Abstract
Radical surgery is currently the first treatment of choice for retroperitoneal soft tissue sarcoma (RSTS). However, the prognosis of RSTS remains poor due to ineffective local control and a high incidence of metastasis after surgical resection. Brachytherapy has been shown to safely provide local radiotherapy for numerous types of cancer when used alone or in combination with surgical resection, but has not been well characterized in the management of RSTS. The aim of this study was to evaluate CT-guided 125I seed implantation for local control and pain relief in the treatment of inoperable RSTS. A total of 23 patients with RSTS were treated with 125I implantation. Pain was assessed using a visual analog scale. Other endpoints were evaluated via computed tomography scan or phone call/e-mail records. The occurrence of complications was assessed preoperatively (baseline) and during postoperatively follow-up or until patient succumbed. All patients were successfully treated with 125I implantation. A mean number of 70.87 radioactive seeds were applied in each patient. During the follow-up, two patients were unaccounted for, local recurrence occurred in three patients, five succumbed and complications were observed in sixteen. The patient's VAS score changed from 7.4 preoperatively to 7.6, 2.3, 2.0, 1.2, 1.5, 1.4 and 2.5 at 24 h, 1, 3, 6, 12, 24 and 36 months after the procedure, respectively. Good local control and significant pain relief after 125I seed implantation was observed in patients with inoperable RSTS. Thus, the present results suggest that this method could be an effective treatment option for patients with inoperable RSTS.
Keywords: brachytherapy, 125I seed, iodine radioisotopes, soft tissue sarcoma, retroperitoneal space
Introduction
Soft tissue sarcoma (STS) is a rare malignancy with an incidence rate of <1% of all adult cancers (1). Among all STS cases, the 10–15% that originate in the retroperitoneum are known as retroperitoneal soft tissue sarcomas (RSTS) (2). The prognosis of RSTS is poor, with a 5-year overall survival (OS) of 20–60% due to the low efficacy of local control and the high incidence of metastasis after resection (2–5). Local reoccurrence is the primary reason for relapse in retroperitoneal sarcoma in up to 90% of relapsing patients, while distant metastasis is the primary cause of tumor-associated mortality in sarcoma (4). Furthermore, local recurrence is common, with a consistent relapse rate of ~5% per year between 60 and 120 months following primary treatment (4). Therefore, it remains a challenge to manage RSTS and long-term local tumor control of RSTS remains a key obstacle (1,6).
Surgery is the most effective treatment for RSTS (7). However, as RSTS are often diagnosed at very advanced stages with anatomic localization and frequent invasion of retroperitoneal adjacent structures, surgery is not suitable for numerous patients (8,9). When surgical en-bloc resection is used as the sole-treatment, the outcomes are poor, with side effects due to the excision of neighboring structures accompanied by the occurrence of positive resection margins despite the aggressive surgical approach (5). A high rate of recurrence typically occurs following surgery (9). Therefore, external radiotherapy in combination with chemotherapy (ERBT) is the current strategy used to reduce the rate of local tumor reoccurrence in these patients. However, an adequate therapeutic dose of ERBT in RSTS may damage the adjacent tissues and organs (10). The adjacent structures, including the small bowel, kidney and stomach, are often radiosensitive and have a low radiation tolerance (10). Additionally, no consistent evidence of a disease-free survival benefit has been shown for neoadjuvant/adjuvant chemotherapy for the majority of histological subtypes, although there may be certain situations where it is advantageous (11). Therefore, treatment options for patients with unresectable RSTS are limited, particularly where the aim is the relief of pain and local RSTS control.
To overcome these problems, different radiotherapeutic techniques have been developed to create a local boost of irradiation that is restricted to the tumor site (9). Brachytherapy (BRT) can deliver to the target tumor a large total radiotherapy dose, relieve pain and decrease complications, and has shown potential for improving local control and pain relief for RSTS (5,11). Accumulating evidence has shown that BRT could be performed in patients with unresectable RSTS as a monotherapy (12). However, the published descriptions of brachytherapy via 125I implantation have been limited to case reports (12–14). Thus, the available literature cannot serve as a guide for the widespread clinical application of brachytherapy using 125I. Additionally, the feasibility, efficacy and safety of 125I seed implantation in patients with unresectable RSTS has not yet been evaluated on a large scale. Herein, we present a brachytherapy 125I treatment technique that was performed in our hospital for unresectable RSTS and the effect of this treatment technique on patient outcomes.
Patients and methods
Patients
Between January 2009 and August 2013, 23 patients with primary, localized recurrent or metastasized, histologically confirmed and unresectable RSTS at the Department of Abdominal Oncology, West China Hospital (Sichuan, China) were recruited into the present study. The patients were reevaluated for eligibility for BRT and were required to be in good general condition, including a normal blood pressure or hypertension controlled by drugs, adequate liver and renal function and adequate hematological function (white blood cell count >3,000/l, platelet count >80,000/l and hemoglobin level >9.5 gm/dl). In addition, the patients were excluded if there was any evidence of cardiac disease (congestive heart failure or history of myocardial infarction within the previous 3 months) and if the patient had a history of acute tumor rupture with hemoperitoneum. Certain patients who would otherwise have been excluded due to poor general condition, but who had no other contraindications, were included when their general status improved and delayed BRT was performed. Pediatric and gynecological sarcomas were excluded due to the uncertain risk posed by the potential displacement of radioactive seeds after intervention therapy. The present reviewed the pathological characteristics of recurrent cases and conducted biopsies for all primary tumors prior to brachytherapy. All suitable patients received the 125I seed implantation therapy at the Department of Abdominal Oncology, West China Hospital, Sichuan University (Chengdu, China). The present study was approved by the Ethics Committee of Sichuan University. Written informed consent was obtained from all patients.
