Abstract
IMPORTANCE
Colorectal cancer (CRC) screening saves lives, but participation rates are low among underserved populations. Knowledge on effective approaches for screening the underserved, including best test type to offer, is limited.
OBJECTIVE
To determine (1) if organized mailed outreach boosts CRC screening compared with usual care and (2) if FIT is superior to colonoscopy outreach for CRC screening participation in an underserved population.
DESIGN, SETTING, AND PARTICIPANTS
We identified uninsured patients, not up to date with CRC screening, age 54 to 64 years, served by the John Peter Smith Health Network, Fort Worth and Tarrant County, Texas, a safety net health system.
INTERVENTIONS
Patients were assigned randomly to 1 of 3 groups. One group was assigned to fecal immunochemical test (FIT) outreach, consisting of mailed invitation to use and return an enclosed no-cost FIT (n = 1593). A second was assigned to colonoscopy outreach, consisting of mailed invitation to schedule a no-cost colonoscopy (n = 479). The third group was assigned to usual care, consisting of opportunistic primary care visit-based screening (n = 3898). In addition, FIT and colonoscopy outreach groups received telephone follow-up to promote test completion.
MAIN OUTCOME MEASURES
Screening participation in any CRC test within 1 year after randomization.
RESULTS
Mean patient age was 59 years; 64% of patients were women. The sample was 41% white, 24% black, 29% Hispanic, and 7% other race/ethnicity. Screening participation was significantly higher for both FIT (40.7%) and colonoscopy outreach (24.6%) than for usual care (12.1%) (P < .001 for both comparisons with usual care). Screening was significantly higher for FIT than for colonoscopy outreach (P < .001). In stratified analyses, screening was higher for FIT and colonoscopy outreach than for usual care, and higher for FIT than for colonoscopy outreach among whites, blacks, and Hispanics (P < .005 for all comparisons). Rates of CRC identification and advanced adenoma detection were 0.4% and 0.8% for FIT outreach, 0.4% and 1.3% for colonoscopy outreach, and 0.2% and 0.4% for usual care, respectively (P < .05 for colonoscopy vs usual care advanced adenoma comparison; P > .05 for all other comparisons). Eleven of 60 patients with abnormal FIT results did not complete colonoscopy.
CONCLUSIONS AND REVELANCE
Among underserved patients whose CRC screening was not up to date, mailed outreach invitations resulted in markedly higher CRC screening compared with usual care. Outreach was more effective with FIT than with colonoscopy invitation.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01191411
Colorectal cancer (CRC) screening saves lives, yet screening rates among underserved populations, such as the uninsured and minorities, are low.1–4 The best CRC screening strategy for underserved populations is unclear. Colonoscopy is known to be the most sensitive test for colorectal neoplasia.5 However, it is unclear on a population level whether colonoscopy should the primary test promoted for boosting screening for all groups for several reasons. Colonoscopy may not be as acceptable as noninvasive tests such as the fecal immunochemical test (FIT) to all populations.6–8 If associated with higher participation rates, programmatic screening with less-invasive tests may result in similar or even better population effectiveness than population colonoscopy screening.5,9 Financial resources and infrastructure required for colonoscopy vs noninvasive screening are markedly different.10 These issues are especially germane for underserved populations because prior work suggests screening participation may differ by type of test offered8 and because safety-net health systems that typically care for underserved populations face financial challenges and have limited colonoscopy capacity.11,12
Identifying the best approach to offering and delivering screening to underserved populations is also critical. The dominant usual care strategy in the United States is opportunistic and office visit–based.13 However, many underserved patients lack the health insurance that would allow regular access to a primary care physician and visit-based screening offers.14,15 Also, the safety-net health systems that often care for underserved populations often have inadequate primary care capacity to deliver effective visit-based screening, particularly in context of competing patient health needs.14–17 Among insured populations, organized outreach programs, such as those that offer mailed invitations to complete FIT screening, have been shown effective for improving screening rates.13,18–22 Mailed outreach efforts with stool testing have been successful in underserved populations, but these efforts used the more cumbersome guaiac fecal occult blood test (gFOBT), which requires multiple stool samples and strict diet restrictions.23–25 Mailed outreach with colonoscopy invitations has also been tested among the underserved, and shown promise.26,27 Whether mailed outreach with the more convenient FIT, which does not require diet restriction and can be performed with 1 sample, is effective in for underserved populations, and how FIT outreach compares with colonoscopy outreach are unknown and merit further study.
Accordingly, we conducted a randomized, comparative effectiveness trial among underserved patients, not up to date with CRC screening. Our aims were to determine (1) if organized mailed outreach boosts screening compared with usual care and (2) if FIT is superior to colonoscopy outreach for screening participation.
