Abstract
Postoperative pain is a critical problem in clinical pain administration. Due to the unclear formation mechanism of postoperative pain, the treatment of postoperative pain is still in the symptomatic treatment stage and lacking satisfactory analgesic effect. Postoperative pain can be simulated by using a rat incision pain model. We observed changes in pain-related behavior of rats affected by the 5-hydroxytryptamine 2A receptor (5-HT2AR) agonist, TCB-2, and antagonist, ketanserin, through intrathecal delivery. The transcription and translation level of potassium-chloride cotransporter 2 (KCC2) in the spinal cord was also measured to investigate the role of the 5-HT2AR-KCC2 pathway in the mechanism of incision pain. Compared with the control group, rats in the incision pain group had decreased mechanical withdrawal threshold (MWT), with significant differences on day 1–7 after surgery, and significant decreases in thermal withdrawal latency (TWL) on days 1, 2, 3 and 6 (P<0.05). Compared with the incision + dimethyl sulfoxide (DMSO) group, MWT and TWL decreased in the incision + ketanserin group on day 1 and 2 (P<0.05). There was no significant difference detected in TWL of incision + TCB-2 group on day 1, while MWT increased significantly compared to the incision + DMSO group (P<0.05). Furthermore, the transcription and translation levels of KCC2 in the incision + ketanserin group decreased significantly in comparison to the incision + DMSO group (P<0.05), while an increase was detected in the incision + TCB-2 group (P<0.05). MWT and TWL decreased in the incision pain rats, accompanied with a decreased transcription and translation level of KCC2. Intrathecal delivery of the 5-HT2AR agonist, TCB-2, alleviated the decreased WMT and inhibited the decreased transcription and translation level of KCC2, while intrathecal delivery of the 5-HT2AR antagonist, ketanserin, aggravated the decreased WMT and transcription and translation levels of KCC2, suggesting the involement of the 5-HT2AR-KCC2 pathway in the formation mechanism of incision pain in rats.
Keywords: incision pain, 5-hydroxytryptamine 2A receptor, potassium-chloride cotransporter 2, mechanical pain, thermal pain
Introduction
Postoperative pain related to tissue damage during an operation is the most common acute pain that occurs immediately after clinical surgery. Postoperative pain may evolve into a persistent postoperative pain, also known as chronic pain, if urgent care is not administrated adequately at its initial stage (1). Postoperative pain may also affect the prognosis by increasing the risk of postoperative complications, prolonged hospital stays and additional hospitalization expenses (2). Therefore, the effective treatment of postoperative pain is of high clinical importance. However, administration of single therapies in the treatment of postoperative pain has shown limitations. In recent years, studies have indicated that multiple targets are involved in the formation mechanism of postoperative pain (3), suggesting the potential clinical efficacy of combinations of multiple analgesics and therapies. One of the most important tasks at present is to clarify the formation mechanism of postoperative pain and to propose novel therapies.
The formation mechanism of postoperative pain involves peripheral sensitization and central sensitization. Peripheral sensitization decreases the afferent threshold of noxious stimulation, while central sensitization enhances the stimulant function and decreases inhibitory function of the pain transmission of central neurons (4). Enhanced stimulant function involved in central sensitization was traditionally considered an important mechanism in the formation of postoperative pain. However, declined function of the inhibitory system has gradually attracted attention in recent years. The descending inhibitory system is an endogenous analgesia system composed of periaqueductal gray (PAG), nucleus raphe magnus (NRM) and dorsal horn of the spinal column(5). Its terminal mainly projects at I–II and IV–V of the spinal cord. Analgesic effects from electroacupuncture stimulation of the descending inhibitory system have been identified (6). Thus, the promotion of postoperative pain relief by enhancing the function of the descending inhibitory system exogenously is imperative.
