Abstract
The prognosis of hepatocellular carcinoma (HCC) is unfavorable following complete tumor resection. The aim of the present study was to identify a molecule able to predict HCC prognosis through comprehensive protein profiling and to elucidate its clinicopathological significance. Comprehensive protein profiling of HCC was performed by liquid chromatography-tandem mass spectrometry. Through the bioinformatic analysis of proteins expressed differentially in HCC and non-HCC tissues, protein disulfide-isomerase A3 (PDIA3) was identified as a candidate for the prediction of prognosis. PDIA3 expression was subsequently examined in 86 cases of HCC by immunostaining and associations between PDIA3 expression levels and clinicopathological characteristics were evaluated. The Ki-67 index and apoptotic cell death of carcinoma cells were examined by immunostaining and terminal deoxynucleotidyl transferase dUTP nick-end labeling assay in 24 cases. The results demonstrated that PDIA3 was expressed in all 86 HCC cases; 56 HCC cases (65%) exhibited high expression of PDIA3 and 30 (35%) exhibited low expression. The disease-free and overall survival times of HCC patients with high PDIA3 expression were significantly shorter than in HCC patients with low expression. Furthermore, increased expression of PDIA3 was associated with an elevated Ki-67 index, indicating increased cancer cell proliferation and a reduction in apoptotic cell death. Taken together, these results suggest that PDIA3 expression is associated with tumor proliferation and decreased apoptosis in HCC, and that increased expression of PDIA3 predicts poor prognosis. PDIA3 may therefore be a key molecule in the development of novel targeting therapies for patients with HCC.
Keywords: protein disulfide-isomerase A3, hepatocellular carcinoma, proliferation, apoptosis, prognosis
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-associated mortality worldwide and its incidence rate is >20 per 100,000 individuals in East Asia (1). Detecting HCC early is difficult and the majority of patients are diagnosed at an advanced stage of the disease (2). The prognosis of patients suffering with HCC remains poor even following complete tumor resection, and recurrence occurs in 70% of cases 5 years after resection (3). Recurrence may be partially predicted by evaluating the proliferation activity of cells in the resected tumor, assessing microvascular invasion and measuring serum levels of α-fetoprotein (4,5). However, recurrent and metastatic cases of HCC are usually refractory to chemotherapy and interventional therapy, and molecular targeting therapy has limited efficacy in such cases (6). It is thus necessary to identify a novel molecule able to predict poor HCC prognosis and to develop novel targeting therapies to treat patients with HCC following tumor resection.
The comprehensive profiling of molecules expressed in human malignant tumors discloses their biology and pathogenesis. Previous studies have performed proteomic analyses of HCC (7–11) and identified candidate proteins for the early diagnosis (7,10) and rapid progression of HCC (9,11). However, only a limited number of patients were examined in these studies.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) has the ability to profile a large number of proteins and formalin-fixed, paraffin-embedded (FFPE) samples may be used for analysis (12). This enables a retrospective study to identify biomarkers for the prognosis of patients with cancer and target molecules to treat HCC, as complete clinical records are available.
The present study aimed to identify a novel biomarker for the prognosis of HCC by investigating the protein disulfide-isomerase A3 (PDIA3), which is highly expressed in HCC, and to investigate the significance of any associations between levels of PDIA3 expression and patient clinicopathological factors.
Materials and methods
HCC cases
The FFPE tumor tissues of 86 cases of HCC were procured from the archives of pathology at Nippon Medical School Hospital (Tokyo, Japan). All patients underwent radical hepatic resection between January 2007 and December 2010. Pathological diagnoses were made following the criteria of the American Joint Committee on Cancer/International Union Against Cancer Tumor-Node-Metastasis classification (13). The present study was performed in accordance with the principles embodied in the Declaration of Helsinki 2013, and the Japanese Society of Pathology Ethics Committee. Informed consent for the study was obtained from all patients.
