Abstract
A number of studies have examined the association between tumor protein 53 (TP53) mutations and the clinical outcome in patients with non-small-cell lung cancer (NSCLC), although these have yielded conflicting results. In the present study, electronic databases updated to September 2015 were searched to find relevant studies. A meta-analysis was performed on the eligible studies, which quantitatively evaluated the association between the TP53 mutations and the survival of patients with NSCLC. Subgroup and sensitivity analyses were performed. A total of 19 studies that involved a total of 6,084 patients with NSCLC were included. When the TP53 mutation group (n=1,406) was compared with the wild-type group (lacking TP53 mutations; n=1,965), the wild-type group was associated with a significantly higher overall survival rate [hazard ratio (HR), 1.26; 95% confidence interval (CI) 1.12–1.41, P<0.0001]. Significant benefits of overall survival in the wild-type group were found in the subgroup involving patients with NSCLC in the early stages, including the I/II phases (HR, 1.93, 95% CI, 1.17–3.19, P=0.01; heterogeneity, I2=0.0%, P=0.976) and patients with adenocarcinoma (HR, 3.06; 95% CI, 1.66–5.62, P<0.0001; heterogeneity: I2=0.0%, P=0.976). This meta-analysis has indicated that TP53 gene alteration may be an indicator of a poor prognosis in patients with NSCLC. Furthermore, the results also suggested that the role of TP53 mutations may differ according to different pathological types and clinical stages. The presence of these mutations may define a subset of patients with NSCLC appropriate for investigational therapeutic strategies.
Keywords: tumor protein 53 mutation, prognosis, non-small cell lung cancer
Introduction
Lung cancer, predominantly non-small-cell lung cancer (NSCLC, comprising 80% of lung cancers), is the leading cause of cancer mortality worldwide (1). Despite the advances made in the diagnosis and treatment of lung cancer in the last few decades, the prognosis of lung cancer remains very poor. Patients with early-stage NSCLC who undergo complete tumor resection develop distant metastases in 50–70% of cases, resulting in 5-year survival rates of ~40% (2–4). Although the tumor-node-metastasis (TNM) staging system is the best prognostic index for resectable NSCLC, patients with the same pathological stage of the disease exhibit a great variability in recurrence and survival rates (5). Therefore, there is an urgent need to identify appropriate molecular markers that are associated with the prognosis of patients with lung cancer.
In a large number of types of human cancer, the tumor protein 53 (TP53) gene is the most frequently mutated gene [identified in ~50% of cases of NSCLC (6,7)]. The TP53 gene contains 11 exons that encode a 53 kDa nuclear phosphoprotein, termed p53, which exerts an essential role in cell cycle control and apoptosis. In response to oncogenic cellular stresses, such as deoxyribonucleic acid (DNA) damage, p53 protein acts as a transcription factor that induces the expression of downstream genes, including p21 and BCL2-associated X protein (BAX), which are involved in cell cycle arrest, DNA repair and apoptosis. It has previously been reported that p53 protein overexpression may be an important prognostic marker of decreased survival rates (8,9). Among these studies, an accumulation of abnormal p53 protein was detected in the cell nuclei by performing routine immunohistochemistry (IHC). However, the measurement of p53 expression by IHC has led to inconsistent conclusions, not only due to variations in the understanding of the term ‘overexpression’, but also since the accumulation of p53 usually corresponds with the class of TP53 gene mutation that results in tumors with a frame-shift or non-sense mutations, and the p53 protein is therefore not generally detectable by IHC (10,11). Furthermore, p53 protein concentrations are increased in certain tumor types that lack any mutations resulting from DNA damage, as would be caused by, e.g. ionizing radiation or chemotherapeutic agents, and this may act as a physiological response to allow for DNA repair (12). Therefore, results obtained from IHC analysis alone are insufficient to permit an evaluation of the prognostic importance of TP53 gene mutation.
In recent years, a large number of studies have been performed to evaluate the impact of TP53 mutations on the prognosis of patients with NSCLC; however, the results of these studies remain controversial (13,14). Several studies indicated that patients with mutations of TP53 survived for a shorter period of time (15–18), whereas others reported that there was no significant correlation between TP53 mutation and the survival rate (19–22). The present study aimed to present a meta-analysis of the available data on the prognostic significance of TP53 gene mutations in patients with NSCLC. Due to the limitations of IHC, this study analyzed data exclusively extracted from studies employing SSCP (single-stranded conformational polymorphism) or DNA sequencing to detect mutations of this gene. The results of the present study may provide a clearer understanding of the prognostic importance of TP53 mutations in NSCLC, and its association with clinicopathological features and clinical outcomes.
Materials and methods
Literature searches
All relevant articles were retrieved by searching the PubMed, Embase and the Central Registry of Controlled Trials of the Cochrane Library databases using a combination of the terms ‘TP53’, ‘p53’, ‘p53 protein’, ‘p53 mutation’, ‘lung’, ‘non-small-cell lung cancer’ and ‘NSCLC’. An additional search in Google Scholar, and a manual search through the reference lists of pertinent reviews, were additionally performed. Two authors (JC. G and J. W.) performed the searches independently of each other. No language or date restrictions were set in the search.
Inclusion and exclusion criteria
Studies considered to be eligible for the present meta-analysis were required to meet the following criteria: i) Published trials of any study design were included that examined the prognostic influence of TP53 mutations in NSCLC; ii) the subjects had not undergone chemotherapy or radiotherapy prior to surgery or biopsy, which might have eliminated the effects due to the TP53 gene; iii) the study had employed DNA techniques for TP53 mutational analysis; iv) the clinical outcomes had been stratified on the basis of TP53 mutation status; and v) information on the primary outcome of survival [i.e. overall survival (OS)] was accessible. Studies failing to meet these inclusion criteria were excluded.