5-Hydroxytryptamine (5-HT) is one of the main neurotransmitters involved in the descending inhibitory system. It functions by binding with specific 5-HT receptors. In recent years, the 5-HT2A receptor (5-HT2AR) has gained attention as a potential new target in pain research. The expression of 5-HT2AR in the descending pain modulation pathway of rat brainstem was detected following an increase in inflammatory pain induced by carrageenan (7). In addition, an agonist of 5-HT2AR may inhibit inflammatory pain (8) and neuropathic pain (9), suggesting that 5-HT2AR is involved in 5-HT descending pain modulation system. Furthermore, 5-HT2AR is a G protein-coupled receptor which could couple with Gq and activate phospholipase C (PLC)-β so as to increase the intracellular Ca2+ concentration and activate protein kinase C (PKC).
GABA is one of the major inhibitory neurotransmitters involved in the descending inhibitory system. GABA can activate the ionic GABAA receptors and cause Cl− internal flow by transferring extracellular Cl− intracellularly, hyperpolarizing neurons so as to inhibit discharging. Internal flow of Cl− depends on the concentration gradient across the cellular membrane, which depends on the potassium-chloride cotransporter 2 (KCC2). KCC2, expressed in neurons specifically, is the primary protein in control of the inhibitory function of the GABAA receptor. It pumps out intracellular chlorine ion to maintain the inhibitory function of GABAA receptors. Reduced KCC2 function on the cell membrane causes an increased concentration of intracellular chlorine ion, activating and depolarizing GABAA receptors, leading to the declined inhibitory function of GABAA receptors and raised excitability of central neural network, resulting in the central sensitization. Studies have reported that reduced function of KCC2 is implicated in the mechanism of neuropathic pain. It also plays an important role in the molecular mechanism of pain sensitization (10,11).
The relationship between 5-HT2AR and KCC2 has been demonstrated in previous studies. Activated 5-HT2AR causes the hyperpolarization of inhibitory postsynaptic potential (IPSP) and increases the expression of KCC2 in the cell membrane through a mechanism mediated by PKC (12). Previous findings demonstrated the decreased expression of spinal cord KCC2 in a rat incisional pain model, while the activity and expression level of KCC2 could be dynamically changed by the phosphorylation of the carboxyl end in the intracellular structure domain of KCC2 (13). Furthermore, PKC-mediated phosphorylation may enhance the activity of KCC2 and reduce the swallowing function (14). Therefore, 5-HT2AR activity may be mediated by PKC to increase the expression level of KCC2, resulting in an analgesic effect.
The aim of the study was to investigate the role of the 5-HT2AR-KCC2 signaling pathway in the formation mechanism of postoperative pain and the therapeutic effect of 5-HT2AR agonist TCB-2 on postoperative pain. We evaluated the effects of the 5-HT2AR agonist, TCB-2, and the 5-HT2AR antagonist, ketanserin, on the mechanical and thermal hyperalgesia behavioral response in incision pain rats. PCR and western blotting were used to measure the transcription and translation levels of KCC2 in the spinal cord. We also investigated the effects of TCB-2 and ketanserin on the expression levels of KCC2 and explored the formation mechanism of postoperative pain. We anticipate that our findings will help provide an experimental basis for the promotion of clinical treatment options for postoperative pain.
Materials and methods
Experimental animals and grouping
A total of 21 male Sprague-Dawley rats of clean class, weighing 200–250 g, were purchased from an Animal Experiment Center of Ruijin Hospital, Affiliated to the School of Medicine, Shanghai Jiaotong University (Shanghai, China). The rats were housed with environmental conditions maintained at 22±1°C and a 12/12-h light-dark cycle. Each rat was treated with intrathecal catheterization and kept in a single cage with free access to standard food and water. Approval for the animal experiments was received from the Animal Ethics Committee of the Second Military Medical University.
Experimental animals were randomly assigned to the sham or incision pain group in order to test the incision pain model. Incision pain rats were then randomly assigned into 3 groups that were treated with either dimethyl sulfoxide (DMSO) (D4540-500 ml; Sigma-Aldrich, St. Louis, MO, USA), TCB-2 (5-HT2AR agonist, 2592; Tocris, Bristol, UK) or ketanserin (5-HT2AR antagonist, S006; Sigma-Aldrich), respectively. Behavioural experiments were conducted between 9 a.m. and 5 p.m.