Implant preparation
Before protocol enrollment, patients were reevaluated with basic history and pathological examination of the tumor/s, physical examination and laboratory tests. Chest/abdomen axial computed tomography (CT) scan of 3-mm slice thickness was performed using a SOMATOM Emotion CT scanner (Siemens Healthcare, Erlangen, Germany) to assess tumor number, location, size, association with adjacent organs and tissues, and any accompanying metastasis. Oncologists and radiologists with >10 years of experience assessed areas at risk for subclinical disease, the optimal puncture route, and the number and distribution of seeds. Any discrepancy in assessment was solved by discussion. For lesions that did not respond to treatment as expected, a post-treatment plan was designed to enhance the radiation dose while considering patient safety and the limitations of this operation, which generally requires a number of sessions.
Operation
For brachytherapy, a metal strip was placed in the body as a surface marker to better visualize the location and orientation of the target tumor. Then the patient was placed in the prone or supine position and a local anesthetic (0.5% lidocaine; Shanghai Zhpharma, Co., Ltd., Shanghai, China) was administered. The physician (usually a radiologist) guided 18-gauge needles (Hakko Trading, Co., Ltd., Shanghai, China) into the predetermined locations under CT imaging. Using a real-time technique, the needle was inserted into the tumor, avoiding important issues like the aortaventralis, inferior vena cava and nerves. Needle tip location in relation to the tumor and surrounding structures was confirmed by CT imaging. A Mick applicator (Mick Radio-Nuclear Instruments, Inc., Mt. Vernon, NY, USA) was used to deposit the radioactive seeds (t1/2, 59.6 days; energy activity range, 0.6–0.8 mCi; mean, 0.78 mCi; Atom-Hitech, Co., Ltd., Beijing, China). The space between permanently implanted seeds was 1.0 cm within rows and the rows were ~1.0 cm apart. Following the operation, a reevaluation for errant seeds was performed via a CT scan of the whole abdomen.
Severe post-procedural pain was controlled with additional moderate lidocaine subcutaneous injections at the procedure location. Hemorrhaging during the operation was controlled by blocking the catheter (Hakko Trading, Co., Ltd.). After the completion of the brachytherapy, the patient remained in the observation room for 2 h to be monitored for any unexpected complications.
Follow-up and assessment indices
Following the 125I implantation, pain remission and local control were considered to be primary outcome indices; complete response and overall survival were deemed secondary outcomes. RECIST guidelines (version 1.1) were used to assess the efficiency, as follows: Complete response (CR), disappearance of all target lesions; Partial response (PR), at least a 30% reduction in the sum of diameters of target lesions; stable disease (SD), neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease (PD); and PD, at least a 20% increase in the sum of diameters of target lesions (15). Duration of overall response (OR) was calculated as CR/PR. As 125I has an effective dose following implantation of six months, local control was defined as patients being free from the disease in the original location after brachytherapy for six months following the procedure. Overall survival was defined as the percentage of patients surviving at the conclusion of the follow-up period. All patients rated their pain status on a visual analog scale (VAS) before and after the procedure: 0 indicated no pain and a score of 10 represented maximal pain. This rating was repeated 24 h after the operation and at 1, 3, 6, 12, 24 and 36 months after operation. Regular CT scans of the abdominal and lesion regions were performed every three months on patients to assess local control and complete response after treatment until August 2014, or until patient mortality or loss to follow-up. A researcher was trained to conduct clinical interviews in person, via phone call or via email to determine overall survival. Interviews collected information about treatment response, primarily in terms of degree of pain, local control, OR and OS. All adverse effects of the procedure for each patient were recorded in this series, including nerve damage, liver or renal damage, drifting seed, stent-tract bleeding, infection or mortality.
Statistical analysis
Data are presented as percentages of patients or as the mean ± standard deviation with ranges. Survival curves were generated using the Kaplan-Meier method. Results of pain relief, renal function and liver function were calculated by the paired t-test. P<0.05 was considered to indicate a statistically significant difference. All data were calculated using SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient demographics
A total of 23 patients with unresectable RSTS were recruited for this study from the Department of Abdominal Oncology. Patient demographics are listed in Table I. The median age of patients at the time of diagnosis was 50.17±14.57 years (range, 19–78 years). Among the 23 patients, 9 were male (39%) and 14 were female (61%). The diameters of tumors ranged between 2.70 and 19.90 cm. The median tumor size was 6.78±3.85 cm. The histologic grades (intermediate and high grade) and the histological types were as follows: Liposarcomas, 6 (26%); leiomyosarcomas, 6 (26%); small round cell liposarcoma, 2 (9%); epithelioid sarcoma, 3 (13%); rhabdomyosarcoma, 2 (9%); malignant fibrous histiocytoma, 1 (4%); synovial sarcoma, 1 (4%); extraskeletal chondrosarcoma, 1 (4%); and extraskeletal osteosarcoma, 1 (4%). Patients received pretreatment with a mean of 1.43±0.99 surgical operations and 0.70±0.97 courses of interventional therapies, such as transarterial chemoembolization (TACE).
Table I.