Outcome measures, data extraction and quality assessment
The primary outcome for the primary meta-analysis was OS. Data for OS were extracted as the hazard ratios (HRs) of patients with TP53 mutations compared with those with wild-type TP53 in NSCLC and its 95% confidence interval (CI) from the subgroup analysis. If the HR and its variance were available directly in an individual trial, these values were subsequently used. However, since a large number of trials did not report this information directly, appropriate data, such as P-values of the log-rank test, were extracted to estimate the log HR and its variance using the previously reported methods (23,24), and the time-to-event data were extracted from the survival curves. Kaplan-Meier curves were read using Engauge Digitizer version 4.1 (free software downloaded from http://sourceforge.net). Data combination was performed using RevMan version 5.1 (free software downloaded from http://www.cochrane.org). The log HR and its variance were pooled using an inverse variance-weighted average, and the results are presented as HR and 95% CI. The data collection and assessment of methodological quality were performed according to the QUORUM and the Cochrane Collaboration guidelines (http://www.cochrane.de). The data on lead author, patient status, study category, pathological type, TP53 mutation status, smoking status and OS were extracted by two investigators (JC. G and J. W.) independently. Three reviewers (JC. G, J. W. and JW X.) used the Newcastle-Ottawa scale specific to cohort study to assess all included studies (25). Discrepancies were discussed with a fourth author (Y.B. Z.) in order to reach a consensus.
Publication bias
An extensive search strategy was designed in order to minimize the potential publication bias. Graphical funnel plots were generated to visually assess a publication bias. The statistical methods used to detect funnel plot asymmetry were the rank correlation test of Begg and Mazumdar (26) and the regression asymmetry test of Egger et al (27).
Statistical analysis
HRs for OS with 95% CIs were pooled. Heterogeneity across the studies was assessed using a forest plot and the inconsistency statistic (I2). The random-effects model was employed in case of potential heterogeneity, and to avoid underestimation of standard errors of pooled estimates in our meta-analyses. All calculations were performed using STATA version 11.0 (Stata Corp., College Station, TX, USA). Subgroup analysis was performed according to the respective study type and treatment line. An HR value <1 represented a greater benefit for those without TP53 mutations in terms of the OS value. All CIs had a two-sided probability coverage of 95%. P<0.05 was considered to indicate a statistically significant value.
Results
Study identification and selection
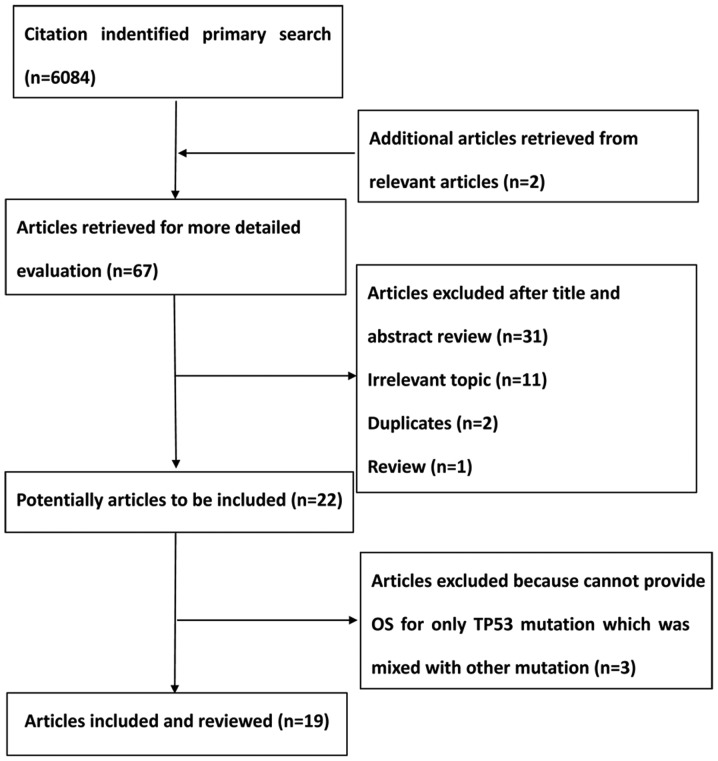
A total of 6,084 citations were identified from the PubMed, Embase and the Central Registry of Controlled Trials of the Cochrane Library databases. Following a review by all the authors, 19 studies (9,15–22,28–39) were identified that fulfilled the inclusion criteria and were eligible with complete and validated data for meta-analysis. Fig. 1 shows a summary of the various stages of the performed literature searches in a flow chart.
Figure 1.
Flow chart showing the stages of the literature searches performed in the present study. OS, overall survival; TP52, tumor protein 53.
Characteristics of the studies and quality assessment
The main characteristics of the 19 studies between 1994 and 2015 that were eligible for the meta-analysis are shown in Table I. Among these studies, 3,371 patients with NSCLC without therapy prior to surgery or biopsy were involved, and these were stratified according to TP53 mutation status. Patients possessing TP53 mutations were categorized as a TP53 mutation cohort (n=1406), whereas the remaining patients had the wild-type TP53 gene (n=1965). The Newcastle-Ottawa scale scores of the included studies were >5, and the methodological quality of the 19 eligible studies is shown in Table II.
Table I.
Characteristics of the included studies for the meta-analyses.