Intrathecal catheterization model
The rats were treated with intrathecal catheterization after being housed for one week and then anaesthetized by isoflurane inhalation and placed in a stereotactic frame. Skin was incised by approximately 1.5 cm on the L3-L4 gaps of the dorsal medialine to expose the superior and inferior articular process and ligamentum flavum. A no. 7 needle was used to puncture the ligamentum flavum, endorhachis and arachnoid until a side swing of the tail or twitch of the hind leg was noted. A polyurethane (PE-10) catheter prefilled with physiological saline was inserted in proximity to the lumbar enlargement of the spinal cord. The exit end of the catheter was taken out through a separate opening between the ears. The catheter and opening were fixed and sutured by polyamide sutures (4–0). The rat was then placed in a single cage with the environment kept warm and quiet. After a 5-day recovery, 20 µl of 2% lidocaine, followed by 10 µl of physiological saline were injected by a microsyringe through the catheter. The rats showed paralysis in the lower extremities and reflection of clipping tails disappeared within 30 sec, indicating the success of the intrathecal catheterization model.
Incision pain model
The incision pain model was conducted after the treatment of intrathecal catheterization, according to Brennan et al (15). The rats were given a subcutaneous injection of 80,000 UI of penicillin and anaesthetized by isoflurane inhalation. Skin was incised by approximately 1 cm on the plantar aspect of the foot. An ophthalmic forceps was used to incise lengthwise on the muscle, which was maintained integrally. Skin incision was sutured by polyamide sutures (5–0) and then the rats were placed in a warm and quiet environment.
Behavioural pain testing
To determine the mechanical withdrawal threshold (MWT), rats with free activities were placed in an overhead plastic box with a steel net bottom. After adaption for 30 min, von Frey (mechanical tactile test package, 58011; Stoelting, Wood Dale, IL, USA) (1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 15.0 and 26.0 g) was used to stimulate the incision from 4.0 g, according to Coull et al (16). Mechanical withdrawal was observed and recorded as O for negative and X for positive. A higher or lower level of stimulation was applied until mechanical withdrawal presented as negative. The last level of stimulation was recorded and the average MWT of each rat was calculated.
Thermal withdrawal latency (TWL)
Ugo Basile (37370; Comerio, Italy) was used to evaluate the TWL of the left foot. Rats were placed on a special glass platform in a separate plastic enclosure and adapted for 30 min. The incision was exposed to a thermal stimulus produced by the infrared ray until the rat withdrew its paw upon feeling pain. The time between exposure of the incision to the stimulus and its withdrawal was recorded in seconds by the computer as TWL. The maximum of TWL was set at 25 sec in case of thermal radiation damage. The test was performed 5 times for each rat and the average TWL was calculated without the highest and the lowest values.
Western blotting
Incision pain rats were sacrificed at 12 h, 1, 3 and 5 days after the surgery. Rats were also sacrificed 6 h after the intrathecal delivery of TCB-2 or ketanserin. Following each sacrifice, the lumbar enlargement was removed for the measurement of KCC2 expression. Each 20-mg tissue sample was cut into sections and mixed with 250 µl histiocytic lysis buffer (RIPA; Solarbio, Shanghai, China), containing a 0.01% protease inhibitor cocktail (Sigma-Aldrich, Shanghai, China). After being fully lysed, the samples were centrifuged at 12,000 RCF for 15 min at 4°C and the supernatant was collected. A BCA protein quantification kit (BCA; Thermo Fisher Scientific, Shanghai, China) was used to quantify the protein contents. The tissue samples were run on a 12% (85 µg/well) SDS-PAGE gel and transferred to a nitrocellulose filter membrane (Millipore, Shanghai, China), electrophoretically. Blots were blocked with 5% skim milk at room temperature for 1 h, followed by incubation with anti-KCC2 and GAPDH (Cell Signaling Technology, Danvers, MA, USA) antibodies, and then incubated with goat anti-mouse or anti-rabbit secondary antibody (Beyotime, Shanghai, China). Enhanced chemiluminescence (ECL; Thermo Fisher Scientific) was used to assess the blots visually.