Comprehensive protein profiling by LC-MS/MS using FFPE tissues
FFPE tissues from 11 HCC cases were used for comprehensive protein profiling. Expressed proteins were profiled in HCC and non-HCC tissues obtained from each case. Each section (10 µm thick) was deparaffinized in xylene and rehydrated through a graded alcohol series of 100, 90, 80 and 70%. Following staining with hematoxylin, the HCC and non-HCC tissues were dissected under a stereoscopic microscope. Protein was extracted from the tissues by lysis buffer [6 M guanidine-hydrochloride, 40 mM Tris (pH 8.2) and 65 mM dithiothreitol (DTT)] and protein concentration was determined by the Bradford method (14). Extracted protein (10 µg) was reduced in 45 mM DTT and 20 mM Tris (2-carboxyethyl) phosphine hydrochloride and alkylated in 100 mM iodoacetamide. The protein was further digested using proteomics-grade trypsin (Agilent Technologies, Inc., Santa Clara, CA, USA) at 37°C for 24 h and the digest was purified in a PepClean™ C-18 spin column. Digested protein (2 µg) was injected into a peptide L-trap column (Chemicals Evaluation and Research Institute, Tokyo, Japan) and further separated through Advanced-nano ultra-high-performance liquid chromatography (AMR, Inc., Tokyo, Japan) using a reverse-phase C-18 column (Zaplous column α, gel particles 3 µm diameter and 10 nm pore size, 0.1×150 mm; AMR, Inc.). The protein solution was run with the gradient concentration of acetonitrile from 5–35% in 0.1% formic acid in acetonitrile at a flow rate of 500 nl/min for 2 h. An amaZon ETD Ion-Trap Mass Spectrometer (Bruker Corporation, Billerica, MA, USA) was used to analyze eluted peptides. Data of the 10 most intense peaks of each full MS scan were acquired. All MS/MS spectral data were analyzed by MASCOT v2.3.01 (Matrix Science Ltd., London, UK). The following parameter settings were used: Trypsin cleavage; allowance of ≤2 missed cleavage peptides; variable modifications of methionine oxidation; peptide mass tolerance; fixed modifications of cysteine carbamidomethylation; and fragment MS/MS tolerance ± 0.5 Da. Amounts of identified proteins were expressed as normalized spectral abundance factor (15). Relative expression levels of identified proteins in HCC tissues relative to those in non-HCC tissues were expressed as ratios of spectral counts (Rsc) in the base 2 logarithmic scale (16). An Rsc >1 indicated that the protein was upregulated in HCC tissue, while an Rsc <-1 indicated that the protein was downregulated in the HCC tissues compared with the non-HCC tissues. An Rsc between −1 and 1 indicated that levels of protein expression were similar in HCC and non-HCC tissue.
Bioinformatic analysis
Functional properties of identified proteins were analyzed by the Database Annotation, Visualization and Integrated Discovery (v6.7) using the Kyoto Encyclopedia of Gene and Genomes (KEGG) database (17). Relative abundance (%) of each functional category was calculated by dividing the number of proteins in each category by the total number of identified proteins.
Reverse-transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from FFPE tissues using the RNeasy FFPE kit (Qiagen, Inc., Valencia, CA, USA). Expression of PDIA3 mRNA and 18S rRNA as a reference was determined by the 7500 Fast Real-Time PCR system (Thermo Fisher Scientific, Inc., Waltham, MA, USA) using cDNA, which was synthesized from 20 ng total RNA by SuperScript® III using the SuperScript® VILO™ cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). TaqMan primers and probes were purchased from Thermo Fisher Scientific, Inc. These consisted of PDIA3 (Hs04194196) and 18S rRNA (Hs03928990), and the details of the primers and probes are available at the manufacturer's website (http://www.thermofisher.com/jp/ja/home.html). qPCR was subsequently performed, beginning with denaturation at 95°C for 10 min, followed by 40 cycles of amplification at 95°C for 15 sec and extension at 60°C for 1 min. The level of expression of PDIA3 mRNA was calculated as the ratio of PDIA3 mRNA to 18S rRNA, and PDIA3 expression in HCC tissue was further calculated as relative increase, compared with that in non-HCC tissue using the 2−ΔΔCq method (18). The expression levels were measured in triplicate.
Immunostaining and scoring
Sections 3-µm thick were used for immunostaining. Following deparaffinization, sections were treated in Histofine® Antigen Activation Liquid (pH 9.0; Nichirei Biosciences, Inc., Tokyo, Japan) at 121°C for 15 min. Endogenous peroxidase was blocked in 0.3% hydrogen peroxide and methanol for 30 min. Sections were then incubated with antibodies for PDIA3 (catalog no. ab13506; dilution, 1:150; Abcam, Tokyo, Japan) and Ki-67 (MIB1; catalog no. M7240; dilution, 1:100; Dako Japan Co., Ltd., Tokyo, Japan) in phosphate-buffered saline containing 1% bovine serum albumin (Sigma-Aldrich Japan K.K., Tokyo, Japan) for 16 h at 4°C. The sections were further incubated with the Histofine Simple Stain™ MAX-PO (R; Nichirei Biosciences, Inc.) for 30 min, and peroxidase activity was visualized by 3,3′-diaminobenzidine. The sections were then counterstained with hematoxylin.