| Pathological type | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author/year | Study type | Methods of detection | Sequence | Patient status | Gender ratio (M/F) | Clinical stage | AC | SCC | Others | TP53 mutation status (sample size) | HR estimation | HR for OS (95% CI)a | Refs. |
| Lee et al, 2015 | Pro | PCR+direct sequencing | Exons | Surgery | 1:1 | I: 67; II/IIIA: 40 | 85 | 22 | 0 | Wild-type (n=107) | Surv. curvesb | 1.38 (0.73–2.61) | (19) |
| 2–11 | 6:3 | I: 40; II/IIIA: 22 | 32 | 34 | 0 | TP53 mutation (n=66) | |||||||
| Molina-Vila et al, 2014 | Retro | PCR+Sanger sequencing | NA | Che | NA | NA | NA | NA | NA | Wild-type (n=225) | HR | 1.45 (0.95–2.22) | (32) |
| NA | NA | TP53 mutation (n=93) | |||||||||||
| Ma et al, 2013 | Pro | PCR+direct sequencing | Exons | Surgery or surgery-Che | 3:7 | I: 115; II: 79; III: 109 | 117 | 156 | 30 | Wild-type (n=303) | HR | 1.08 (0.86–1.37) | (21) |
| 4–8 | 6:6 | I: 58; II: 51; III: 112 | 58 | 136 | 27 | TP53 mutation (n=221) | |||||||
| Scoccianti et al, 2012 | Pro | DHPLC+2th PCR+bi-directional sequencing | Exons | Surgery | 4:9 | I: 90; II: 28; III: 9 | 85 | 41 | 3 | Wild-type (n=129) | HR | 0.95 (0.64–1.40) | (20) |
| 4–10 | 5:7 | I: 82; II: 32; III: 8 | 48 | 69 | 4 | TP53 mutation (n=121) | |||||||
| Chien et al, 2010 | Retro | PCR+direct sequencing | Exons | Surgery | NA | NA | NA | NA | NA | Wild-type (n=216) | HR | 1.16 (0.87–1.55) | (22) |
| 5–8 | TP53 mutation (n=90) | ||||||||||||
| Regina et al, 2009 | Pro | PCR+direct sequencing | Exons | Surgery | 5:6 | I/II: 18; III: 11; IV: 4 | 22 | 7 | 4 | Wild-type (n=33) | HR | 0.67 (0.44–1.00) | (30) |
| 5–8 | 9 | I/II: 10; III: 5; IV: 5 | 10 | 6 | 4 | TP53 mutation (n=20) | |||||||
| Kosaka et al, 2009 | Pro | PCR+direct sequencing | Exons | Surgery | 1:4 | I: 158; II–V: 76 | 234 | 0 | 0 | Wild-type (n=234) | Surv. curves | 1.50 (1.02–2.50) | (15) |
| 4–10 | 2:3 | I: 77; II–V: 65 | 142 | 0 | 0 | TP53 mutation (n=142) | |||||||
| Ludovini et al, 2008 | Pro | PCR | Exons 5–8 | Surgery | 0:6 | I/II: 31; III: 4 | 18 | 12 | 5 | Wild-type (n=76) | HR | 2.3 (0.80–6.60) | (37) |
| 7:2 | I/II: 29; III: 12 | 10 | 25 | 6 | TP53 mutation (n=41) | ||||||||
| Tsao et al, 2007 | Pro | PCR+direct sequencing | Exons | Surgery-Observation | NA | NA | NA | NA | NA | Wild-type (n=40) | HR | 1.15 (0.75–1.77) | (9) |
| 5–9 | TP53 mutation (n=200) | ||||||||||||
| Ahrendt et al, 2003 | Pro | PCR+direct sequencing | Exons 5–9 | Surgery | 1 | I: 48; II: 19; III: 17 | 34 | 25 | 25 | Wild-type (n=84) | HR | 1.56 (1.0–2.4) | (34) |
| 1;8 | I: 58; II: 28; III: 18 | 39 | 52 | 13 | TP53 mutation (n=104) | ||||||||
| Bria et al, 2015 | Retro | Multiple PCR+direct sequencing | Exons | Gefitini b-Surgery/surgery | NA | III/IV: 7 | NA | NA | NA | Wild-type (n=8) | HR | 1.36 (0.24–7.26) | (49) |
| 5–8 | III/IV: 11 | TP53 mutation (n=11) | |||||||||||
| Tomizawa et al, 1999 | Pro | PCR-SSCP sequencing | Exons | Surgery | NA | I: 61 | NA | NA | NA | Wild-type (n=61) | Surv. curves | 2.21 (0.78–6.23) | (18) |
| 5–8 | I: 39 | TP53 mutation (n=39) | |||||||||||
| Vega et al, 1997 | Pro | PCR-SSCP sequencing | Exons | Surgery | NA | I: 30; II: 4; III: 21 | 40 | 17 | 7 | Wild-type (n=64) | Surv. curves | 1.46 (0.60–3.55) | (33) |
| 5–9 | I: 7; II: 1; III: 9 | 3 | 12 | 2 | TP53 mutation (n=17) | ||||||||
| Huang et al, 1997 | Pro | PCR-SSCP sequencing | Exons | Surgery | 2:3 | I: 46; II: 10; III: 37 | 67 | 22 | 1 | Wild-type (n=93) | Surv. curves | 1.34 (0.76–2.37) | (31) |
| 5–8 | 4:1 | I: 24; II: 7; III: 20 | 21 | 27 | 6 | TP53 mutation (n=51) | |||||||
| Ohno et al, 1997 | Pro | PCR-SSCP sequencing | Exons | Surgery | 1:2 | I: 29; II: 11; III: 13 | 12 | 39 | 2 | Wild-type (n=53) | Surv. curves | 2.02 (0.75–5.44) | (36) |
| 5–9 | 1.6 | I: 8; II: 5; III: 8 | 8 | 11 | 2 | TP53 mutation (n=21) | |||||||
| Fukuyama et al, 1996 | NA | PCR-SSCP sequencing | Exons | Surgery | 1.2 | I/II: 69; III/IV: 33 | 69 | 21 | 2 | Wild-type (n=102) | HR | NA | (16) |
| 5–8 | 5.3 | I/II: 38; III/IV: 19 | 25 | 26 | 6 | TP53 mutation (n=57) | |||||||
| Top et al, 1995 | Pro | PCR-SSCP sequencing | Exons | NA | 1.8 | I: 10; II: 6; III: 1 | 14 | 2 | 1 | Wild-type (n=17) | Surv. curves | 2.35 (0.65–8.51) | (28) |
| 5–8 | 3.6 | I: 22; II: 7; III: 8 | 18 | 7 | 12 | TP53 mutation (n=37) | |||||||
| Mitsudomi et al, 1995 | Pro | PCR-SSCP sequencing | Exons | NA | NA | 27 | 10 | NA | Wild-type (n=82) | HR | 1.18 (0.60–2.30) | (35) | |
| 5–8 | 13 | 7 | NA | TP53 mutation (n=44) | |||||||||
| Kashii et al, 1994 | NA | PCR-SSCP sequencing | Exons | Surgery | 1.1 | I: 25; II: 5; III: 7; IV:1 | 27 | 4 | 7 | Wild-type (n=38) | HR | 2.0 (0.88–4.55) | (29) |
| 5–9 | 1.6 | I: 9; II: 4; III: 16; IV:2 | 20 | 5 | 6 | TP53 mutation (n=31) | |||||||
HR represents the ratio of the HR of the TP53 mutation/wild-type in patients with non-small-cell lung carcinoma
Surv. curves are represented by the HR and its CI acquired from the survival curves. CI, confidence interval; Che, chemotherapy (non-specific); RT, radiotherapy; PCR, polymerase chain reaction; SSCP, single-stranded conformational polymorphism; M, male; F, female; TP53, tumor protein 53 gene; OS, overall survival; HR, hazard ratio; NA, not available; Pro, prospective; Retro, retrospective; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Table II.