Real-time PCR assay
Total RNA was extracted from tissue samples and quantified, using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). A reverse transcription kit (Fermentas, Shanghai, China) was used to synthesize cDNA through a total volume of 25 µl [12 µl RNA-primer mix, 5 µl 5X RT reaction buffer, 1 µl 25 mM dNTPs, 1 µl 25 U/µl RNase inhibitor, 1 µl 200 U/µl M-MLV Rtase, 1 µl Oligo(dt)18 and 4 µl DNase-free ddH2O]. Then, cDNA was amplified through a total PCR system of 25 µl (12.5 µl SYBR-Green mix, 0.5 µl forward primer, 0.5 µl reverse primer, 9.5 µl ddH2O and 2 µl cDNA template) with the PCR conditions consisting of 10 min at 95°C, and then 15 sec at 95°C and 45 sec at 60°C, for 40 cycles. Amplification kinetic curves were obtained through 15 sec at 95°C, 1 min at 60°C, 15 sec at 95°C and 15 sec at 60°C.
Statistical analysis
Experimental data were analyzed by SPSS 16.0 statistical software (Chicago, IL, USA), using two-way RM analysis of variance (ANOVA), and was presented as mean ± SEM. Bonferroni correction was applied in pairwise comparisons. One-way ANOVA was used for intra-group comparisons. P<0.05 was considered to indicate a statistically significant difference. Graphs were produced by GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Incision pain model in rats
Changes in MWT in the incision pain rats
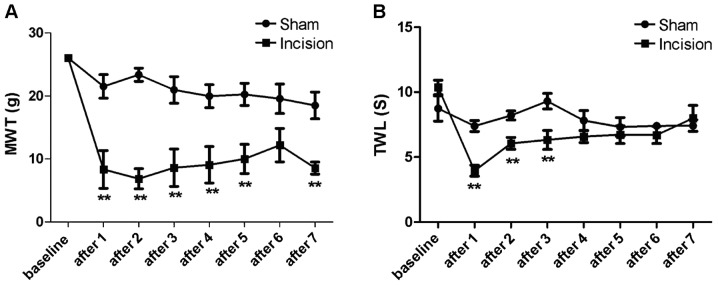
As shown in Fig. 1A, MWT of rats in the incision group (n=7) decreased on days 1, 2, 3, 4, 5 and 7 after surgery, with statistical significance (P<0.05), compared with that of the sham group (n=7). Although there was no statistical significance (P>0.05) detected in the MWT between the sham and incision groups on day 6, the incision group showed a much lower MWT in comparison to the sham group overall.
Figure 1.
Changes in MWT and TWL in the sham and incision groups were detected using the rat incision pain model at baseline and 1–7 days after. (A) The MWT in the incision group showed a decrease compared with the sham group (**P<0.01). (B) Decreases in TWL were detected in the incision group compared with the sham group (**P<0.01). MWT, mechanical withdrawal threshold; TWL, thermal withdrawal latency.
Changes in TWL in the incision pain rats
As shown in Fig. 1B, TWL of rats in the incision group (n=7) decreased from day 1 to 3 after surgery, with statistical significance (P<0.05) compared with that of the sham group (n=7). However, there was no statistical significance (P>0.05) detected in the MWT between the two groups from day 4 to 7.
Behavioural changes of incision pain rats after intrathecal delivery of TCB-2 and ketanserin
Changes in MWT in incision pain rats after intrathecal delivery of TCB-2 and ketanserin
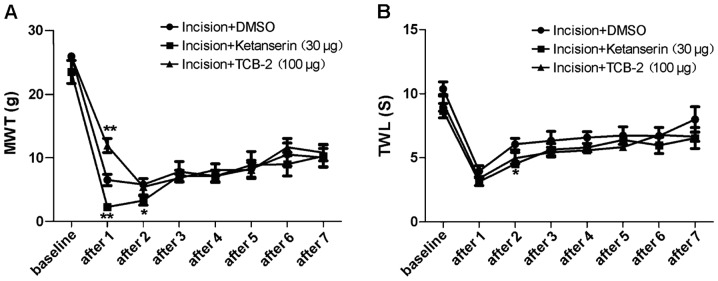
Rats were delivered 5-HT2AR agonist, TCB-2 (100 µg), or antagonist, ketanserin (30 µg), by intrathecal administration on day 1 after surgery. As shown in Fig. 2A, MWT of the incision + TCB-2 group (n=10) increased and MWT of the incision + ketanserin group (n=6) decreased on day 1 after delivery, compared with the incision + DMSO group (n=7), both with a significant difference (P<0.05).