Pathological characteristics and distribution of events in the 23 RSTS patients in this study.
| Variable | Data |
|---|---|
| Age (years) | 50.17±14.57 |
| Gender (male/female) | 9/14 (39/61) |
| Histological subtype | |
| Liposarcoma | 6 (26) |
| Leiomyosarcoma | 6 (26) |
| Small round cell liposarcoma | 2 (9) |
| Epithelioid sarcoma | 3 (13) |
| Other typesa | 6 (26) |
| Location of lesions | |
| Pararenal space | 6 (26) |
| Lumbosacral anterior area | 2 (9) |
| Paravertebral area | 3 (13) |
| Posterior pancreatic area | 2 (9) |
| Para aortic region | 9 (39) |
| Portal vein adjacent area | 1 (4) |
| Initial presentation | |
| Primary | 2 (9) |
| Recurrent | 15 (65) |
| Metastasis | 6 (26) |
| Tumor size (cm) | 6.78±3.85 |
| <5 | 4 (17) |
| 5–10 | 14 (61) |
| >10 | 5 (22) |
| No. prior intervention operations | 0.70±0.97 |
| No. prior surgeries | 1.43±0.99 |
| 0 | 3 (13) |
| 1 | 11 (48) |
| >1 | 9 (39) |
| No. of sessions | 2.57±1.43 |
| 1 | 7 (30) |
| >1 | 16 (70) |
| No. of seeds | |
| First time procedure | 70.87±52.28 |
| Subsequent procedures | 46.32±30.73 |
| Energy activity (mean, mCi) | 0.78 (0.6–0.8) |
| Follow-up (months) | 20.87±13.22 |
| Coagulation function (s) | |
| PT | 11.56±2.44 |
| APTT | 28.13±7.38 |
| TT | 19.15±1.81 |
Data are presented as the mean ± standard deviation or as n (%).
Other histologies included one case each of rhabdomyosarcoma, malignant fibrous histiocytoma, synovial sarcoma, extraskeletal chondrosarcoma, extraskeletal osteosarcoma and ovarian carcinosarcoma. RSTS, retroperitoneal soft tissue sarcoma; RPT, prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time.
CT-guided 125I implantation is a feasible, safe and effective treatment for RSTS
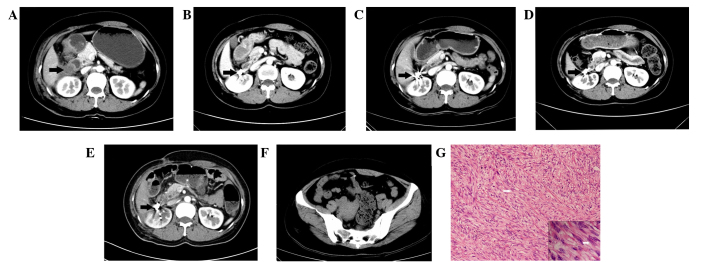
We successfully implanted CT-guided 125I seeds in 23 RSTS patients (Fig. 1). A mean of 70.87±52.28 seeds were implanted in RSTS (range, 10–210) in the first session and an average number of 46.32±30.73 seeds were used during post-treatment sessions (Table I). No patients were recalled to collect data specifically for this study. All data was obtained from medical records and imaging. All of the patients were treated, with satisfactorily outcomes. The P-values of alpha-fetoprotein and TBil were 0.023 and 0.015 (Table II), which were P<0.05 and were considered statistically significant. However, there was no clinical significance as the pre-operation and post-operation concentrations of alpha-fetoprotein and TBil were in the normal range. Thus, no clinically significant damage to renal and liver functions was detected, indicating that CT-guided 125I implantation is a safe treatment for RSTS.
Figure 1.
Representative computed tomography (CT) scan and pathology of retroperitoneal soft tissue sarcoma (RSTS). Brachytherapy using CT-guided 125I seed implantation in a 44-year-old woman with RSTS. High-density spots representing 125I seeds were observed. (A) Axial CT showed the location and relationship with surrounding tissue of RSTS before operation (black arrow). (B-E) Axial enhanced CT images at 3, 12, 24 and 36 months after operation. (F) Seed drafted was observed in the pelvic cavity after the operation. (G) Histology of leiomyosarcoma in perirenal space with (stain, hematoxylin and eosin) (magnification, ×200 and ×400) staining showing spindle cells (white arrow) and indicating that the leiomyosarcoma was derived from smooth muscle.
Table II.
Pre-operative and one month post-operative blood test.