Quality assessment of eligible studies using the Newcastle-Ottawa quality assessment scale.
| First author/year | Selectiona | Comparabilityb | Outcomec | Total (quality) scored | Refs. |
|---|---|---|---|---|---|
| Lee et al, 2015 | 4 | 2 | 1 | 7 | (19) |
| Molina-Vila et al, 2014 | 3 | 1 | 2 | 6 | (31) |
| Ma et al, 2013 | 4 | 2 | 1 | 7 | (21) |
| Scoccianti et al, 2012 | 4 | 2 | 2 | 8 | (20) |
| Chien et al, 2010 | 3 | 1 | 2 | 6 | (22) |
| Regina et al, 2009 | 4 | 2 | 3 | 9 | (29) |
| Kosaka et al, 2009 | 4 | 2 | 2 | 8 | (15) |
| Ludovini et al, 2008 | 4 | 2 | 2 | 8 | (36) |
| Tsao et al, 2007 | 3 | 1 | 3 | 7 | (9) |
| Ahrendt et al, 2003 | 4 | 2 | 2 | 8 | (33) |
| Bria et al, 2015 | 4 | 2 | 2 | 8 | (49) |
| Tomizawa et al, 1999 | 4 | 2 | 3 | 9 | (18) |
| Vega et al, 1997 | 4 | 2 | 3 | 9 | (32) |
| Huang et al, 1997 | 4 | 2 | 3 | 9 | (30) |
| Ohno et al, 1997 | 4 | 2 | 3 | 9 | (35) |
| Fukuyama et al, 1996 | 4 | 2 | 3 | 9 | (16) |
| Top et al, 1995 | 4 | 2 | 3 | 9 | (27) |
| Mitsudomi et al, 1995 | 3 | 1 | 2 | 6 | (34) |
| Kashii et al, 1994 | 4 | 2 | 3 | 9 | (28) |
Selection was based on a score of 0–4 points, as follows: First point, representativeness of the exposed cohort (1 point, truly or somewhat representative of the average level in the community; 0 points, selected group of users, or no description of the derivation of the cohort); second point, selection of the non-exposed cohort (1 point, drawn from the same community as the exposed cohort; 0 point, drawn from a different source or no description of the derivation of the non-exposed cohort); third point, ascertainment of exposure (1 point, secure record or structured interview; 0 point, written self-report or no description); Fourth point, demonstration that outcome of interest was not present at the start of the study (1 point, yes; 0 point, no).
Comparability, rated as 0–2 points (2 points, study controls for the most important factor and any additional factor; 1 point, study controls for the most important factor or any additional factor; 0 point, study controls without the most important factor or any additional factor).
Outcome, rated as 0–3 points: First point, assessment of outcome (1 point, independent blind assessment or record linkage; 0 point, self-report or no description); second point, was follow-up long enough for outcomes to occur? (1 point, yes; 0 point, no); third point, adequacy of follow-up of cohorts (1 point, complete follow-up or subjects lost to follow-up unlikely to introduce bias; 0 point, follow-up rate <80% and no description of those lost, or no statement).
The quality score was ranked as low (≤5 points) or high (≥6 points).
Meta-analyses of the wild-type and TP53 mutation groups in terms of OS
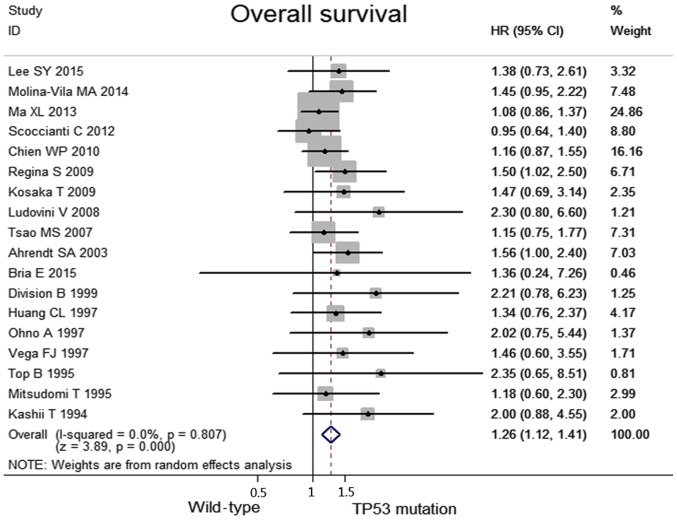
No heterogeneity was observed among the included studies regarding the OS (I2=0.0%, P=0.81). Taken together, when compared with the TP53 mutation group, the wild-type group was associated with significantly higher OS values (HR, 1.26; 95% CI, 1.12–1.41, P<0.0001; Fig. 2). Data concerning the response rates were unavailable in the majority of the studies; consequently, they were not referred to as outcome endpoints.
Figure 2.
Meta-analyses of overall survival between the wild-type and the TP53 mutation groups in patients with non-small cell lung cancer. CI, confidence interval; HR, hazard ratio; TP53, tumor protein 53.