The intensity and proportion of stained tumor cells were semi-quantitated
Cytoplasmic staining was considered a positive reaction. If no tumor cell staining occurred, intensity and proportion were scored as 0. Tumor cell staining intensity was categorized into 3 grades: 1, weak; 2, moderate; and 3, strong. The proportional score was divided into 3 grades: 1, <10%; 2, 10–50%; and 3, >50%. The total score was calculated as the sum of the intensity and proportional scores. Two investigators evaluated the score in a blind manner. HCC tissue with a mean total score ≥5 was classified as having high expression and HCC tissue with a mean total score <5 was classified as having low expression. The Ki-67 index was calculated as the percentage of Ki-67-positive cells in 1,000 tumor cells in the areas of the highest nuclear labeling (so-called ‘hot spots’), which was determined using a microscope (magnification, ×40).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay
Apoptotic cell death of HCC was determined by TUNEL assay using an Apoptag® Peroxidase In Situ Apoptosis Detection kit (EMD Millipore, Billerica, MA, USA). Nuclear staining was considered a positive result. The TUNEL index was calculated as the percentage of TUNEL-positive cells in 1,000 carcinoma cells in the areas of highest nuclear labeling under a microscope (magnification ×40).
Statistical analysis
All data are presented as the mean ± standard error. The data of two groups were compared by the Mann-Whitney U-test. Clinicopathological parameters were analyzed by the χ2 test and Fisher's exact test. Cumulative survival rate was calculated using the Kaplan-Meier method and the significance of differences in survival rate were analyzed by the log-rank test. P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using GraphPad Prism v5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Comprehensive profiling of proteins
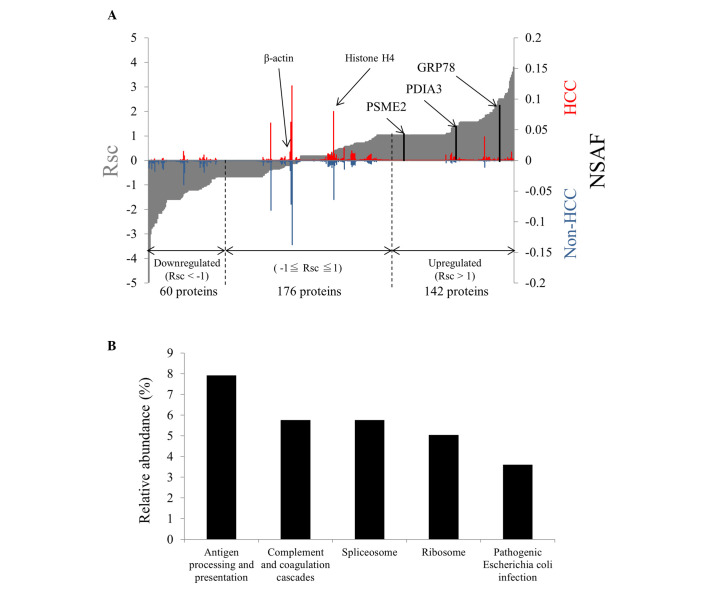
A total of 378 proteins were identified from the FFPE tissues, 295 from the HCC tissues and 270 proteins in the non-HCC tissues. A total of 187 proteins were identified in HCC and non-HCC. In total, 142 proteins were upregulated (Rsc >1) in the HCC tissues compared with the non-HCC tissues and 60 proteins were downregulated (Rsc <-1; Fig. 1A). Overall, 176 proteins were equally expressed in the HCC and non-HCC tissues, and housekeeping gene products, including β-actin and histone H4, were equally expressed.
Figure 1.
Protein expression and functional annotation. (A) NSAF and Rsc of the identified proteins in the HCC and non-HCC tissues. The proteins are plotted from the left to the right on the x-axis in ascending order of Rsc value. A higher Rsc indicates higher expression in HCC relative to non-HCC. (B) Relative abundance (%) of proteins categorized by the Kyoto Encyclopedia of Genes and Genomes. Relative abundance is the percentage of the number of annotated proteins in the total number of upregulated proteins. NSAF, normalized spectral abundance factor; Rsc, ratios of spectral counts; HCC, hepatocellular carcinoma; PSME2, proteasome activator complex subunit 2; PDIA3, protein disulfide-isomerase A3; GRP78, glucose-regulated protein 78 kDa.
The functional properties of the identified proteins were analyzed using the KEGG database. Among the upregulated proteins, the most abundant functional category was antigen processing and presentation (Fig. 1B), and 11 proteins out of 142 upregulated proteins (7.7%) were classified within this category (Table I). None of the protein among the downregulated proteins (0/60, 0%) and equally expressed proteins (0/176, 0%) was classified in this functional category. It was thus speculated that the upregulation of proteins involved in antigen processing and presentation was a characteristic feature of HCC. Among 11 proteins in the antigen processing and presentation category, the clinicopathological significance of PDIA3 in HCC is unknown, therefore PDIA3 expression at the mRNA level was investigated.
Table I.