| Parameter | Pre-operative | Post-operative | P-value |
|---|---|---|---|
| Liver function | |||
| TBil (µmol/l) | 13.11±5.57 | 9.80±3.24 | 0.015 |
| ALT (IU/l) | 22.78±18.26 | 22.63±14.07 | 0.278 |
| AST IU/l) | 24.30±7.06 | 22.42±7.85 | 0.122 |
| Renal function | |||
| Urea (mmol/l) | 4.75±1.44 | 4.02±1.40 | 0.389 |
| Creatinine (µmol/l) | 71.91±16.10 | 62.97±22.82 | 0.192 |
| Uric acid (µmol/l) | 307.87±86.91 | 276.28±106.65 | 0.652 |
| Tumor markers | |||
| Alpha-fetoprotein (ng/ml) | 3.41±2.22 | 3.58±2.03 | 0.023 |
| Carcino-embryonic antigen (ng/ml) | 1.76±1.38 | 1.55±1.25 | 0.445 |
| Carbohydrate antigen 1–25 (U/ml) | 24.11±29.38 | 28.98±29.03 | 0.139 |
| Carbohydrate antigen 199 (U/ml) | 10.01±6.54 | 12.89±7.39 | 0.601 |
Data presented as the mean ± standard deviation. TBil, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
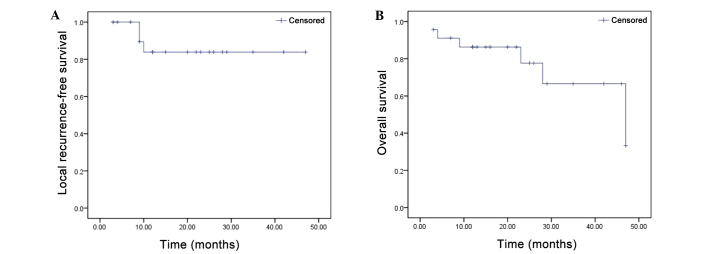
Local recurrence was detected in two patients at 9 months and one patient at 10 months after the operation. The early OR of brachytherapy, evaluated at 90 days from the second cycle of completed treatment, was observed in all 23 patients (100%). Two patients did not complete the follow-up period of the study. Local recurrence was detected in three patients during the follow-up period (20.87±13.22 months). Therefore, local control by 125I seed implantation was 87.0% (Fig. 2A). The median overall survival was 21.56±14.16 months (Fig. 2B). The VAS scores were increased from 7.4±3.2 preoperatively to 7.6±3.0 by 24 h after the operation, but remained at low levels throughout the follow-up period: 2.3±2.6 at one month, 2.0±2.7 at 3 months, 1.2±1.3 at 6 months, 1.5±1.3 at 12 months, 1.4±1.0 at 24 months and 2.5±0.8 at 36 months. Therefore, the mean VAS scores differed significantly from the preoperative baseline at each postoperative time point and all P-values were <0.05 (Table III), demonstrating that patients received significant pain relief one month after brachytherapy.
Figure 2.
(A) Local recurrence-free survival in patients with inoperable retroperitoneal soft tissue sarcoma (RSTS) treated with 125I seed implantation (n=23). (B) Kaplan-Meier overall survival curve of the 23 patients with unresectable RSTS after 125I implantation therapy.
Table III.
VAS scores of patients pre-and post-operatively.
| Post-op time (months) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Pre-op | Post-op 24 h | 1 | 3 | 6 | 12 | 24 | 36 |
| Patients (n) | 24 | 24 | 24 | 24 | 20 | 16 | 8 | 4 |
| VAS score | 7.4±3.2 | 7.6±3.0 | 2.0±2.6 | 2.0±2.7 | 1.2±1.3 | 1.5±1.3 | 1.4±1.0 | 2.5±0.8 |
| P-value | – | P=0.26 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | P=0.006 |
VAS scores presented as the mean ± standard deviation. P-value vs. pre-op. VAS, visual analog scale.
Adverse events
Four asymptomatic seed drafts were observed in three patients. One seed was drafted to the liver and the remainder were drafted to the pelvic cavity (Fig. 1). However, no serious complications were detected. None of these patients exhibited clinical symptoms associated with the procedure. One patient with light radioactive intestinal bleeding was observed three months after the operation and received further treatment in the form of a rectal diversion operation. Some other complications were observed in some of the 23 patients, including fever in 4 patients and nausea in 2 patients (Table IV). These complications were solved following symptomatic treatments.
Table IV.
Complications detected among 23 patients during follow-up.
| Complication | Patients (n) |
|---|---|
| Seed draft | 3 |
| Stent-tract bleeding | 1 |
| Fever | 4 |
| Enterobrosis | 0 |
| Radioactive intestines, bleeding | 1 |
| Vascular perforation | 0 |
| Nerve damage | 0 |
| Loss of appetite | 2 |
| Diarrhea | 1 |
| Ventosity | 2 |
| Nausea | 2 |
Discussion
In the present study, 23 patients were successfully treated with CT-guided 125I seeds implanted via different approaches relative to their RSTS tumors. Among this patient group, only one severe complication was observed during the follow-up period. Four drafting seeds were detected in 3/23 patients. Good local control (87.0%), OR (100%) and median overall survival (21.56±14.16 months) values were achieved by brachytherapy in all recruited patients. The present findings demonstrated a significant improvement in the management of patients with inoperable RSTS by CT-guided 125I seed implantation.
The average number of seeds we used (mean=70.87) in the present study was more than that used by Li et al, who used 30 seeds in the first session in one case (13), though less than Kumar and Good, who used 229 seeds (12). Unlike the present study, these studies were case reports without any statistical significance. The number of sessions (n=2.57) in the present study was similar to Chen et al (14) (n=2.6) and Li et al (13) (n=2). During follow-up, it was found that the mortality rate in the present study was 21.7% (5/23), and the number of adverse events was 16/23, which was more than previous similar studies (12,16). Notably, one patient had radioactive intestinal bleeding, which was not observed in previous studies (12–14). This may have been due to the patients having had several previous external-beam radiation therapy (EBRT) treatments, and the relatively high activity of the seeds that were used in this study. Based on the results in this patient, we speculate that, for safety, lower activity seeds should be applied in cases where the target tumor is close to the aorta or intestines. The discrepancy in overall survival and other complications may be due in part to the smaller number of cases in the study by Chen et al (14). Furthermore, previous studies were limited to case reports and did not have as large a sample size (12,16). Lastly, the follow-up period in the present study was longer compared with the previous studies. VAS scores were elevated 24 h after the operation, while they remained low during follow-up periods from 1 to 24 months, suggesting that the duration of radiation treatment may be too short to relieve cancer pain and that the puncture might increase pain in the local region. Notably, pain was found to be elevated at 36 months, supporting the observation that 125I seeds have ~180 days of effective radiation cover following implantation, and that the residual sarcoma tissue may progress after the duration of the effective radiation has elapsed.