Subgroup analyses and sensitivity analyses
When stratifying patients according to clinical stage (early stage, including the I/II stages, vs. advanced stage, including the II–IV stages), pathological type (adenocarcinoma vs. non-adenocarcinoma) and methods of detection (PCR-SSCP vs. others), the observed results indicated that significant benefits of OS in the wild-type group were identified in the subgroup involving patients with NSCLC in the early stage, including the I/II phase (HR, 1.93; 95% CI, 1.17–3.19; P=0.01; heterogeneity: I2=0.0%, P=0.976) and patients with adenocarcinoma (HR, 3.06; 95% CI, 1.66–5.62, P=0.00; heterogeneity: I2=0.0%, P=0.976). No significant differences were identified with the methods of detection. All the results from the above subgroups are shown in Table III.
Table III.
Summary of the results of the subgroup analyses results.
| Effect size | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Subgroup | No. of studies | HR (95% CI)a | Z | P-value | I2 | P-value |
| Overall survival | PCR-SSCP and other methods | 11 | 1.21 (1.07–1.38) | 3.03 | 0.002 | 0.0% | 0.702 |
| PCR-SSCP | 7 | 1.56 (1.15–2.12) | 2.86 | 0.004 | 0.0% | 0.880 | |
| Adenocarcinoma | 4 | 3.06 (1.66–5.62) | 3.60 | 0.000 | 0.0% | 0.976 | |
| Non-adenocarcinoma | 5 | 1.25 (0.57–2.74) | 0.56 | 0.574 | 0.0% | 0.990 | |
| Early stage (I/II) | 4 | 1.93 (1.17–3.19) | 2.56 | 0.011 | 0.0% | 0.976 | |
| Advanced stage (II/III/IV) | 4 | 0.76 (0.55–1.05) | 1.09 | 0.095 | 0.0% | 0.781 | |
HR represents the ratio of the HR of the TP53 mutation/wild-type in patients with non-small-cell lung carcinoma. CI, confidence interval; I2, inconsistency statistic.
Publication bias
With regard to the publication bias, the funnel plot revealed an almost symmetrical distribution, as shown in Fig. 3. This therefore suggested that no clear publication bias was present in this meta-analysis.
Figure 3.
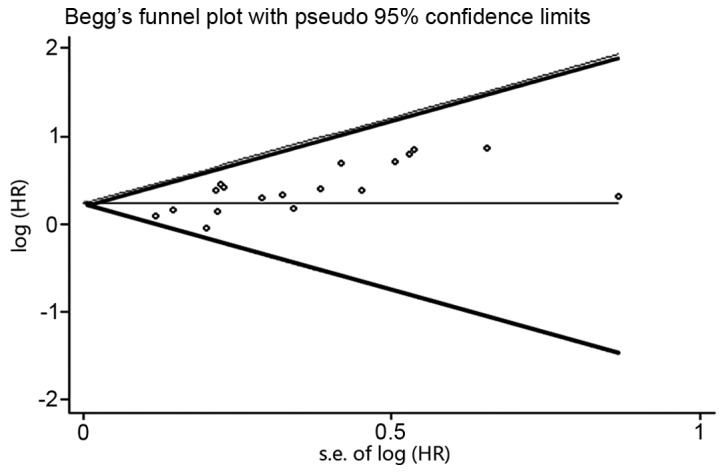
Begg's funnel plots to determine the extent of publication bias in the present study. HR, hazard ratio.
Discussion
For patients with NSCLC, the association of TP53 mutations with prognostic significance has yet to be fully elucidated. A meta-analysis incorporating all the available data from correlative studies provides a useful method for addressing this question. We performed the present study, and identified that the patients with TP53 mutations indeed have markedly worse survival rates compared with those without the mutations, especially for patients with NSCLC in the early stages, or with adenocarcinoma.
Theoretically, SSCP is less sensitive as a technique compared with direct dideoxynucleotide sequencing, as it failed to identify mutations in 14–38% of the tumors in which TP53 mutations were detected by the latter technique (40,41). However, when the subgroup analysis in methods of detection was performed in the present study, the two methods revealed a very similar, significant predictive value of TP53 mutations, which indicated that these methods were not the key factor affecting the association between TP53 mutations and the survival rate. Based on data compiled in the International Agency for Research on Cancer (IARC) TP53 database (http://p53.iarc.fr/), mutations were significantly (P<0.01) associated with histology (21). In the study of Ludovini et al (37), 55% of the patients with NSCLC possessed TP53 mutations, and the incidence of the mutations was higher in squamous-cell carcinomas and in smokers compared with those in adenocarcinomas and non-smokers, as previously reported by Fong et al (42). In the studies of Fukuyama et al (16) and Kashii et al (29), it was stated that TP53 mutations were an unfavorable prognostic factor in patients with adenocarcinoma, although not in patients with squamous cell carcinoma (SCC), in spite of its higher frequency (16,29), a conclusion which has been borne out by the results in the present study. On further analysis, the tumors with wild-type TP53 more often had a K-ras mutation (P=0.036), which is known to constitute an unfavorable prognostic factor in lung adenocarcinoma. Huang et al (31) reported that it is important to evaluate mutations of TP53 and K-ras simultaneously, for the purpose of predicting the prognosis of patients and determining appropriate treatments, particularly in patients with adenocarcinoma. Furthermore, TP53 gene mutations are considered to occur relatively early in the dysplastic epithelium in the histogenesis of SCC, whereas they may occur relatively late in adenocarcinoma, as suggested by the above results, hence providing a different impact on the prognosis of patients (43). In addition, for SCC, Vega et al (33) identified a markedly poor clinical evolution when the TP53 mutation was located in exon 5 (an independent parameter of borderline importance), with the group of patients with SCC having this alteration exhibiting the worst prognosis. These facts may suggest that TP53 mutations exert a different role in adenocarcinoma compared with SCC.