Upregulated proteins in the antigen processing and presentation category.
| Spectral counting | |||||
|---|---|---|---|---|---|
| ID | Protein | AAa | HCC | Non-HCC | Rsc |
| HSP71 | Heat shock 70 kDa protein 1A/1B | 641 | 14 | 0 | 3.8 |
| HSP76 | Heat shock 70 kDa protein 6 | 643 | 12 | 0 | 3.6 |
| 1A01 | HLA class I histocompatibility antigen, A-1 α chain | 365 | 6 | 0 | 2.72 |
| HS71L | Heat shock 70 kDa protein 1-like | 64 | 5 | 0 | 2.51 |
| HS90B | Heat shock protein HSP 90-β | 724 | 5 | 0 | 2.51 |
| GRP78 | 78 kDa glucose-regulated protein | 654 | 8 | 1 | 2.23 |
| HS90A | Heat shock protein 90-α | 732 | 5 | 1 | 1.66 |
| HSP7C | Heat shock cognate 71 kDa protein | 646 | 10 | 3 | 1.59 |
| PDIA3 | Protein disulfide-isomerase A3 | 505 | 6 | 2 | 1.34 |
| HLAE | HLA class I histocompatibility antigen, α chain E | 358 | 1 | 0 | 1.03 |
| PSME2 | Proteasome activator complex subunit 2 | 239 | 1 | 0 | 1.03 |
Number of AAs. AA, amino acid; HCC, hepatocellular carcinoma; Rsc, ratio of spectral counts; HLA, human leukocyte antigen.
RT-qPCR analysis of PDIA3 (data not shown)
The expression of PDIA3 in HCC tissues was verified by TaqMan probes in 11 cases of HCC and non-HCC. The relative expression of PDIA3 mRNA was significantly elevated in the HCC tissues (3.43±2.93) compared with the non-HCC tissues (1.20±0.81; P<0.05). Protein profiling and quantitation of mRNA confirmed the upregulation of PDIA3 in HCC. The clinicopathological significance of increased PDIA3 expression was subsequently examined in all cases of HCC.
Cases of HCC and immunostaining of PDIA3
Immunostaining was performed in 86 HCC cases. Of these, 51 patients were men and 35 were women, with a mean age of 68 years (range, 34–87). Hepatitis B surface antigen (HBsAg) was positive in 26 cases and hepatitis C virus (HCV) was positive in 51 cases. A total of 6 cases were positive for HBsAg and HCV. Local recurrence was noted in 47 cases and 6 patients exhibited metastasis to the lungs, brain and lymph nodes. Early recurrence or metastasis within 6 months of surgery was reported in 13 cases (15%).
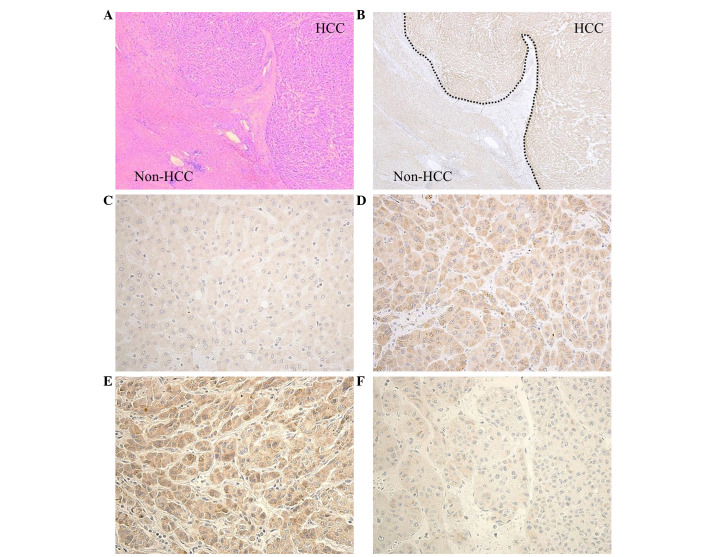
Representative immunostaining for PDIA3 in the HCC and non-HCC tissues is presented in Fig. 2A. The HCC tissues demonstrated potent staining for PDIA3, while the non-HCC tissues exhibited weak staining (Fig. 2B). At a higher magnification, cytoplasm of the non-HCC cells exhibited a vaguely positive reaction (Fig. 2C), while the cytoplasm of HCC cells exhibited a clear positive reaction (Fig. 2D). Staining intensity varied among the HCC tissues; 56 cases (65%) were classified as high expression (Fig. 2E) and 30 cases (35%) were classified as low expression (Fig. 2F).
Figure 2.
Expression of PDIA3 in HCC tissue. (A) The representative histology of HCC with trabecular growth pattern and non-HCC tissue. Hematoxylin & eosin staining (magnification, ×40). (B) Intense expression of PDIA3 was observed in the HCC tissue compared with the non-HCC tissue (magnification, ×40). (C) Healthy hepatocytes exhibited only weak PDIA3 staining (magnification, ×400). (D) Clear cytoplasmic staining occurred in HCC cells (magnification, ×400). Representative immunostaining of HCC tissue with (E) high expression and (F) low expression of PDIA3 (magnification, ×400). PDIA3, protein disulfide-isomerase A3; HCC, hepatocellular carcinoma.