Collectively, the present data suggest that CT-guided 125I seed implantation is feasible, safe and effective for patients with unresectable RSTS. In the following sections, we report the operative procedure and thereby provide clinical guidelines for patients with retroperitoneal sarcoma.
Surgical resection remains the standard primary treatment for patients with RSTS, and may improve overall survival. By contrast, the primary outcome aims of en-bloc resection for patients with obstinate RSTS are to achieve pain relief and local control. Considering the palliative aims, surgery may not be the optimal treatment option, particularly in patients with multiple unresectable metastases, an unfavorable overall prognosis or poor performance status. It has been well-documented that surgical resection of tumors with sizes >5 cm (5,7) and high-grade histology (17) is accompanied by a high probability of loco-regional recurrence and distant metastases within the first two years (13). This is due to the difficulty in surgical resection of RSTS of achieving complete excision; there is often a positive postoperative margin, owing to adjacent structures like the gastrointestinal tract (3). Unless there is further treatment, 90% of patients will succumb to recurrent tumors (18,19). Bonvalot et al (20) once pointed out that no long-term overall survival benefit has been demonstrated in patients that have undergone resection of uninvolved organs. Furthermore, Mullinax et al (19) reported that 13 patients (4%) succumbed in the perioperative setting, while 3 succumbed intraoperatively. These numbers are high (though reportedly acceptable, per the authors) and should be taken into consideration when evaluating this approach for patients with retroperitoneal sarcomas. In addition, poor tissue healing further limits the role of repeated surgery as a therapeutic option for this disease. Lastly, though common surgical oncological principles prevail, every operation will be different. Therefore, an experienced surgical team should plan each operation after careful study, as soft-tissue sarcoma may occur at any site. Thus, for certain patients, less invasive therapies may be more effective for managing local disease recurrence.
Chemotherapy is widely used for treating advanced cancer. Although particular subtypes of soft-tissue sarcoma are sensitive to chemotherapeutic agents, neoadjuvant/adjuvant chemotherapy has not yet been shown to confer a disease-free survival benefit for the majority of histological subtypes (11). Palliative systemic chemotherapy is the cornerstone therapy; however, the response rate is 20–30% and the median overall survival is generally lower than 12 months (21). Furthermore, since the use of adjuvant chemotherapy remains controversial, there are no standard guidelines for systemic chemotherapy in patients with RSTS (19). A meta-analysis of adjuvant chemotherapy did not demonstrate an overall survival advantage, although progression-free survival was improved (22), suggesting that RSTS may be insensitive to adjuvant chemotherapy. Consistently, a previous study showed that the outcome of therapeutic chemotherapy for RSTS was unsatisfactory in terms of overall survival (23). In addition, chemotherapy usually results in complications such as vomiting, diarrhea and decreased platelet count. Collectively, these previous results indicate that chemotherapy is not a good choice for patients with RSTS.
Radiotherapy is another well-established modality in the management of RSTS and radiation treatment is generally considered beneficial. Preoperative EBRT is able to facilitate marginally negative resection and postoperative radiation treatment can diminish local recurrence and may improve survival (18,24). However, the role of ERBT in primary retroperitoneal sarcoma has, to date, been only poorly defined due to a lack of randomized clinical series (3,9,10). Furthermore, the role of radiotherapy in relieving pain is limited. Since the 125I provides a source of continuous low dose radiation, it may be more effective than daily pulsed high dose irradiation in treating the hypoxic portion of large, slow-growing necrotic tumors (12). Unfortunately, in the retroperitoneum and spinal region, the required curative doses exceed 45–50 Gy, which is neither easily nor safely delivered without a high risk of radiation-induced gastrointestinal, genitourinary or spinal cord complications (7). EBRT was excluded as it might cause skin injury (13). The higher doses required for local control and the inherent normal-tissue-tolerance limitations of external beam radiation therapy may explain the failure of this modality to adequately control retroperitoneal soft-tissue sarcomas (11,12,23). Therefore, radiotherapy, as a monotherapy, may not be suitable for patients with RSTS.
There are other options for patients with unresectable RSTS, including different interventional radiology therapies. These interventional radiology approaches have common advantages; for example, they are simple, cheap, have high safety, good efficacy, low invasiveness and few complications (25). However, there are also a number of drawbacks associated with them.
Percutaneous ethanol injection has been clinically applied for several decades and has proven to be a safe technique, but its effects have been limited by alcohol tolerance and local blood flow (26). Furthermore, multiple sessions may be required, leading to a prolonged treatment time.