Mitsudomi et al (17) identified a much greater prognostic effect of TP53 mutations in patients with more advanced disease (stages IIIB and IV). There is a tendency that the prognostic value of TP53 is more significant for patients with early-stage disease compared with those in the advanced stage, as included in the present study. Furthermore, Tomizawa et al (18) reported that, although TP53 expression has no correlation with the survival rate, the presence of TP53 mutations in tumors was significantly associated with decreased survival rates. A prospective study also suggested that TP53 mutation predicts poor survival in patients with stage I NSCLC, although not in patients with advanced NSCLC (44). Similarly, stage I patients with wild-type TP53 in the study by Chien et al (22) had better overall survival rates for lung cancer compared with those who bore TP53 mutations, although such a result was not identified in patients with advanced NSCLC (22). The present study suggests that TP53 mutations are associated with a higher risk of eventual patient mortality in patients with stage I NSCLC. From a biological viewpoint, TP53 and K-ras mutations may represent very early events in lung carcinogenesis (20), which consequently have an important role for prediction at early stage. When tumors progress and become increasingly complex, it is difficult for tumor behavior to be defined by a single genetic abnormality. At the present time, the obtained results do not readily provide the explanation for these discrepancies.
TP53 mutations were also classified into two groups: Disruptive and non-disruptive (45), on the basis of the degree of disturbance of the protein structure predicted from the crystal structure of the TP53-DNA complex (46). Poeta et al (45) reported that a disruptive TP53 alteration, as compared with the wild-type, had an independent, significant association with decreased survival. In the study of Lee et al (19), neither disruptive nor non-disruptive mutations were significantly associated with the survival rate of the patients. However, these various TP53 genotypes were not mentioned in other studies that were included in this meta-analysis. Therefore, in future studies, it will be important to take into consideration TP53 mutations and the TP53 genotype in assessing the prognosis and predictive importance of the gene status of TP53 in NSCLC.
Notably, to the best of our knowledge, this is the first study to comprehensively answer the prognostic value of TP53 mutations detected by molecular techniques in patients with NSCLC. Nevertheless, there exist several limitations. First, data for the objective response rate (ORR) and the disease control rate (DCR) were not available in all the included studies, and an absence of the short-term prognosis value does not preclude that mutations have significance as predictors of the response to specific forms of therapies. Secondly, after searching in the PubMed, Embase and the Central Registry of Controlled Trials of the Cochrane Library databases, publication bias remains, since positive results tend to be accepted by journals, whereas negative results are often rejected, or not even submitted. In addition, since p53 mutations occur frequently in the so-called ‘hot-spot’ region of exons 5–8, only the hot-spot will have been examined to evaluate the frequency of TP53 mutations in the majority of studies, whereas meta-analyses have determined that 13.6% of the mutations occur outside exons 5–8 (47–49). Therefore, further studies are warranted to ensure the robustness of the conclusions of the present study.
In conclusion, TP53 mutations may be an indicator for poor prognosis in only a subset of patients. The present study also suggested that the role of TP53 alterations may therefore differ between that observed in adenocarcinomas and SCC. The presence of these mutations may define a subset of patients with NSCLC appropriate for investigational therapeutic strategies. In the future, it may be possible to apply our expanding knowledge of the molecular genetics of these lesions in order to improve the survival rates and quality of life of patients suffering from this disease.
Acknowledgments
The present study was supported by grants from the National Natural Science foundation of China (no. 81570008, to Yanbin Zhou), the Natural Science Foundation of Guangdong Province of China (no. 2014A030313052; to Yanbin Zhou), and Science and Technology Program of Guangzhou, China (no. 2014J4100132; to Yanbin Zhou).
Glossary
Abbreviations
- NSCLC
non-small cell lung cancer
- TNM
tumor-node-metastasis
- DNA
deoxyribonucleic acid
- IHC
immunohistochemistry
- SSCP
single-stranded conformational polymorphism
- OS
overall survival
- HR
hazard ratio
- CI
confidence interval
- IARC
international agency for research on cancer
- SCC
squamous cell carcinoma
- BAX
Bcl2-associated X protein
- ORR
objective response rate
- DCR
disease control rate
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ihde DC, Minna JD. Non-small cell lung cancer. Part II: Treatment. Curr Probl Cancer. 1991;15:105–154. doi: 10.1016/0147-0272(91)90012-Y. [DOI] [PubMed] [Google Scholar]
- 3.Farjah F, Flum DR, Ramsey SD, Heagerty PJ, Symons RG, Wood DE. Multi-modality mediastinal staging for lung cancer among medicare beneficiaries. J Thorac Oncol. 2009;4:355–363. doi: 10.1097/JTO.0b013e318197f4d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein I, Marcel V, Olivier M, Oren M, Rotter V, Hainaut P. Understanding wild-type and mutant p53 activities in human cancer: New landmarks on the way to targeted therapies. Cancer Gene Ther. 2011;18:2–11. doi: 10.1038/cgt.2010.63. [DOI] [PubMed] [Google Scholar]