Association between PDIA3 expression and clinicopathological factors
Associations between PDIA3 expression and patient clinicopathological factors were examined in 86 cases of HCC (Table II). No significant correlations were observed between expression levels of PDIA3 and any patient characteristics. However, low PDIA3 was associated with poorly differentiated HCC and a smaller tumor size, though these associations were not significant (P>0.05). Local recurrence in the residual liver occurred in 29 cases (52%) of HCC with high expression and in 18 cases (60%) of low expression. Distant metastasis occurred in 5 cases (9%) with high expression and in 1 case (3%) of low expression.
Table II.
Associations between clinicopathological factors and PDIA3 expression in hepatocellular carcinoma.
| PDIA3 expression | ||||
|---|---|---|---|---|
| Characteristic | n | High (n=56) | Low (n=30) | P-value |
| Gender | 0.819 | |||
| Male | 51 | 34 | 17 | |
| Female | 35 | 22 | 13 | |
| Age, years | 0.482 | |||
| <65 | 31 | 22 | 9 | |
| ≥65 | 55 | 34 | 21 | |
| HBsAg | 0.806 | |||
| Positive | 26 | 16 | 10 | |
| Negative | 60 | 40 | 20 | |
| HCV | 0.819 | |||
| Positive | 51 | 34 | 17 | |
| Negative | 35 | 22 | 13 | |
| Cirrhosis | 0.251 | |||
| Yes | 51 | 36 | 15 | |
| No | 35 | 20 | 15 | |
| Preoperative AFP, ng/ml | 1 | |||
| <20 | 44 | 29 | 15 | |
| ≥20 | 42 | 27 | 15 | |
| Preoperative DCP, mAU/ml | 0.645 | |||
| <40 | 32 | 22 | 10 | |
| ≥40 | 54 | 34 | 20 | |
| Tumor size, cm | 0.143 | |||
| <5 | 59 | 35 | 24 | |
| ≥5 | 27 | 21 | 6 | |
| Tumor number | 0.24 | |||
| 1 | 54 | 32 | 20 | |
| <1 | 32 | 24 | 8 | |
| Vascular invasion | 0.815 | |||
| Positive | 31 | 21 | 10 | |
| Negative | 55 | 35 | 20 | |
| UICC stage | 0.287 | |||
| I | 35 | 19 | 16 | |
| II | 44 | 31 | 13 | |
| III | 6 | 5 | 1 | |
| IV | 1 | 1 | 0 | |
| Differentiation | 0.066 | |||
| Well | 17 | 15 | 2 | |
| Moderate | 54 | 31 | 23 | |
| Poor | 15 | 10 | 5 | |
| Local recurrence | 0.503 | |||
| Yes | 47 | 29 | 18 | |
| No | 39 | 27 | 12 | |
| Distal metastasis | 0.66 | |||
| Yes | 6 | 5 | 1 | |
| No | 80 | 51 | 29 | |
HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; PDIA3, protein disulfide-isomerase A3; AFP, α-fetoprotein; DCP, des-gamma-carboxy prothrombin; UICC, Union for International Cancer Control.
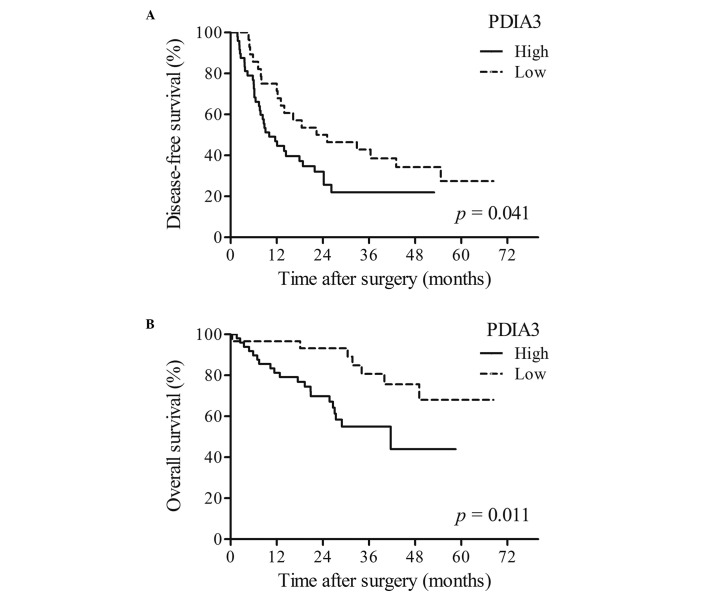
Disease-free survival (DFS) and overall survival time (OS) of patients with HCC
The DFS and OS times significantly differed between HCC patients with high and low expression of PDIA3. OS and DFS times were significantly shorter in patients with high PDIA3 expression (P<0.05; Fig. 3A) compared with that of patients with low PDIA3 expression (P<0.05; Fig. 3B).