Methods
Study Setting
The study was conducted from January 2011 through February 2012 at the John Peter Smith Health Network (JPS), a system of 13 community-and hospital-based primary care clinics and a tertiary care hospital that provides services to residents of Fort Worth and Tarrant County, Texas. To serve the uninsured, JPS offers a medical assistance program for uninsured residents of Tarrant County that provides access to primary and specialty care, including surgery and cancer care, on a sliding pay scale.
Inclusion/Exclusion Criteria
We included men and women, aged 54 to 64 years, who were uninsured but enrolled in the JPS medical assistance program for the uninsured. We excluded patients meeting 1 or more of the following criteria: (1) up-to-date CRC screening; (2) no address or phone number on file; (3) primary language other than English or Spanish; (4) history of CRC, inflammatory bowel disease, or colorectal polyps; (5) no recent health system visit (defined as any visit within the 8-month period prior to randomization); or (6) incarceration. Up-to-date CRC screening was defined as having a fecal occult blood test within 1 year, sigmoidoscopy or barium enema within 5 years, or colonoscopy within 8 years. Colonoscopy within 8 years rather than 10 years was used in our screening up-to-date definition because health system data were not available beyond 8 years.
Interventions
Eligible patients were randomly assigned to FIT outreach, colonoscopy outreach, or usual care. The FIT and colonoscopy outreach interventions both included (1) a mailed 1-page English and Spanish invitation to complete no-cost screening, including information on risk for CRC based on age; (2) 2 prerecorded automated phone messages, one delivered at the time of invitation mailing alerting patients to expect the invitation, and the other delivered 2 weeks after the mailing reminding patients to complete screening; (3) as many as 2 “live” telephone reminders for patients who had not completed screening within 3 weeks of invitation; and (4) aid with scheduling and understanding preparation for colonoscopy.
Patients randomized to FIT outreach were simultaneously mailed a 1-sample FIT test (OC-Auto FIT CHEK, Polymedco), English and Spanish instructions on how to perform the test, and a postage-paid return envelope for the kit. The invitation letter for colonoscopy outreach included a phone number to call and schedule screening.
Returned FIT kits were processed by the health system clinical laboratory in accordance with manufacturer instructions, using a 50-mg hemoglobin/mL buffer or higher cutoff for abnormal. Patients with an abnormal FIT result were referred for diagnostic colonoscopy.
Screening and diagnostic colonoscopy completion was facilitated in several ways: (1) phone triage to assess whether the patient could have colonoscopy scheduled over the phone or required a precolonoscopy clinic visit; (2) provision of bowel preps (via mail or clinic pickup); and (3) appointment reminders and review of preparation instructions 5 to 7 days prior to colonoscopy appointments. FIT and colonoscopy results were communicated to patients either through the mail or clinic visits, and to primary health care providers by mail. Patients with abnormal FIT results who were unreachable by telephone were sent certified letters with the test results and a recommendation to schedule colonoscopy.
Usual care included opportunistic, clinic visit–based offers to complete screening with gFOBT, colonoscopy, barium enema, or sigmoidoscopy at the discretion of primary care providers. FIT and colonoscopy outreach groups also could receive usual care screening through primary care visits. Usual care screening offers were not interfered with by the screening outreach team. Prior to the study intervention, usual care included office visit–based offers for screening with gFOBT, colonoscopy, sigmoidoscopy, or barium enema by primary care providers. No screening reminders were in use prior to the study.
Sample Size, Randomization, and Analytic Approach
Two a priori principles guided sample size allocation for this study. The first was a prespecified goal to detect a difference of 10% or more in screening participation rates for usual care vs any outreach invitation as well as a difference of 10% or more in screening for FIT vs colonoscopy outreach. These differences were based on predicted CRC participation rates of 10% for usual care, 20% or higher for FIT and colonoscopy outreach groups combined, 20% or higher for colonoscopy outreach alone, and 30% or higher for FIT outreach alone.28–30 The second principle was maximizing the number of patients offered screening outreach, balanced against the anticipated colonoscopy capacity of the health system, as well as resources provided by the primary granting agency for outreach, including support of screening outreach team members, reimbursement for FIT kits, and screening/diagnostic colonoscopies. Accordingly, we a priori planned to assign 1600 patients to FIT outreach, 480 patients to colonoscopy outreach, and all remaining eligible patients to usual care (predicted to be at least 3920 patients prior to project initiation). Given these planned assignments, we predicted over 90% power to detect statistically significant differences for our 2 primary comparisons, assuming a prespecified alpha of 0.025 for each comparison.
