Abstract
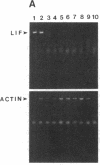
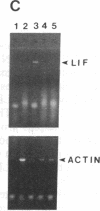
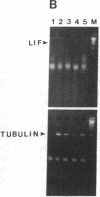
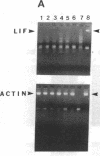
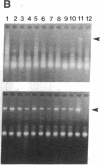
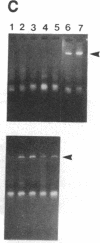
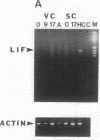
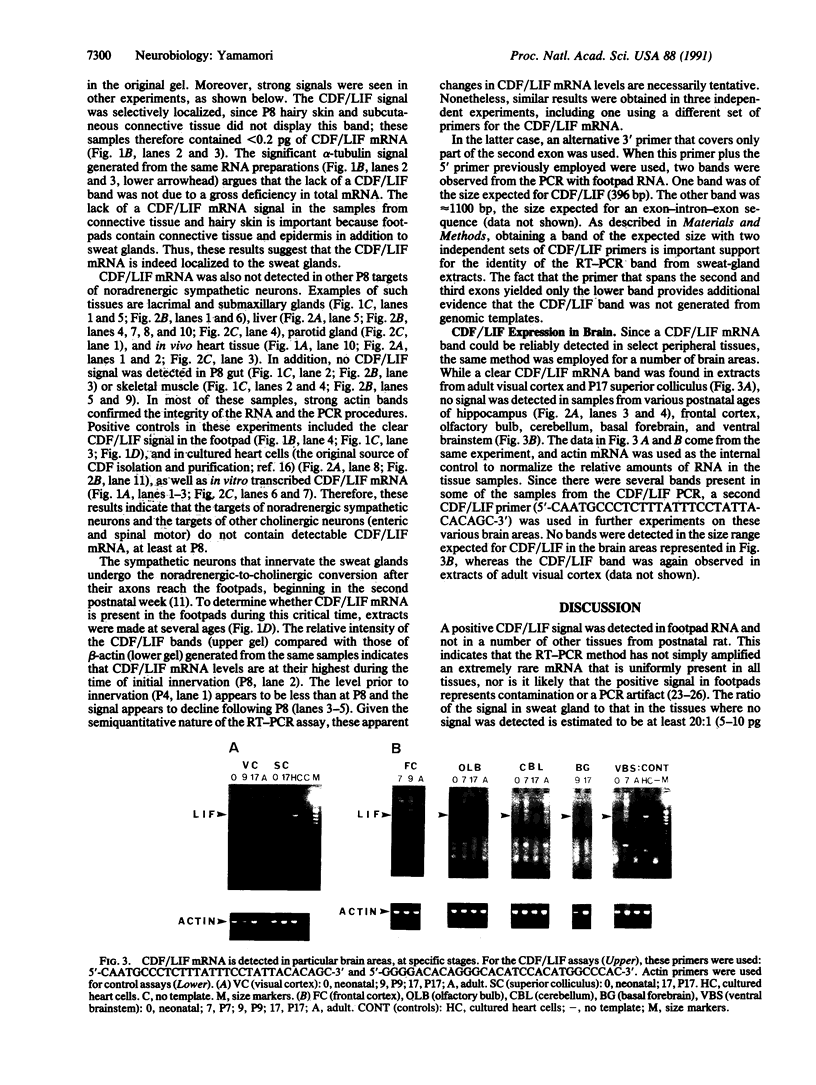
Sympathetic neurons display considerable plasticity in the neurotransmitter and neuropeptide phenotypes they express in vitro and in vivo. The cholinergic differentiation factor (CDF, also known as leukemia inhibitory factor, LIF) induces cultured rat sympathetic neurons to become cholinergic, without affecting their survival or growth. To understand the role of this factor in normal development, it is essential to determine where it is produced in situ. To localize CDF/LIF mRNA, a semiquantitative, reverse transcription-polymerase chain reaction method was employed. Actin and tubulin mRNA were used as internal controls, and two different sets of CDF/LIF primers were compared. In postnatal rat peripheral tissues, CDF/LIF mRNA was selectively localized in the target area of developing, sympathetic cholinergic neurons; the mRNA was not detected in the targets of sympathetic noradrenergic neurons. This finding supports the hypothesis that CDF/LIF is a target-derived neuronal differentiation factor. In postnatal rat brain, CDF/LIF mRNA is localized selectively in two parts of the visual system, visual cortex and superior colliculus. Thus, CDF/LIF may play a role in this system as well.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. E., Schleifer L. S., Black I. B. Partial purification and characterization of a membrane-derived factor regulating neurotransmitter phenotypic expression. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1080–1083. doi: 10.1073/pnas.86.3.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conquet F., Brûlet P. Developmental expression of myeloid leukemia inhibitory factor gene in preimplantation blastocysts and in extraembryonic tissue of mouse embryos. Mol Cell Biol. 1990 Jul;10(7):3801–3805. doi: 10.1128/mcb.10.7.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada K. Hormonal control of neurotransmitter choice in sympathetic neurone cultures. Nature. 1980 Oct 9;287(5782):553–555. doi: 10.1038/287553a0. [DOI] [PubMed] [Google Scholar]
- Fukada K. Purification and partial characterization of a cholinergic neuronal differentiation factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8795–8799. doi: 10.1073/pnas.82.24.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing D. P., Gough N. M., King J. A., Hilton D. J., Nicola N. A., Simpson R. J., Nice E. C., Kelso A., Metcalf D. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). EMBO J. 1987 Dec 20;6(13):3995–4002. doi: 10.1002/j.1460-2075.1987.tb02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Williams R. L. The pleiotropic actions of leukemia inhibitory factor. Cancer Cells. 1989 Nov;1(3):77–80. [PubMed] [Google Scholar]