- 5.Singhal S, Vachani A, Antin-Ozerkis D, Kaiser LR, Albelda SM. Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non-small cell lung cancer: A review. Clin Cancer Res. 2005;11:3974–3986. doi: 10.1158/1078-0432.CCR-04-2661. [DOI] [PubMed] [Google Scholar]
- 6.Toyooka S, Tsuda T, Gazdar AF. The TP53 gene, tobacco exposure, and lung cancer. Hum Mutat. 2003;21:229–239. doi: 10.1002/humu.10177. [DOI] [PubMed] [Google Scholar]
- 7.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: Clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 8.Steels E, Paesmans M, Berghmans T, Branle F, Lemaitre F, Mascaux C, Meert AP, Vallot F, Lafitte JJ, Sculier JP. Role of p53 as a prognostic factor for survival in lung cancer: A systematic review of the literature with a meta-analysis. Eur Respir J. 2001;18:705–719. doi: 10.1183/09031936.01.00062201. [DOI] [PubMed] [Google Scholar]
- 9.Tsao MS, Aviel-Ronen S, Ding K, Lau D, Liu N, Sakurada A, Whitehead M, Zhu CQ, Livingston R, Johnson DH, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25:5240–5247. doi: 10.1200/JCO.2007.12.6953. [DOI] [PubMed] [Google Scholar]
- 10.Bodner SM, Minna JD, Jensen SM, D'Amico D, Carbone D, Mitsudomi T, Fedorko J, Buchhagen DL, Nau MM, Gazdar AF, et al. Expression of mutant p53 proteins in lung cancer correlates with the class of p53 gene mutation. Oncogene. 1992;7:743–749. [PubMed] [Google Scholar]
- 11.Lohmann D, Ruhri C, Schmitt M, Graeff H, Höfler H. Accumulation of p53 protein as an indicator for p53 gene mutation in breast cancer. Occurrence of false-positives and false-negatives. Diagn Mol Pathol. 1993;2:36–41. doi: 10.1097/00019606-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Harris CC, Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993;329:1318–1327. doi: 10.1056/NEJM199310283291807. [DOI] [PubMed] [Google Scholar]
- 13.Mitsudomi T, Hamajima N, Ogawa M, Takahashi T. Prognostic significance of p53 alterations in patients with non-small cell lung cancer: A meta-analysis. Clin Cancer Res. 2000;6:4055–4063. [PubMed] [Google Scholar]
- 14.Huncharek M, Kupelnick B, Geschwind JF, Caubet JF. Prognostic significance of p53 mutations in non-small cell lung cancer: A meta-analysis of 829 cases from eight published studies. Cancer Lett. 2000;153:219–226. doi: 10.1016/S0304-3835(00)00381-5. [DOI] [PubMed] [Google Scholar]
- 15.Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T. Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol. 2009;4:22–29. doi: 10.1097/JTO.0b013e3181914111. [DOI] [PubMed] [Google Scholar]
- 16.Fukuyama Y, Mitsudomi T, Sugio K, Ishida T, Akazawa K, Sugimachi K. K-ras and p53 mutations are an independent unfavourable prognostic indicator in patients with non-small-cell lung cancer. Br J Cancer. 1997;75:1125–1130. doi: 10.1038/bjc.1997.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsudomi T, Oyama T, Kusano T, Osaki T, Nakanishi R, Shirakusa T. Mutations of the p53 gene as a predictor of poor prognosis in patients with non-small-cell lung cancer. J Natl Cancer Inst. 1993;85:2018–2023. doi: 10.1093/jnci/85.24.2018. [DOI] [PubMed] [Google Scholar]
- 18.Tomizawa Y, Kohno T, Fujita T, Kiyama M, Saito R, Noguchi M, Matsuno Y, Hirohashi S, Yamaguchi N, Nakajima T, Yokota J. Correlation between the status of the p53 gene and survival in patients with stage I non-small cell lung carcinoma. Oncogene. 1999;18:1007–1014. doi: 10.1038/sj.onc.1202384. [DOI] [PubMed] [Google Scholar]
- 19.Lee SY, Jeon HS, Hwangbo Y, Jeong JY, Park JY, Lee EJ, Jin G, Shin KM, Yoo SS, Lee J, et al. The influence of TP53 mutations on the prognosis of patients with early stage non-small cell lung cancer may depend on the intratumor heterogeneity of the mutations. Mol Carcinog. 2015;54:93–101. doi: 10.1002/mc.22077. [DOI] [PubMed] [Google Scholar]
- 20.Scoccianti C, Vesin A, Martel G, Olivier M, Brambilla E, Timsit JF, Tavecchio L, Brambilla C, Field JK, Hainaut P. European Early Lung Cancer Consortium: Prognostic value of TP53, KRAS and EGFR mutations in nonsmall cell lung cancer: The EUELC cohort. Eur Respir J. 2012;40:177–184. doi: 10.1183/09031936.00097311. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Rousseau V, Sun H, Lantuejoul S, Filipits M, Pirker R, Popper H, Mendiboure J, Vataire AL, Le Chevalier T, et al. Significance of TP53 mutations as predictive markers of adjuvant cisplatin-based chemotherapy in completely resected non-small-cell lung cancer. Mol Oncol. 2014;8:555–564. doi: 10.1016/j.molonc.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chien WP, Wong RH, Cheng YW, Chen CY, Lee H. Associations of MDM2 SNP309, transcriptional activity, mRNA expression, and survival in stage I non-small-cell lung cancer patients with wild-type p53 tumors. Ann Surg Oncol. 2010;17:1194–1202. doi: 10.1245/s10434-009-0853-2. [DOI] [PubMed] [Google Scholar]
- 23.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21:3337–3351. doi: 10.1002/sim.1303. [DOI] [PubMed] [Google Scholar]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Smith G Davey, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Top B, Mooi WJ, Klaver SG, Boerrigter L, Wisman P, Elbers HR, Visser S, Rodenhuis S. Comparative analysis of p53 gene mutations and protein accumulation in human non-small-cell lung cancer. Int J Cancer. 1995;64:83–91. doi: 10.1002/ijc.2910640203. [DOI] [PubMed] [Google Scholar]
- 29.Kashii T, Mizushima Y, Lima C, Noto H, Sato H, Saito H, Kusajima Y, Kitagawa M, Sugiyama S, Kobayashi M. Evaluation of prognostic-significance of p53 gene alterations in patients with surgically resected lung-cancer. Int J Oncol. 1995;6:123–128. [PubMed] [Google Scholar]