Transcatheter embolization (TAE) or TACE has shown varying degrees of efficacy. A tumor may have a number of feeding arteries other than the main feeders, and the vascularly-rich bottom portion of the tumor does not respond effectively to TAE (26). Nevertheless, TAE has been shown to be effective for the treatment of rapidly growing tumors (26). Unfortunately, RSTS is not an ideal target for TAE due to its slow growth (12,26). TACE has increased intratumoral chemotherapeutic concentration, reduced systemic toxicity and increased local effects, and thus has improved therapeutic results when compared with systemic chemotherapy (27). With TACE, embolization of the tumor feeding vessels slows blood flow, creates ischemia and increases the contact time between the chemotherapeutic agent and the tumor cells (27). Unfortunately, RSTS are less vascular compared with numerous other tumor types, reducing the efficacy of the therapy while leaving the patient just as vulnerable to the most common complications associated with the toxicity of the chemotherapeutic agents, including nausea, neutropenia, myelosuppression and bacteremia (28). Furthermore, for osteosarcoma, the intraarterial infusion of cisplatin did not improve the local tumor response (29).
Radiofrequency ablation (RFA) has been widely used for two decades in the treatment of various neoplasms, including renal cancer located in the retroperitoneum (30,31). Successful treatment of retroperitoneal lymph nodes has also been reported. However, the application of RFA requires particular caution be observed because of the high risk of thermal damage to neighboring organs, such as the bowel, nerves and nearby vessels (32). Therefore, considering the uncertain effectiveness and difficulty in avoiding complications, these intervention therapies may not be a good choice for RSTS.
Traditionally, interstitial implants were performed with 226Ra needles (31). Due to radiation safety considerations, however, 226Ra has largely been replaced by other radionuclides (33). Currently, the majority of interstitial brachytherapy treatments are delivered using different radioactive sources, such as 192Ir, 103Pd and 125I (33).
192Ir is ideal for temporary brachytherapy. It decays with a half-life of 73.83 days and emits gamma rays with an average energy of ~370 keV. 192Ir is most commonly used in the form of a wire as a transient brachytherapy without any ‘fixicity’ problems when compared with permanent implantation seeds like 125I and 103Pd (34). 125I decays with a half-life of 59.4 days and emits photons with an average energy of 27.4–35.5 keV (35). 125I is commercially available in the form of small ‘seed’ sources for interstitial permanent implants. If rectal toxicity is a concern, the very low dose rate of 125I should favor 125I implants (36). Furthermore, the dose homogeneity inside the target volume is very high with 125I (36). Crucially, 125I is an ideal isotope to use for large volume irradiation of retroperitoneal tumors close to the spinal cord due to its low gamma photon energy of 35.5 keV (37), which results in a rapidly decreasing radiation dose outside of the implanted volume (12).
103Pd is ideal for use as a permanent interstitial source, similar to 125I. 103Pd decays with a half-life of 17.0 days and emits photons with an average energy of 21–30 keV. A 103Pd source is similar in size and encapsulation to 125I sources (33). Although it also offers the practical advantage of low energy, reducing the dose to surrounding organs and minimizing shielding requirements, the difference of the half-lives between 103Pd (17 days) and 125I (61 days) is marked (33). In addition, 125I has been in practical use for longer than 103Pd (33).
Brachytherapy is an established method of safely providing local adjuvant radiotherapy that may be used alone or in combination with resection for prostate cancer, breast cancer, cervical cancer and soft tissue sarcomas. Compared with surgery, 125I has fewer complications, a larger application field and requires fewer procedures to be conducted under sedation or a short general anesthetic (38). Compared with chemotherapy, 125I implantation has fewer toxic complications (16). Compared with ERBT, 125I implantation has an advantage as brachytherapy inflicts less radiation damage to adjacent structures such as the bowel and genitourinary tract (10). For certain tumors, consecutive radiotherapy can be performed by repeatedly implanting 125I seeds, and the curative effects are better than external radiotherapy (13). Compared with vascular intervention therapies, 125I is not limited by the size of the cancer or the distribution of the vessels (39). In comparison with RFA, 125I has the advantage of avoiding damage to critical structures, such as blood vessels and nerves (39). Compared with other radioactivity sources, 125I has a long half-life, a low level of radiant energy that is steadily released over 200 days following implantation and is suitable for targeting slowly growing tumors such as RSTS (34).
There were several limitations in the present study. The first limitation is a shortage of a treatment planning system, which limits the user's ability to manipulate the isodose lines manually to ensure adequate target coverage and spare critical structures (14). Secondly, due to the difficulties in maintaining optimum implant geometry in the irregular anatomy of the retroperitoneal space, bones, arteries and veins, the ability to deliver an optimum dose to the tumor is often limited. This difficulty has also been observed in the results of previous studies (34). Although peripheral nerves are generally tolerant of radiation, the high doses of radiation adjacent to the sources may be injurious (14). By adjusting implant geometry to avoid this complication, the likelihood of hot or cold spots occurring within the tumor bed may be increased (14). Third, patients with large and closely applied dorsal veins and vessels are at particular risk for seed migration to the lung. Fourth, certain patients succumbed during the follow-up period. Fifth, the present patients were recruited from a single center, and the sample size was relatively small due to the rarity of RSTS. Sixth, we did not perform an arm-to-arm study, comparing CT-guided 125I implantation with any other treatments. Seven, to the best of our knowledge, there has been no economic assessment of 125I implantation for RSTS. Finally, seed migration may cause cold or hot spots, move to important structures like the bladder and urethra, or move into a vascular structure (34).
Several methods to remedy the drawbacks outlined above were implemented. First, we implanted seeds with a 1-cm interval seed-to-seed (0.8 cm raw-to-raw) to produce an array with the best possible coverage. Second, in order maximize patient retention in the study we facilitated a close patient relationship, improved patient communication and maximum kindness of care by not only maintaining contact with patients via phone and/or e-mail but also maintaining contact with their family. Third, multidisciplinary clinical teams with a substantial knowledge and experience in the management of sarcomas worked together on this project. The team included surgeons, oncologists, radiologists and pathologists. Fourth, to decrease the ‘cold spots’, we usually performed a post-therapy plan by supplementing 125I seeds as necessary in the target location to enhance the radiation dose.