- Guthrie K. M., Leon M. Induction of tyrosine hydroxylase expression in rat forebrain neurons. Brain Res. 1989 Sep 11;497(1):117–131. doi: 10.1016/0006-8993(89)90977-3. [DOI] [PubMed] [Google Scholar]
- Hughes T., Janssen J. W., Morgan G., Martiat P., Saglio G., Pignon J. M., Pignatti F. P., Mills K., Keating A., Gluckman E. False-positive results with PCR to detect leukaemia-specific transcript. Lancet. 1990 Apr 28;335(8696):1037–1038. doi: 10.1016/0140-6736(90)91102-g. [DOI] [PubMed] [Google Scholar]
- Iacovitti L., Evinger M. J., Joh T. H., Reis D. J. A muscle-derived factor(s) induces expression of a catecholamine phenotype in neurons of cultured rat cerebral cortex. J Neurosci. 1989 Oct;9(10):3529–3537. doi: 10.1523/JNEUROSCI.09-10-03529.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchin P. A., Szotyori Z., Fromholc C., Almond N. Avoidance of PCR false positives [corrected]. Nature. 1990 Mar 15;344(6263):201–201. doi: 10.1038/344201a0. [DOI] [PubMed] [Google Scholar]
- Landis S. C., Keefe D. Evidence for neurotransmitter plasticity in vivo: developmental changes in properties of cholinergic sympathetic neurons. Dev Biol. 1983 Aug;98(2):349–372. doi: 10.1016/0012-1606(83)90365-2. [DOI] [PubMed] [Google Scholar]
- Landis S. C. Target regulation of neurotransmitter phenotype. Trends Neurosci. 1990 Aug;13(8):344–350. doi: 10.1016/0166-2236(90)90147-3. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Farmer S., Racaniello V. R., Sharp P. A. Nucleotide sequence and evolution of a mammalian alpha-tubulin messenger RNA. J Mol Biol. 1981 Sep 5;151(1):101–120. doi: 10.1016/0022-2836(81)90223-0. [DOI] [PubMed] [Google Scholar]
- Martinou J. C., Le Van Thai A., Cassar G., Roubinet F., Weber M. J. Characterization of two factors enhancing choline acetyltransferase activity in cultures of purified rat motoneurons. J Neurosci. 1989 Oct;9(10):3645–3656. doi: 10.1523/JNEUROSCI.09-10-03645.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C., Moisand A., Weber M. J. Acetylcholine metabolism by cultured neurons from rat nodose ganglia: regulation by a macromolecule from muscle-conditioned medium. Neuroscience. 1984 Dec;13(4):1373–1386. doi: 10.1016/0306-4522(84)90306-3. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J., Wainer B. H., Levey A. I. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience. 1983 Dec;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Nawa H., Yamamori T., Le T., Patterson P. H. Generation of neuronal diversity: analogies and homologies with hematopoiesis. Cold Spring Harb Symp Quant Biol. 1990;55:247–253. doi: 10.1101/sqb.1990.055.01.027. [DOI] [PubMed] [Google Scholar]