- 30.Regina S, Valentin JB, Lachot S, Lemarié E, Rollin J, Gruel Y. Increased tissue factor expression is associated with reduced survival in non-small cell lung cancer and with mutations of TP53 and PTEN. Clin Chem. 2009;55:1834–1842. doi: 10.1373/clinchem.2009.123695. [DOI] [PubMed] [Google Scholar]
- 31.Huang CL, Taki T, Adachi M, Konishi T, Higashiyama M, Kinoshita M, Hadama T, Miyake M. Mutations of p53 and K-ras genes as prognostic factors for non-small cell lung cancer. Int J Oncol. 1998;12:553–563. doi: 10.3892/ijo.12.3.553. [DOI] [PubMed] [Google Scholar]
- 32.Molina-Vila MA, Bertran-Alamillo J, Gascó A, Mayo-de-las-Casas C, Sánchez-Ronco M, Pujantell-Pastor L, Bonanno L, Favaretto AG, Cardona AF, Vergnenègre A, et al. Nondisruptive p53 mutations are associated with shorter survival in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2014;20:4647–4659. doi: 10.1158/1078-0432.CCR-13-2391. [DOI] [PubMed] [Google Scholar]
- 33.Vega FJ, Iniesta P, Caldés T, Sanchez A, López JA, de Juan C, Diaz-Rubio E, Torres A, Balibrea JL, Benito M. p53 Exon 5 mutations as a prognostic indicator of shortened survival in non-small-cell lung cancer. Br J Cancer. 1997;76:44–51. doi: 10.1038/bjc.1997.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahrendt SA, Hu Y, Buta M, McDermott MP, Benoit N, Yang SC, Wu L, Sidransky D. p53 mutations and survival in stage I non-small-cell lung cancer: Results of a prospective study. J Natl Cancer Inst. 2003;95:961–970. doi: 10.1093/jnci/95.13.961. [DOI] [PubMed] [Google Scholar]
- 35.Mitsudomi T, Oyama T, Nishida K, Ogami A, Osaki T, Nakanishi R, Sugio K, Yasumoto K, Sugimachi K. p53 nuclear immunostaining and gene mutations in non-small-cell lung cancer and their effects on patient survival. Ann Oncol. 1995;6:S9–S13. doi: 10.1093/annonc/6.suppl_3.S9. (Suppl 3) [DOI] [PubMed] [Google Scholar]
- 36.Ohno A, Hirashima T, Kubo A, Masuda N, Takada M, Fujiwara H, Yasumitsu T, Kikui M, Fukuoka M, Nakagawa K. p53 status and prognosis in stage I–IIIa non-small cell lung cancer. Int J Oncol. 1997;10:521–528. doi: 10.3892/ijo.10.3.521. [DOI] [PubMed] [Google Scholar]
- 37.Ludovini V, Pistola L, Gregorc V, Floriani I, Rulli E, Piattoni S, Di Carlo L, Semeraro A, Darwish S, Tofanetti FR, et al. Plasma DNA, microsatellite alterations, and p53 tumor mutations are associated with disease-free survival in radically resected non-small cell lung cancer patients: A study of the perugia multidisciplinary team for thoracic oncology. J Thorac Oncol. 2008;3:365–373. doi: 10.1097/JTO.0b013e318168c7d0. [DOI] [PubMed] [Google Scholar]
- 38.Horio Y, Takahashi T, Kuroishi T, Hibi K, Suyama M, Niimi T, Shimokata K, Yamakawa K, Nakamura Y, Ueda R, et al. Prognostic significance of p53 mutations and 3p deletions in primary resected non-small cell lung cancer. Cancer Res. 1993;53:1–4. [PubMed] [Google Scholar]
- 39.Schiller JH, Adak S, Feins RH, Keller SM, Fry WA, Livingston RB, Hammond ME, Wolf B, Sabatini L, Jett J, et al. Lack of prognostic significance of p53 and K-ras mutations in primary resected non-small-cell lung cancer on E4592: A laboratory ancillary study on an eastern cooperative oncology group prospective randomized trial of postoperative adjuvant therapy. J Clin Oncol. 2001;19:448–457. doi: 10.1200/JCO.2001.19.2.448. [DOI] [PubMed] [Google Scholar]
- 40.Tolbert DM, Noffsinger AE, Miller MA, DeVoe GW, Stemmermann GN, Macdonald JS, Fenoglio-Preiser CM. p53 immunoreactivity and single-strand conformational polymorphism analysis often fail to predict p53 mutational status. Mod Pathol. 1999;12:54–60. [PubMed] [Google Scholar]
- 41.Meinhold-Heerlein I, Ninci E, Ikenberg H, Brandstetter T, Ihling C, Schwenk I, Straub A, Schmitt B, Bettendorf H, Iggo R, Bauknecht T. Evaluation of methods to detect p53 mutations in ovarian cancer. Oncology. 2001;60:176–188. doi: 10.1159/000055316. [DOI] [PubMed] [Google Scholar]
- 42.Fong KM, Kida Y, Zimmerman PV, Ikenaga M, Smith PJ. Loss of heterozygosity frequently affects chromosome 17q in non-small cell lung cancer. Cancer Res. 1995;55:4268–4272. [PubMed] [Google Scholar]
- 43.Sozzi G, Miozzo M, Donghi R, Pilotti S, Cariani CT, Pastorino U, Porta G Della, Pierotti MA. Deletions of 17p and p53 mutations in preneoplastic lesions of the lung. Cancer Res. 1992;52:6079–6082. [PubMed] [Google Scholar]
- 44.Ahrendt SA, Hu Y, Buta M, McDermott MP, Benoit N, Yang SC, Wu L, Sidransky D. p53 mutations and survival in stage I non-small-cell lung cancer: Results of a prospective study. J Natl Cancer Inst. 2003;95:961–970. doi: 10.1093/jnci/95.13.961. [DOI] [PubMed] [Google Scholar]
- 45.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, Ridge JA, Goodwin J, Kenady D, Saunders J, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–2561. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: Understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 47.Chiba I, Takahashi T, Nau MM, D'Amico D, Curiel DT, Mitsudomi T, Buchhagen DL, Carbone D, Piantadosi S, Koga H, et al. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Lung Cancer Study Group. Oncogene. 1990;5:1603–1610. [PubMed] [Google Scholar]
- 48.Soussi T, Béroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1:233–240. doi: 10.1038/35106009. [DOI] [PubMed] [Google Scholar]
- 49.Bria E, Pilotto S, Amato E, Fassan M, Novello S, Peretti U, Vavalà T, Kinspergher S, Righi L, Santo A, et al. Molecular heterogeneity assessment by next-generation sequencing and response to gefitinib of EGFR mutant advanced lung adenocarcinoma. Oncotarget. 2015;6:12783–12795. doi: 10.18632/oncotarget.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]