All eligible patients were randomly assigned to 1 of the 3 groups at baseline using SAS PROC PLAN (SAS Institute Inc). Group allocation was concealed because all patients were randomized at a single time point. Outreach patients were blind to presence of alternate interventions; usual care patients were blind to presence of group assignment altogether.
The intent-to-screen principle was used to analyze all eligible patients for the primary outcome: screening participation. Screening participation was defined as completion of any CRC screening test within 1 year of follow-up after randomization. Screening participation was measured by querying administrative claims data for presence of Current Procedural Terminology (CPT) codes consistent with exposure to colonoscopy, sigmoidoscopy, gFOBT, or barium enema during the follow-up period. Tests were included in the primary outcome of test participation, regardless of indication. Thus, some tests noted on follow-up might have been performed for diagnostic workup of gastrointestinal signs and symptoms such as rectal bleeding rather than screening purposes. Colonoscopy and FIT outreach patients also had test exposure for colonoscopy and FIT, respectively, recorded as part of our tracking activities. There were 2 prespecified primary comparisons: usual care vs any outreach invitation and FIT outreach vs colonoscopy outreach.
We also examined whether screening participation results were similar within sex and race/ethnicity strata. Additionally, we measured rates of adenoma, advanced adenoma, CRC, and abnormal FIT results. Colonoscopy completion among patients with abnormal FIT findings was also recorded through review of colonoscopy claims and confirmation of colonoscopy appointments facilitated by the screening coordination team. Deaths during follow-up were assessed based on recording within the health system’s administrative data for all 3 groups at the end of follow-up.
Continuous variables were compared with analysis of variance for 3-group analyses, and 2-sample t tests for 2-group analyses. Categorical variable comparisons were performed with χ2 or Fisher exact tests. The number needed to invite (NNI) was defined as the number of patients who needed to be invited to accomplish 1 additional screening, equivalent to the inverse of the absolute risk difference in screening rates between groups. For our 2 primary comparisons, P < .025 was considered statistically significant. For all other comparisons, a P < .05was considered statistically significant. All analyses were performed using SAS software, version 9.2 (SAS Institute Inc).
Waiver of Informed Consent
A key feature in this study design was a waiver of informed consent granted for eligibility and group assignment. This allowed us to avoid the potential selection/volunteer bias toward inclusion of patients particularly interested in screening or research that can occur when consent is required. The study was approved by the institutional review boards at JPS and University of Texas Southwestern Medical Center, and registered as ClinicalTrials.gov NCT01191411. The full trial protocol is available upon request.
Results
Study Population
Overall, 5994 patients were randomly assigned to FIT outreach, colonoscopy outreach, or usual care (Figure 1). Twenty-four randomized patients were subsequently found to not meet the age inclusion criteria, resulting in a final intent-to-treat sample size of 5970 patients: 1593 for FIT outreach, 479 for colonoscopy outreach, and 3898 for usual care. Overall, mean patient age was 59 years; 64% of patients were women. The sample was 41% white, 24% black, 29% Hispanic, and 7% other race/ethnicity. The primary language was Spanish for 17% of all patients. There were no statistically significant differences in demographic characteristics across the 3 groups (Table 1).
Figure 1. CONSORT Diagram.
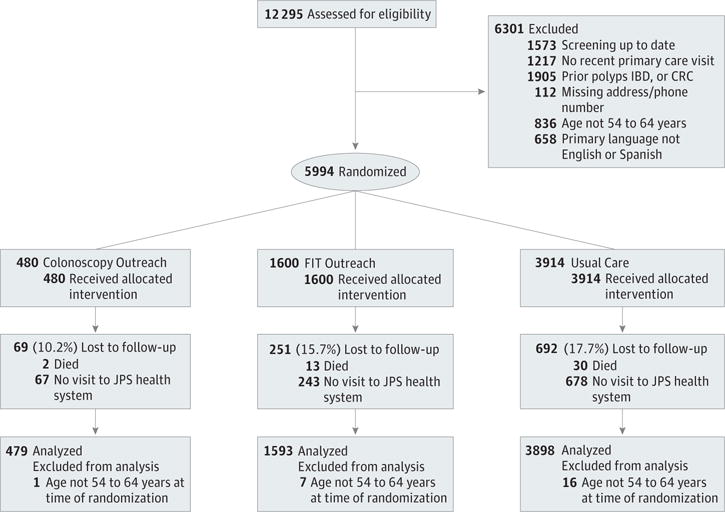
Study recruitment and follow-up are depicted. CRC indicates colorectal cancer; FIT, fecal immunochemical test; IBD, inflammatory bowel disease; JPS, John Peter Smith Health Network.