- Nudel U., Zakut R., Shani M., Neuman S., Levy Z., Yaffe D. The nucleotide sequence of the rat cytoplasmic beta-actin gene. Nucleic Acids Res. 1983 Mar 25;11(6):1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H. Control of cell fate in a vertebrate neurogenic lineage. Cell. 1990 Sep 21;62(6):1035–1038. doi: 10.1016/0092-8674(90)90379-s. [DOI] [PubMed] [Google Scholar]
- Patterson P. H. Environmental determination of autonomic neurotransmitter functions. Annu Rev Neurosci. 1978;1:1–17. doi: 10.1146/annurev.ne.01.030178.000245. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Landis S. C. Characterization of a target-derived neuronal cholinergic differentiation factor. Neuron. 1990 Dec;5(6):899–910. doi: 10.1016/0896-6273(90)90350-o. [DOI] [PubMed] [Google Scholar]
- Rao M. S., Landis S. C., Patterson P. H. The cholinergic neuronal differentiation factor from heart cell conditioned medium is different from the cholinergic factors in sciatic nerve and spinal cord. Dev Biol. 1990 May;139(1):65–74. doi: 10.1016/0012-1606(90)90279-r. [DOI] [PubMed] [Google Scholar]
- Saadat S., Sendtner M., Rohrer H. Ciliary neurotrophic factor induces cholinergic differentiation of rat sympathetic neurons in culture. J Cell Biol. 1989 May;108(5):1807–1816. doi: 10.1083/jcb.108.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotzinger R. J., Landis S. C. Acquisition of cholinergic and peptidergic properties by sympathetic innervation of rat sweat glands requires interaction with normal target. Neuron. 1990 Jul;5(1):91–100. doi: 10.1016/0896-6273(90)90037-g. [DOI] [PubMed] [Google Scholar]
- Schotzinger R. J., Landis S. C. Cholinergic phenotype developed by noradrenergic sympathetic neurons after innervation of a novel cholinergic target in vivo. Nature. 1988 Oct 13;335(6191):637–639. doi: 10.1038/335637a0. [DOI] [PubMed] [Google Scholar]
- Singer-Sam J., Robinson M. O., Bellvé A. R., Simon M. I., Riggs A. D. Measurement by quantitative PCR of changes in HPRT, PGK-1, PGK-2, APRT, MTase, and Zfy gene transcripts during mouse spermatogenesis. Nucleic Acids Res. 1990 Mar 11;18(5):1255–1259. doi: 10.1093/nar/18.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl J., Gearing D. P., Willson T. A., Brown M. A., King J. A., Gough N. M. Structural organization of the genes for murine and human leukemia inhibitory factor. Evolutionary conservation of coding and non-coding regions. J Biol Chem. 1990 May 25;265(15):8833–8841. [PubMed] [Google Scholar]
- Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991 May;14(5):165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Wong V., Kessler J. A. Solubilization of a membrane factor that stimulates levels of substance P and choline acetyltransferase in sympathetic neurons. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8726–8729. doi: 10.1073/pnas.84.23.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Fukada K., Aebersold R., Korsching S., Fann M. J., Patterson P. H. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science. 1989 Dec 15;246(4936):1412–1416. doi: 10.1126/science.2512641. [DOI] [PubMed] [Google Scholar]