Although 125I implantation does not represent a cure for RSTS, the principal goals of 125I implantation of significant pain relief and good local control have been achieved. The present results suggest that CT-guided 125I implantation is safe, feasible and effective for the treatment of patients with obstinate RSTS. However, familiarity with local anatomy, experience and skill are prerequisites for success. The present findings may aid physicians who are determining the appropriate management of their patients. Additional large-scale and multicenter studies are required.
Acknowledgements
The present study was supported by the National Nature Science Foundation of China (grant no. 81470141) and the Science & Technology Department of Sichuan Province (grant no. 2014SZ0002-8).
References
- 1.Lewis JJ, Brennan MF. Soft tissue sarcomas. Curr Probl Surg. 1996;33:817–872. doi: 10.1016/S0011-3840(96)80013-X. [DOI] [PubMed] [Google Scholar]
- 2.Karakousis CP, Gerstenbluth R, Kontzoglou K, Driscoll DL. Retroperitoneal sarcomas and their management. Arch Surg. 1995;130:1104–1109. doi: 10.1001/archsurg.1995.01430100082016. [DOI] [PubMed] [Google Scholar]
- 3.Windham TC, Pisters PW. Retroperitoneal sarcomas. Cancer Control. 2005;12:36–43. doi: 10.1177/107327480501200105. [DOI] [PubMed] [Google Scholar]
- 4.Heslin MJ, Lewis JJ, Nadler E, Newman E, Woodruff JM, Casper ES, Leung D, Brennan MF. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: Implications for management. J Clin Oncol. 1997;15:2832–2839. doi: 10.1200/JCO.1997.15.8.2832. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins MP, Alvaranga JC, Thomas JM. The management of retroperitoneal soft tissue sarcomas. Eur J Cancer 32A. 1996:622–626. doi: 10.1016/0959-8049(95)00638-9. [DOI] [PubMed] [Google Scholar]
- 6.Jaques DP, Coit DG, Hajdu SI, Brennan MF. Management of primary and recurrent soft-tissue sarcoma of the retroperitoneum. Ann Surg. 1990;212:51–59. doi: 10.1097/00000658-199007000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: Analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGrath PC. Retroperitoneal sarcomas. Semin Surg Oncol. 1994;10:364–368. doi: 10.1002/ssu.2980100509. [DOI] [PubMed] [Google Scholar]
- 9.Dziewirski W, Rutkowski P, Nowecki ZI, Salamacha M, Morysiński T, Kulik A, Kawczyńska M, Kasprowicz A, Lyczek J, Ruka W. Surgery combined with intraoperative brachytherapy in the treatment of retroperitoneal sarcomas. Ann Surg Oncol. 2006;13:245–252. doi: 10.1245/ASO.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Classen J, Hehr T, Lamprecht U, Zumbrägel A, Bamberg M, Budach W. Hyperfractionated 192Ir brachytherapy for recurrent retroperitoneal sarcoma: A technique for delivery of local tumor boost dose. Strahlenther Onkol. 2003;179:118–122. doi: 10.1007/s00066-003-0998-z. [DOI] [PubMed] [Google Scholar]
- 11.Strauss DC, Hayes AJ, Thomas JM. Retroperitoneal tumours: Review of management. Ann R Coll Surg Engl. 2011;93:275–280. doi: 10.1308/003588411X571944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar PP, Good RR. Interstitial 125I implantation in the retreatment of retroperitoneal soft tissue sarcoma. Report of a case. Acta Radiol Oncol. 1986;25:37–39. doi: 10.3109/02841868609136375. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Wang Y, Liu B, Li Z, Wang W. (125)I brachytherapy seeds implantation for inoperable low-grade leiomyosarcoma of inferior vena cava. Korean J Radiol. 2013;14:278–282. doi: 10.3348/kjr.2013.14.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ME, Zhang B, Li HP. PET/CT-Guided radioactive 125I seeds implanted in retroperitoneal sarcoma. Guang Dong Yi Xue. 2012;33:3. (In Chinese) [Google Scholar]
- 15.Eisenhauer E, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Wang Y, Liu B, Li Z, Wang W. 125I Brachytherapy Seeds Implantation for Inoperable Low-Grade Leiomyosarcoma of Inferior Vena Cava. Korean J Radiol. 2013;14:278–282. doi: 10.3348/kjr.2013.14.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuhaus SJ, Barry P, Clark MA, Hayes AJ, Fisher C, Thomas JM. Surgical management of primary and recurrent retroperitoneal liposarcoma. Br J Surg. 2005;92:246–252. doi: 10.1002/bjs.4802. [DOI] [PubMed] [Google Scholar]
- 18.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 19.Mullinax JE, Zager JS, Gonzalez RJ. Current diagnosis and management of retroperitoneal sarcoma. Cancer Control. 2011;18:177–187. doi: 10.1177/107327481101800305. [DOI] [PubMed] [Google Scholar]
- 20.Bonvalot S, Rivoire M, Castaing M, Stoeckle E, Le Cesne A, Blay JY, Laplanche A. Primary retroperitoneal sarcomas: A multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–37. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 21.D'Adamo DR. Appraising the current role of chemotherapy for the treatment of sarcoma. Semin Oncol. 2011;38:S19–S29. doi: 10.1053/j.seminoncol.2011.09.004. (Suppl 3) [DOI] [PubMed] [Google Scholar]
