.He L, Chen X, Zhou M, Zhang D, Yang J, Yang M, Zhou D. Radix/rhizoma notoginseng extract (Sanchitongtshu) for ischemic stroke: A randomized controlled study. Phytomedicine. 2011;18:437–442. doi: 10.1016/j.phymed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Zheng X, Deng YH, Feng Y, Liu Y, Yang L, Huang Y, Sun J, Liang W, Guan Y. Pharmacokinetics and safety of ginsenoside Rd following a single or multiple intravenous dose in healthy Chinese volunteers. J Clin Pharmacol. 2010;50:285–292. doi: 10.1177/0091270009344334. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Wang Y, Qin L, Yu Y, Wang C. Saponins of Panax notoginseng: Chemistry, cellular targets and therapeutic opportunities in cardiovascular diseases. Expert Opin Investig Drugs. 2014;23:523–539. doi: 10.1517/13543784.2014.892582. [DOI] [PubMed] [Google Scholar]
- 18.Uzayisenga R, Ayeka PA, Wang Y. Anti-diabetic potential of Panax notoginseng saponins (PNS): A review. Phytother Res. 2014;28:510–516. doi: 10.1002/ptr.5026. [DOI] [PubMed] [Google Scholar]
- 19.Cicero AF, Bandieri E, Arletti R. Orally administered Panax notoginseng influence on rat spontaneous behaviour. J Ethnopharmacol. 2000;73:387–391. doi: 10.1016/S0378-8741(00)00277-4. [DOI] [PubMed] [Google Scholar]
- 20.Huang YS, Yang ZC, Yan BG, Hu XC, Li AN, Crowther RS. Improvement of early postburn cardiac function by use of Panax notoginseng and immediate total eschar excision in one operation. Burns. 1999;25:35–41. doi: 10.1016/S0305-4179(98)00129-6. [DOI] [PubMed] [Google Scholar]
- 21.Wang XJ, Ichikawa H, Konishi T. Antioxidant potential of qizhu tang, a Chinese herbal medicine, and the effect on cerebral oxidative damage after ischemia reperfusion in rats. Biol Pharm Bull. 2001;24:558–563. doi: 10.1248/bpb.24.558. [DOI] [PubMed] [Google Scholar]
- 22.Li XH, Li SH. Effects of total saponins of Sanchi (Panax pseudo-ginseng var. notoginseng) on TNF, NO and its mechanisms. Zhong Cao Yao. 1999;307:514–515. (In Chinese) [Google Scholar]
- 23.Liu S, Chen JX. Study on effects of raw and cooked Panax notoginseng on blood lipids. Acta Pharmacology Sinica. 1984;5:100–103. [PubMed] [Google Scholar]
- 24.Chen W, Dang Y, Zhu C. Simultaneous determination of three major bioactive saponins of Panax notoginseng using liquid chromatography-tandem mass spectrometry and a pharmacokinetic study. Chin Med. 2010;5:12. doi: 10.1186/1749-8546-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JR, Yao LF, Gao WN, Liu Y, Yick PW, Liu L, Jiang ZH. Quantitative comparison and metabolite profiling of saponins in different parts of the root of Panax notoginseng. J Agric Food Chem. 2014;62:9024–9034. doi: 10.1021/jf502214x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Li Z, Zhao X, Liu W, Liu Y, Yang J, Li X, Fan X, Cheng Y. A network study of Chinese medicine xuesaitong injection to elucidate a complex mode of action with multicompound, multitarget and multipathway. Evid Based Complement Alternat Med. 2013;2013:652373. doi: 10.1155/2013/652373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin F, Yu SL, Zheng Y, Gao YH. Simultaneous determination of ginsenoside Rg1 and ginsenoside Re in total saponins of fibrous roots of Panax notoginseng by HPLC-MS/MS. Zhongguo Yao Fang. 2010;21:2922–2924. (In Chinese) [Google Scholar]
- 28.Zhang C, Bao J, Li X, Zheng Y. HPLC determination of the amount of ginsenosides in different parts of Panax ginseng C.A.Mey. and P. quiquefolius L. and P. notoginseng (Burk) F.H.Chen. Yao Wu Fen Xi Za Zhi. 2005;25:1190–1194. (In Chinese) [Google Scholar]
- 29.Jiang YQ, Wang Q, Ma SP, Danf XD. Quantitative determination of saponins in the root of Panax pseudoginseng var.notoginseng by HPLC-ELSD and UV spectrophotometry. Zhong Cao Yao. 2000;31:737–739. (In Chinese) [Google Scholar]
- 30.Wan JB, Wang YT, Li SP. Sanqi (Panax notoginseng) In: Li SP, Wang YT, editors. Pharmacological Activity-Based Quality Control of Chinese Herbs. Nova Science Publishers; New York: 2008. pp. 179–203. [Google Scholar]
- 31.Wan JB, Zhang QW, Ye WC, Wang YT. Quantification and separation of protopanaxatriol and protopanaxadiol type saponins from Panax notoginseng with macroporous resins. Sep Pur Tech. 2008;60:198–205. doi: 10.1016/j.seppur.2007.08.007. [DOI] [Google Scholar]
- 32.Xu SY, editor. Methodology of Pharmacological Experiments. 2nd. People's Health Publishing House; Beijing: 1994. pp. 886–887. (In Chinese) [Google Scholar]
- 33.Qiao CL, Ding YF, Yang CR. Pharmacologic research progress of notoginseng total saponins. Zhongguo Xian Dai Zhong Yao. 2012;11:25–30. (In Chinese) [Google Scholar]
- 34.Guo WJ, Yang M, Zhu JG, et al. New progress on pharmacological study of Panax notoginsenoside on the cardiovascular effects. Xian Dai Shi Pin Yu Yao Pin Za Zhi. 2007;2:1–4. (In Chinese) [Google Scholar]