Figure 3.
DFS and OS times of patients with HCC. (A) The DFS time of HCC patients with high PDIA3 expression was significantly shorter (P=0.041) than that of HCC patients with low expression. (B) The OS time of HCC patients with high PDIA3 expression was also significantly shorter (P=0.011) than that of HCC patients with low expression. DFS, disease-free survival; OD, overall survival; HCC, hepatocellular carcinoma; PDIA3, protein disulfide-isomerase A3.
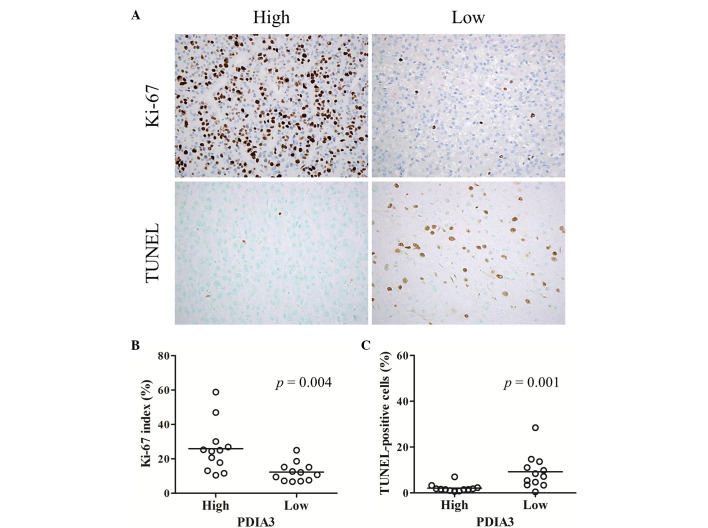
Ki-67 index and TUNEL indices
Proliferation activity and apoptotic cell death were examined in 24 cases of HCC. Representative immunostaining of Ki-67 and TUNEL in HCC tissues with high and low expression of PDIA3 was performed (Fig. 4A). The Ki-67 index in the HCC tissues with high expression was significantly greater than those in the HCC tissues with low PDIA3 expression (P<0.05; Fig. 4B), indicating increased cell proliferation in HCC tissues with high expression of PDIA3. By contrast, the TUNEL index in the HCC tissues with high expression was significantly lower than those in the HCC tissues with low expression (P<0.05; Fig. 4C), indicating decreased cell apoptosis in HCC tissues with high PDIA3 expression.
Figure 4.
Ki-67 labeling index and TUNEL index in HCC. (A) Representative immunostaining of Ki-67 and TUNEL assay in HCC tissues with high and low expression of PDIA3 (magnification, ×400). Scatter plots of (B) Ki-67 index and (C) TUNEL index in HCC tissue with high and low PDIA3 expression. These results suggest that cell proliferation is increased and cell apoptosis is decreased in HCC tissues with a high expression of PDIA3. HCC, hepatocellular carcinoma; PDIA3, protein disulfide-isomerase A3; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling.
Discussion
In the current study, 378 proteins were identified from HCC and non-HCC tissues by LC-MS/MS. Proteins involved in antigen processing and presentation were the most abundant among the upregulated proteins, and proteins in this category were not detected among the downregulated and equally expressed proteins. These upregulated proteins, including PDIA3, are usually present in the endoplasmic reticulum (ER) (19) and the enrichment of ER proteins in HCC has been reported in a previous protein profiling study (7). It is therefore plausible that the upregulation of ER proteins is a characteristic feature of HCC.
Among the proteins classified in the category of antigen processing and presentation, the clinicopathological significance of increased PDIA3 expression in HCC was previously unknown. The current study therefore examined PDIA3 expression in HCC and investigated its association with various patient clinicopathological features. The DFS and OS times of HCC patients with high expression of PDIA3 were significantly shorter than those of patients with low expression, thus PDIA3 expression may be considered as a potential biomarker for the poor prognosis of HCC.
PDIA3, also known as ERp57 or glucose-regulated protein 58 kDa, is a thiol-oxidoreductase chaperone belonging to the PDI family (20). The molecule is involved in multiple cellular functions, including the folding of newly synthesized proteins and assembly of major histocompatibility complex I. Previous studies have reported the upregulation of PDIA3 protein and mRNA levels in HCC (7,10), which is consistent with the results of the current study. However, in previous proteomic studies, PDIA3 was not identified as a candidate biomarker indicating early recurrence and metastasis of HCC (9,11). This may be partially due to patient selection as these studies were conducted with patients in which recurrence and metastasis occurred within 6 months of the treatment, while only 15% of patients in the current study experienced recurrence and metastasis within this same time frame. Therefore, PDIA3 may not be a suitable marker for patients with rapidly progressing HCC.