- 22.Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults, corp-author. Meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997;350:1647–1654. [PubMed] [Google Scholar]
- 23.Lewis JJ, Benedetti F. Adjuvant therapy for soft tissue sarcomas. Surg Oncol Clin N Am. 1997;6:847–862. [PubMed] [Google Scholar]
- 24.Hines OJ, Nelson S, Quinones-Baldrich WJ, Eilber FR. Leiomyosarcoma of the inferior vena cava: Prognosis and comparison with leiomyosarcoma of other anatomic sites. Cancer. 1999;85:1077–1083. doi: 10.1002/(SICI)1097-0142(19990301)85:5<1077::AID-CNCR10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Ryder SD. British Society of Gastroenterology: Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52:iii1–iii8. doi: 10.1136/gut.52.suppl_3.iii1. (Suppl 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai Y, Habe K, Imada M, Hakamada A, Isoda KI, Yamanishi K, Uchida A, Mizutani H. A case of a large dermatofibrosarcoma protuberans successfully treated with radiofrequency ablation and transcatheter arterial embolization. J Dermatol. 2004;31:42–46. doi: 10.1111/j.1346-8138.2004.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 27.Chu JP, Chen W, Li JP, Zhuang WQ, Huang YH, Huang ZM, Yang JY. Clinicopathologic features and results of transcatheter arterial chemoembolization for osteosarcoma. Cardiovasc Intervent Radiol. 2007;30:201–206. doi: 10.1007/s00270-005-0302-y. [DOI] [PubMed] [Google Scholar]
- 28.Avritscher R, Javadi S. Transcatheter intra-arterial limb infusion for extremity osteosarcoma: Technical considerations and outcomes. Tech Vasc Interv Radiol. 2011;14:124–128. doi: 10.1053/j.tvir.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Winkler K, Bielack S, Delling G, Salzer-Kuntschik M, Kotz R, Greenshaw C, Jürgens H, Ritter J, Kusnierz-Glaz C, Erttmann R. Effect of intraarterial versus intravenous cisplatin in addition to systemic doxorubicin, high-dose methotrexate, and ifosfamide on histologic tumor response in osteosarcoma (study COSS-86) Cancer. 1990;66:1703–1710. doi: 10.1002/1097-0142(19901015)66:8<1703::AID-CNCR2820660809>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 30.Zhao M, Li X, Wang J, Li W, Huang Z. Retroperitoneal schwannoma treated with percutaneous computed tomography-guided radiofrequency ablation. J Neurosurg Spine. 2012;17:173–176. doi: 10.3171/2012.4.SPINE111061. [DOI] [PubMed] [Google Scholar]
- 31.Shariat SF, Raptidis G, Masatoschi M, Bergamaschi F, Slawin KM. Pilot study of radiofrequency interstitial tumor ablation (RITA) for the treatment of radio-recurrent prostate cancer. Prostate. 2005;65:260–267. doi: 10.1002/pros.20242. [DOI] [PubMed] [Google Scholar]
- 32.Keil S, Bruners P, Brehmer B, Mahnken AH. Percutaneous radiofrequency ablation for treatment of recurrent retroperitoneal liposarcoma. Cardiovasc Intervent Radiol. 2008;31:S213–S216. doi: 10.1007/s00270-007-9263-7. (Suppl 2) [DOI] [PubMed] [Google Scholar]
- 33.Nath R. Response to “Comments on ‘Dosimetry of interstitial brachytherapy sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No. 43’”. [Med. Phys. 22, 209–234 (1995)] Med Phys. 1995;22:209–234. doi: 10.1118/1.597556. [DOI] [PubMed] [Google Scholar]
- 34.Stone NN, Stock RG. Complications following permanent prostate brachytherapy. Eur Urol. 2002;41:427–433. doi: 10.1016/S0302-2838(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 35.Sloboda RS, Menon GV. Experimental determination of the anisotropy function and anisotropy factor for model 6711 I-125 seeds. Med Phys. 2000;27:1789–1799. doi: 10.1118/1.1287285. [DOI] [PubMed] [Google Scholar]
- 36.Nickers P, Thissen B, Jansen N, Deneufbourg JM. 192Ir or 125I prostate brachytherapy as a boost to external beam radiotherapy in locally advanced prostatic cancer: A dosimetric point of view. Radiother Oncol. 2006;78:47–52. doi: 10.1016/j.radonc.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Dutreix A, Wambersie A. Letter: Specification of gamma-ray brachytherapy sources. Br J Radiol. 1975;48:1034–1035. doi: 10.1259/0007-1285-48-576-1034. [DOI] [PubMed] [Google Scholar]
- 38.Zhang P, Li HP. Application of radioactive 125I seeds in abdominal malignant solid tumors. Yingxiang Zhenduan Yu Jieru Fangshexue. 2011;20:313–316. (In Chinese) [Google Scholar]
- 39.Holloway CL, Delaney TF, Alektiar KM, Devlin PM, O'Farrell DA, Demanes DJ. American Brachytherapy Society (ABS) consensus statement for sarcoma brachytherapy. Brachytherapy. 2013;12:179–190. doi: 10.1016/j.brachy.2012.12.002. [DOI] [PubMed] [Google Scholar]