- 35.Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang JT. Nootropic mechanisms of ginsenoside Rg1-influence on neuronal plasticity and neurogenesis. Yao Xue Xue Bao. 2005;40:385–388. (In Chinese) [PubMed] [Google Scholar]
- 37.Zhong ZD, Wang CM, Wang W, Shen L, Chen ZH. Major hypoglycemic ingredients of Panax notoginseng saponins for treating diabetes. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45:235–239. (In Chinese) [PubMed] [Google Scholar]
- 38.Peng L, Sun S, Xie LH, Wicks SM, Xie JT. Ginsenoside Re: Pharmacological effects on cardiovascular system. Cardiovasc Ther. 2012;30:e183–1e88. doi: 10.1111/j.1755-5922.2011.00271.x. [DOI] [PubMed] [Google Scholar]
- 39.Ye R, Zhao G, Liu X. Ginsenoside Rd for acute ischemic stroke: Translating from bench to bedside. Expert Rev Neurother. 2013;13:603–613. doi: 10.1586/ern.13.51. [DOI] [PubMed] [Google Scholar]
- 40.He K, Yan L, Pan CS, Liu YY, Cui YC, Hu BH, Chang X, Li Q, Sun K, Mao XW, et al. ROCK-dependent ATP5D modulation contributes to the protection of notoginsenoside NR1 against ischemia-reperfusion-induced myocardial injury. Am J Physiol Heart Circ Physiol. 2014;307:H1764–H1776. doi: 10.1152/ajpheart.00259.2014. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Qu JT, Zhao X, Guo Y, Mao HP. Vasodilator effect of oroxylin A on thoracic aorta isolated from rats. Zhong Xi Yi Jie He Xue Bao. 2012;10:880–885. doi: 10.3736/jcim20120808. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 42.Martinsen A, Baccelli C, Navarro I, Abad A, Quetin-Leclercq J, Morel N. Vascular activity of a natural diterpene isolated from Croton zambesicus and of a structurally similar synthetic trachylobane. Vascul Pharmacol. 2010;52:63–69. doi: 10.1016/j.vph.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 43.McFadzean I, Gibson A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br J Pharmacol. 2002;135:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slish DF, Arvigo R, Balick MJ. Alseis yucatanensis: A natural product from Belize that exhibits multiple mechanisms of vasorelaxation. J Ethnopharmacol. 2004;92:297–302. doi: 10.1016/j.jep.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Stone DJ, Johns RA. Endothelium-dependent effects of halothane, enflurane and isoflurane on isolated rat aortic vascular rings. Anesthesiology. 1989;71:126–132. doi: 10.1097/00000542-198907000-00021. [DOI] [PubMed] [Google Scholar]
- 46.Furchgott RF. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–194. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- 47.Vanhoutte PM, Rubanyi GM, Miller VM, Houston DS. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- 48.Corvol P, Alhenc-Gelas F, Soubrier F. The vascular endothelium, a site of production and metabolism of vasoactive peptides. Med Sci (Paris) 1993;9:1050–1060. doi: 10.4267/10608/2808. (In French) [DOI] [Google Scholar]
- 49.Negro R. Endothelial effects of antihypertensive treatment: Focus on irbesartan. Vasc Health Risk Manag. 2008;4:89–101. doi: 10.2147/VHRM.S1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hellsten Y, Nyberg M, Jensen LG, Mortensen SP. Vasodilator interactions in skeletal muscle blood flow regulation. J Physiol. 2012;590:6279–6305. doi: 10.1113/jphysiol.2012.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang GZ, Luo XF, Sun B, Hou YL, Li LP, Zheng DD, Qiao GF. Effects and mechanism of the flavonoid glycoside of Polygonum aviculare L. on vascular endothelium relaxation. Haerbin Yi Ke Da Xue Xue Bao. 2010;4:315–318. (In Chinese) [Google Scholar]
- 52.Li YJ, Zhou JW, Bin K. The diastolic mechanism of Gualou Xiebai Banxia Decoction. Zhong Yao Yao Li Yu Lin Chuang. 2010;4:5–7. (In Chinese) [Google Scholar]
- 53.Li XL, Zou XM, Nie G, Song ML, Li G. Roles of neuronal nitric oxide synthase and inducible nitric oxide synthase in intestinal transplantation of rats. Transplant Proc. 2013;45:2497–2501. doi: 10.1016/j.transproceed.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 54.Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/S0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 55.Marinko M, Novakovic A, Nenezic D, Stojanovic I, Milojevic P, Jovic M, Ugresic N, Kanjuh V, Yang Q, He GW. Nicorandil directly and cyclic GMP-dependently opens K+ channels in human bypass grafts. J Pharmacol Sci. 2015;128:59–64. doi: 10.1016/j.jphs.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Fang WT, Li HJ, Zhou LS, Su LQ. Prostacyclin signal pathway: Research advances. Guo Ji Yao Xue Yan Jiu Za Zhi. 2010;4:276–278. (In Chinese) [Google Scholar]
- 57.Zhou P, Wang HP, Jiang HD. The Extracts of Cortex Eucammiae induces both directly and endothelium-dependent relaxation in rat thoracic aorta. Zhongguo Xian Dai Ying Yong Yao Xue. 2007;3:182–185. (In Chinese) [Google Scholar]