The expression of PDIA3 has been observed in various types of human cancer, including ovarian, mammary, uterine, pulmonary and gastric cancer (21,22). Increased PDIA3 expression was associated with a poor prognosis in adenocarcinoma of the uterine cervix (23). However, its downregulation was associated with poor outcomes in squamous cell carcinoma of the uterine cervix (24), and loss of expression of PDIA3 is correlated with more aggressive forms of gastric cancer (25). Therefore, although PDIA3 is expressed in various types of cancer, the pathogenetic role of PDIA3 may vary among various types of human cancer, depending on the primary organs affected and the histology of the cancer.
In the present study, the shorter DFS and OS times observed in HCC patients with high expression of PDIA3 appeared to be associated with an increase in proliferation and reduction in apoptotic cell death of cancer cells. In addition to its function as a chaperone, PDIA3 is involved in the ER stress signaling pathway, otherwise known as the ‘unfolded protein response’ (20). It has been demonstrated that PDIA3 protects cells from ER-stress-induced apoptosis and that silencing PDIA3 induces apoptosis (26). However, the direct signaling pathway from PDIA3 to cell proliferation is not well known. Increased proliferation of HCC may, in part, be due to the interaction of PDIA3 with the mammalian target of rapamycin (mTOR) (27). The mTOR pathway serves an important role in the carcinogenesis of HCC (28,29) and increased expression of PDIA3 may induce cell proliferation through enhanced interaction with mTOR. Further studies are required to determine the precise molecular mechanism of cell proliferation and interaction between PDIA3 and mTOR in order to establish an effective targeting therapy.
In conclusion, the present study performed comprehensive protein profiling of HCC by LC-MS/MS using FFPE tissues and determined that expression was upregulated in HCC. Increased PDIA3 expression predicted poor prognosis and was associated with an increase in cell proliferation and a reduction of apoptosis. To the best of our knowledge, the current study is the first to demonstrate the significance of PDIA3 in the prognosis of HCC, and may enable an effective targeting strategy to be developed to treat patients with refractory and metastatic HCC.
Acknowledgements
The authors would like to thank Mr. Kiyoshi Teduka, Mr. Takenori Fujii, Ms. Yoko Kawmoto, Ms. Kiyoko Kawahar and Ms. Taeko Suzuki for technical assistance (Department of Integrated Diagnostic Pathology, Nippon Medical School, Tokyo, Japan). The present study was supported by grants-in-aid for Clinical Rebiopsy Bank Project for Comprehensive Cancer Therapy Development to Professor Zenya Naito from the Ministry of Education, Culture, Sport, Science, and Technology, Japan (S1311022).
Glossary
Abbreviations
- HCC
hepatocellular carcinoma
- PDIA3
protein disulfide-isomerase A3
- AFP
alpha fetoprotein
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- FFPE
formalin-fixed paraffin-embedded
- NSAF
normalized spectral abundance factor
- Rsc
ratios of spectral counts
- KEGG
Kyoto Encyclopedia of Gene and Genomes
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labeling
- DFS
disease-free survival
- OS
overall survival
- ER
endoplasmic reticulum
- mTOR
mammalian target of rapamycin
References
- 1.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: Consider the population. J Clin Gastroenterol. 2013;47:S2–S6. doi: 10.1097/MCG.0b013e3182872f29. (Suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roxburgh P, Evans TR. Systemic therapy of hepatocellular carcinoma: Are we making progress? Adv Ther. 2008;25:1089–1104. doi: 10.1007/s12325-008-0113-z. [DOI] [PubMed] [Google Scholar]
- 3.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56:S75–S87. doi: 10.1016/S0168-8278(12)60009-9. (Suppl 1) [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases: Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Prognostic molecular markers in hepatocellular carcinoma: A systematic review. Eur J Cancer. 2007;43:979–992. doi: 10.1016/j.ejca.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: A daunting challenge. Hepatology. 2011;54:757–759. doi: 10.1002/hep.24569. [DOI] [PubMed] [Google Scholar]
- 7.Chignard N, Shang S, Wang H, Marrero J, Bréchot C, Hanash S, Beretta L. Cleavage of endoplasmic reticulum proteins in hepatocellular carcinoma: Detection of generated fragments in patient sera. Gastroenterology. 2006;130:2010–2022. doi: 10.1053/j.gastro.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 8.Corona G, De Lorenzo E, Elia C, Simula MP, Avellini C, Baccarani U, Lupo F, Tiribelli C, Colombatti A, Toffoli G. Differential proteomic analysis of hepatocellular carcinoma. Int J Oncol. 2010;36:93–99. [PubMed] [Google Scholar]