Figure 2.
Changes in MWT and TWL in incision pain rats were recorded after intrathecal delivery of the 5-HT2AR agonist, TCB-2, and the 5-HT2AR antagonist, ketanserin, at baseline and 1–7 days after. (A) MWT increased in the incision+TCB-2 group, while it decreased in the incision+ketanserin group after 1 day (*P<0.05, **P<0.01), compared with the incision+DMSO group. (B) Changes in TWL were detected in incision pain rats after intrathecal delivery of the 5-HT2AR agonist, TCB-2, and the 5-HT2AR antagonist, ketanserin (*P<0.05), compared with the incision+DMSO group. MWT, mechanical withdrawal threshold; TWL, thermal withdrawal latency; 5-HT2AR, 5-hydroxytryptamine 2A receptor; DMSO, dimethyl sulfoxide.
Changes in TWL in the incision pain rats after intrathecal delivery of TCB-2 and ketanserin
As shown in Fig. 2B, TWL of the incision + TCB-2 group (n=10) showed no significant difference in comparison to the incision + DMSO group (P>0.05, n=7). The rats in the incision + ketanserin group (n=6) showed a significantly decreased TWL on day 2 after surgery compared with the incision + DMSO group (P<0.05, n=7).
Changes in KCC2 expression in the incision pain rats
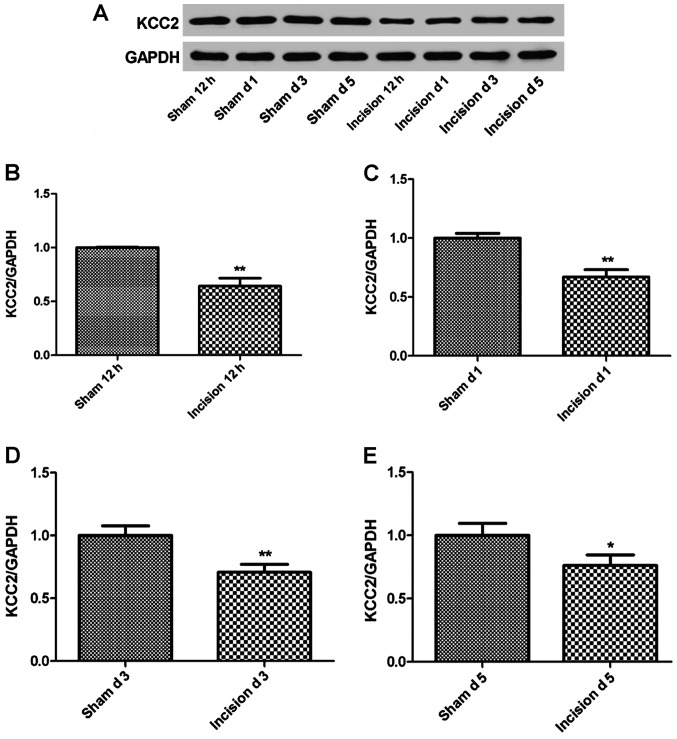
The KCC2 expression in the incision pain rats (n=7) was measured using western blot analysis at 12 h, 1, 3 and 5 days after the surgery. As shown in Fig. 3, the expression level of KCC2 demonstrated a significant decrease in the incision group compared to the sham group after surgery (P<0.05 or P<0.01).
Figure 3.
The expression of KCC2 in lumbar enlargement was measured using western blotting. (A) The expression of KCC2 was prepared on SDS-PAGE. (B) Relative expression of KCC2 between the sham group and the incision group was measured 12 h after the surgery (**P<0.01). (C) Relative expression of KCC2 in the sham and incision groups was measured 1 day after the surgery (**P<0.01). (D) Relative expression of KCC2 in the sham group and the incision group was measured 3 days after the surgery (**P<0.01). (E) Relative expression of KCC2 in the sham group and the incision group was measured 5 days after the surgery (*P<0.05). KCC2, potassium-chloride cotransporter 2.
Effect of TCB-2 and ketanserin on the expression of KCC2 after intrathecal delivery
The transcription and translation levels of KCC2 were measured using quantitative PCR and western blotting, respectively. As shown in Figs. 4 and 5, the transcription and translation levels were decreased in the incision + ketanserin group (P<0.05 or P<0.01), indicating the inhibitory effect of ketanserin on the expression level of KCC2. On the other hand, significant increases in the transcription and translation levels were detected in the incision + TCB-2 group (P<0.05 or P<0.01).
Figure 4.
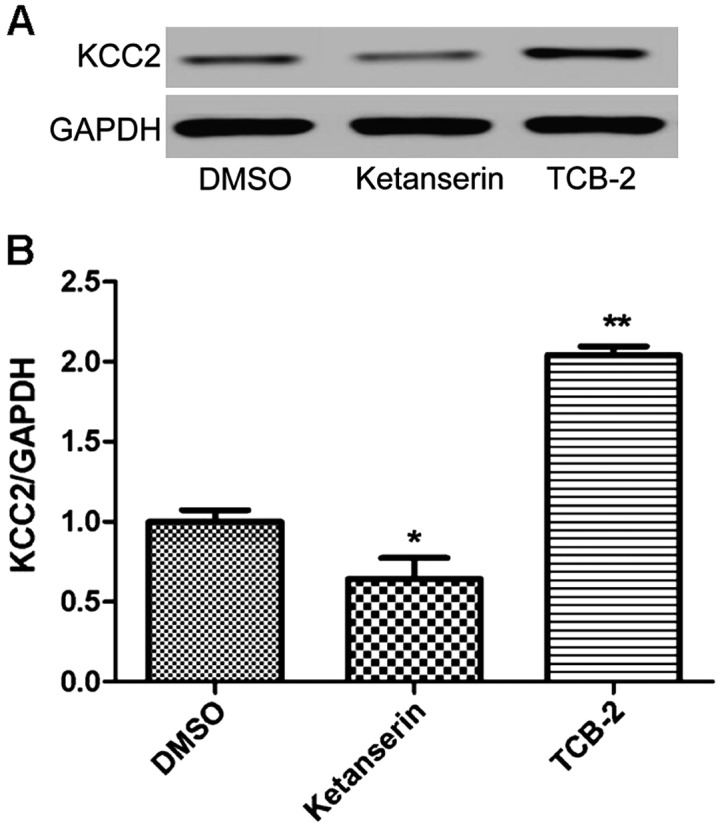
Effects of the 5-HT2AR agonist, TCB-2, and the 5-HT2AR antagonist, ketanserin, on the expression of KCC2 were evaluated using western blotting after intrathecal delivery. (A) The expression of KCC2 was prepared on SDS-PAGE. (B) Relative expression of KCC2 was measured 6 h after intrathecal delivery of TCB-2 or ketanserin, compared with the incision+DMSO group (*P<0.05, **P<0.01). 5-HT2AR, 5-hydroxytryptamine 2A receptor; KCC2, potassium-chloride cotransporter 2; DMSO, dimethyl sulfoxide.
Figure 5.