- 9.Huang X, Zeng Y, Xing X, Zeng J, Gao Y, Cai Z, Xu B, Liu X, Huang A, Liu J. Quantitative proteomics analysis of early recurrence/metastasis of huge hepatocellular carcinoma following radical resection. Proteome Sci. 2014;12:22. doi: 10.1186/1477-5956-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teramoto R, Minagawa H, Honda M, Miyazaki K, Tabuse Y, Kamijo K, Ueda T, Kaneko S. Protein expression profile characteristic to hepatocellular carcinoma revealed by 2D-DIGE with supervised learning. Biochim Biophys Acta. 2008;1784:764–772. doi: 10.1016/j.bbapap.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Yokoo H, Kondo T, Okano T, Nakanishi K, Sakamoto M, Kosuge T, Todo S, Hirohashi S. Protein expression associated with early intrahepatic recurrence of hepatocellular carcinoma after curative surgery. Cancer Sci. 2007;98:665–673. doi: 10.1111/j.1349-7006.2007.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustafsson OJ, Arentz G, Hoffmann P. Proteomic developments in the analysis of formalin-fixed tissue. Biochim Biophys Acta. 2015;1854:559–580. doi: 10.1016/j.bbapap.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal Chem. 2005;77:6218–6224. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
- 16.Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, Resing KA, Ahn NG. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics. 2005;4:1487–1502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.da W Huang, Sherman BT, Stephens R, Baseler MW, Lane HC, Lempicki RA. DAVID gene ID conversion tool. Bioinformation. 2008;2:428–430. doi: 10.6026/97320630002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Paulsson K, Wang P. Chaperones and folding of MHC class I molecules in the endoplasmic reticulum. Biochim Biophys Acta. 2003;1641:1–12. doi: 10.1016/S0167-4889(03)00048-X. [DOI] [PubMed] [Google Scholar]
- 20.Turano C, Gaucci E, Grillo C, Chichiarelli S. ERp57/GRP58: A protein with multiple functions. Cell Mol Biol Lett. 2011;16:539–563. doi: 10.2478/s11658-011-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Celli CM, Jaiswal AK. Role of GRP58 in mitomycin C-induced DNA cross-linking. Cancer Res. 2003;63:6016–6025. [PubMed] [Google Scholar]
- 22.Chay D, Cho H, Lim BJ, Kang ES, Oh YJ, Choi SM, Kim BW, Kim YT, Kim JH. ER-60 (PDIA3) is highly expressed in a newly established serous ovarian cancer cell line, YDOV-139. Int J Oncol. 2010;37:399–412. doi: 10.3892/ijo_00000688. [DOI] [PubMed] [Google Scholar]
- 23.Liao CJ, Wu TI, Huang YH, Chang TC, Wang CS, Tsai MM, Lai CH, Liang Y, Jung SM, Lin KH. Glucose-regulated protein 58 modulates cell invasiveness and serves as a prognostic marker for cervical cancer. Cancer Sci. 2011;102:2255–2263. doi: 10.1111/j.1349-7006.2011.02102.x. [DOI] [PubMed] [Google Scholar]
- 24.Chung H, Cho H, Perry C, Song J, Ylaya K, Lee H, Kim JH. Downregulation of ERp57 expression is associated with poor prognosis in early-stage cervical cancer. Biomarkers. 2013;18:573–579. doi: 10.3109/1354750X.2013.827742. [DOI] [PubMed] [Google Scholar]
- 25.Leys CM, Nomura S, LaFleur BJ, Ferrone S, Kaminishi M, Montgomery E, Goldenring JR. Expression and prognostic significance of prothymosin-alpha and ERp57 in human gastric cancer. Surgery. 2007;141:41–50. doi: 10.1016/j.surg.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Corazzari M, Lovat PE, Armstrong JL, Fimia GM, Hill DS, Birch-Machin M, Redfern CP, Piacentini M. Targeting homeostatic mechanisms of endoplasmic reticulum stress to increase susceptibility of cancer cells to fenretinide-induced apoptosis: The role of stress proteins ERdj5 and ERp57. Br J Cancer. 2007;96:1062–1071. doi: 10.1038/sj.bjc.6603672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramírez-Rangel I, Bracho-Valdés I, Vazquez-Macías A, Carretero-Ortega J, Reyes-Cruz G, Vázquez-Prado J. Regulation of mTORC1 complex assembly and signaling by GRp58/ERp57. Mol Cell Biol. 2011;31:1657–1671. doi: 10.1128/MCB.00824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: Current state and future trends. J Hepatol. 2014;60:855–865. doi: 10.1016/j.jhep.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang Z, Shi G, Jin J, Guo H, Guo X, Luo F, Song Y, Jia X. Dual PI3K/mTOR inhibitor NVP-BEZ235-induced apoptosis of hepatocellular carcinoma cell lines is enhanced by inhibitors of autophagy. Int J Mol Med. 2013;31:1449–1456. doi: 10.3892/ijmm.2013.1351. [DOI] [PubMed] [Google Scholar]