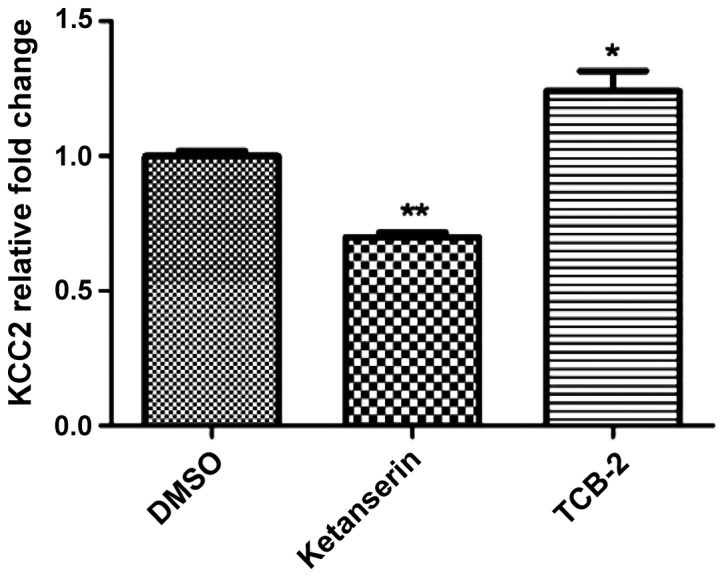
Effects of the 5-HT2AR agonist, TCB-2, and the 5-HT2AR antagonist, ketanserin, on the transcription level of KCC2, compared with the incision+DMSO group. The transcription of KC22 was measured using quantitative PCR after intrathecal delivery. (*P<0.05, **P<0.01). 5-HT2AR, 5-hydroxytryptamine 2A receptor; KCC2, potassium-chloride cotransporter 2; DMSO, dimethyl sulfoxide.
Discussion
In this study, we applied the classic rat incision pain model and demonstrated significant decrease in MWT and TWL. Decreased MWT and TWL lasted for 3 and 7 days, respectively, indicating a successful simulation of postoperative pain in this model. Given the unclear formation mechanism of postoperative pain, it remains one of the most challenging problems in clinical pain therapeutics. We explored the mechanism of pain sensitization by using a rat incision pain model in order to provide an experimental basis for the promotion of clinical treatment of postoperative pain.
We found that the MWT of the incision pain rats increased on day 1 after the delivery of 5-HT2AR agonist, TCB-2, while the TWL showed no significant change. On the other hand, the MWT and TWL of rats decreased on day 1 and 2 after the delivery of 5-HT2AR antagonist, ketanserin, respectively, indicating that the activation of 5-HT2AR alleviated mechanical hyperalgesia caused by incision pain, while the antagonism of 5-HT2AR aggravated mechanical and thermal hyperalgesia. The mechanism of TCB-2 in pain sensitization may involve the activation of 5-HT2AR, leading to the increased expression of downstream signaling molecules, which may be downregulated by ketanserin. 5-HT2AR is a G protein-coupled receptor which mediates a series of functions by the activation of PKC after coupling with Gq. The activation of 5-HT2AR was reported to induce the expression of KCC2 on the cell membrane through the mediation of PKC, resulting in the recovery of endogenous inhibitory function in a spinal cord injury (SCI) model (12). In the present study, alleviated pain sensitization was detected after the intrathecal delivery of 5-HT2AR agonist TCB-2. By contrast, aggravated mechanical and thermal hyperalgesia were observed after the delivery of 5-HT2AR antagonist, ketanserin. Thus, we suggest that the 5-HT2AR-KCC2 pathway may be involved in the formation of incision pain.
The study of 5-HT2AR in pain research has, thus far, mainly focused on neuropathic pain and inflammatory pain models. 5-HT2AR belongs to the group of 5-HT receptors involved in the descending inhibitory system. It was reported that a significant increase in the expression of 5-HT2AR in the descending pain modulation pathway of rat brainstem was detected during inflammatory pain induced by carrageenan (7). An agonist of 5-HT2AR may inhibit inflammatory pain (8) or neuropathic pain (9), suggesting that 5-HT2AR is involved in the 5-HT descending pain modulation system. KCC2, another member of the inhibitory function system, is also a hotspot in pain research (16,17). KCC2 is directly involved in neuropathic pain pathways and plays a crucial role in the molecular mechanism of pain sensitization. Previous studies detected a decreased expression of spinal cord KCC2 in a rat incisional pain model, indicating the participation of 5-HT2AR-KCC2 signaling pathway in the formation of SCI. However, the mechanism of the 5-HT2AR-KCC2 pathway involvement in the rat incision pain model has not been clearly elucidated. We found that the mechanical hyperalgesia caused by incision pain may be alleviated by TCB-2, while aggravated mechanical and thermal hyperalgesia were induced by ketanserin, suggesting that the expression of KCC2 may be regulated by the 5-HT2AR-KCC2 pathway to participate in the formation of incision pain. We explored the mechanism of 5-HT2AR-KCC2 pathway in the formation of incision pain through animal behaviour. Further study should be conducted to characterize and define the relationship between KCC2 expression and formation of incision pain.
In conclusion, we succeeded in simulating the descending inhibitory system in a rat incision pain model by demonstrating decreased MWT and TWL. The 5-HT2AR agonist, TCB-2, showed mitigative effects on postoperative pain which could be exacerbated by the 5-HT2AR antagonist, ketanserin, indicating the relationship between 5-HT2AR and the formation of incision pain.
References
- 1.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101:77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 2.Pavlin DJ, Chen C, Penaloza DA, Polissar NL, Buckley FP. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesth Analg. 2002;95:627–634. doi: 10.1097/00000539-200209000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, Carter T, Cassidy CL, Chittenden EH, Degenhardt E, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Dirks J, Møiniche S, Hilsted KL, Dahl JB. Mechanisms of postoperative pain: Clinical indications for a contribution of central neuronal sensitization. Anesthesiology. 2002;97:1591–1596. doi: 10.1097/00000542-200212000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Dostrovsky JO, Shah Y, Gray BG. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. II. Effects on medullary dorsal horn nociceptive and nonnociceptive neurons. J Neurophysiol. 1983;49:948–960. doi: 10.1152/jn.1983.49.4.948. [DOI] [PubMed] [Google Scholar]
- 6.Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197:183–186. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- 7.Xie H, Ma F, Zhang YQ, Gao X, Wu GC. Expression of 5-HT2A receptor mRNA in some nuclei of brain stem enhanced in monoarthritic rats. Brain Res. 2002;954:94–99. doi: 10.1016/S0006-8993(02)03347-4. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto K, Imbe H, Kimura A, Donishi T, Tamai Y, Senba E. Activation of central 5HT2A receptors reduces the craniofacial nociception of rats. Neuroscience. 2007;147:1090–1102. doi: 10.1016/j.neuroscience.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Obata H, Saito S, Sasaki M, Goto F. Interactions of 5-HT2 receptor agonists with acetylcholine in spinal analgesic mechanisms in rats with neuropathic pain. Brain Res. 2003;965:114–120. doi: 10.1016/S0006-8993(02)04145-8. [DOI] [PubMed] [Google Scholar]
- 10.Lavertu G, Côté SL, De Koninck Y. Enhancing K-Cl co-transport restores normal spinothalamic sensory coding in a neuropathic pain model. Brain. 2014;137:724–738. doi: 10.1093/brain/awt334. [DOI] [PubMed] [Google Scholar]
- 11.Cramer SW, Baggott C, Cain J, Tilghman J, Allcock B, Miranpuri G, Rajpal S, Sun D, Resnick D. The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Mol Pain. 2008;4:36. doi: 10.1186/1744-8069-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos R, Sadlaoud K, Boulenguez P, Buttigieg D, Liabeuf S, Brocard C, Haase G, Bras H, Vinay L. Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci USA. 2013;110:348–353. doi: 10.1073/pnas.1213680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Lee HH, Walker JA, Williams JR, Goodier RJ, Payne JA, Moss SJ. Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J Biol Chem. 2007;282:29777–29784. doi: 10.1074/jbc.M705053200. [DOI] [PubMed] [Google Scholar]
- 15.Brennan TJ, Van der Meulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 16.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 17.Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem. 2005;5:547–555. doi: 10.2174/1568026054367629. [DOI] [PMC free article] [PubMed] [Google Scholar]