Table 1.
Demographic Characteristics by Group Assignmenta
| Characteristic | Usual Care (n = 3898) | Colonoscopy Outreach (n = 479) | FIT Outreach (n = 1593) | P Value |
|---|---|---|---|---|
| Age, mean (SD), y | 59 (3) | 59 (3) | 59 (3) | .93 |
| Sex | ||||
| Female | 2517 (65) | 286 (60) | 993 (62) | .05 |
| Male | 1381 (35) | 193 (40) | 600 (38) | |
| Race/ethnicity | ||||
| White | 1600 (41) | 195 (41) | 653 (41) | .19 |
| Black | 913 (23) | 128 (27) | 370 (23) | |
| Hispanic | 1140 (29) | 122 (25) | 445 (28) | |
| Other | 245 (6) | 34 (7) | 125 (8) | |
| Primary language | ||||
| English | 3253 (83) | 410 (86) | 1322 (83) | .06 |
| Spanish | 645 (17) | 69 (14) | 271 (17) |
Abbreviation: FIT, fecal immunochemical test.
Unless otherwise noted, data are reported as number (percentage) of patients.
Primary Outcome: Screening Participation
Screening participation was 40.7% (95% CI, 38.3%–43.1%) for FIT outreach, 24.6% (95% CI, 20.8%–28.5%) for colonoscopy outreach, and 12.1% (95% CI, 11.1%–13.1%) for usual care (Figure 2). Differences were statistically significant for any outreach vs usual care, and for FIT vs colonoscopy outreach (P < .001 for all comparisons). The NNI to accomplish 1 additional screening over usual care was 8 for colonoscopy and 3.5 for FIT outreach.
Figure 2. CRC Screening Participation For Usual Care, Colonoscopy Outreach, and FIT Outreach.
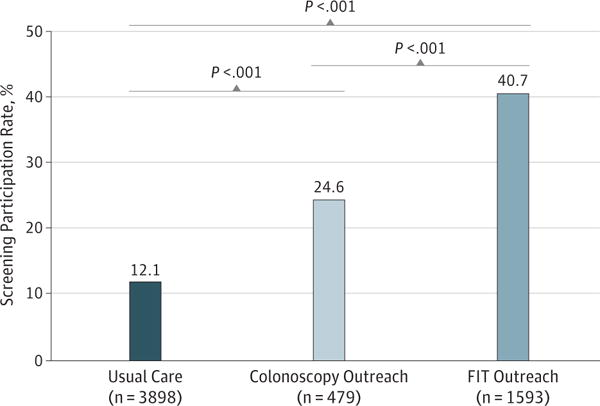
CRC indicates colorectal cancer; FIT, fecal immunochemical test.
Screening Participation Stratified by Sex and Race
Within sex and race/ethnicity strata, both FIT and colonoscopy outreach approaches were superior to usual care for increasing screening (Table 2). Additionally, compared with colonoscopy, FIT outreach was associated with statistically significantly higher screening among men, women, whites, blacks, and Hispanics (Table 2).
Table 2.
Screening Participation Overall, and by Race/Ethnicity and Sex
| Group | Usual Carea | Coloa | FITa | P Value | |||
|---|---|---|---|---|---|---|---|
| Usual Care vs Any Outreach | Usual Care vs Colo | Usual Care vs FIT | Colo vs FIT | ||||
| All groups (n = 5970) | 3898 471 (12.1) (11.1–13.1) |
479 118(24.6) (20.8–28.5) |
1593 648 (40.7) (38.3–43.1) |
<.001 | <.001 | <.001 | <.001 |
| Men (n = 2174) | 1381 148(10.7) (9.1–12.3) |
193 45 (23.3) (17.4–29.3) |
600 232 (38.7) (34.8–42.6) |
<.001 | <.001 | <.001 | <.001 |
| Women (n = 3796) | 2517 323 (12.8) (11.5–14.1) |
286 73 (25.5) (20.5–30.6) |
993 416(41.9) (38.8–45.0) |
<.001 | <.001 | <.001 | <.001 |
| Whites (n = 2448) | 1600 151 (9.4) (8.0–10.9) |
195 40 (20.5) (14.8–26.2) |
653 223 (34.2) (30.5–37.8) |
<.001 | <.001 | <.001 | <.001 |
| Blacks (n = 1411) | 913 139(15.2) (12.9–17.6) |
128 34(26.6) (18.9–34.2) |
370 159 (43) (37.9–48.0) |
<.001 | .002 | <.001 | .001 |
| Hispanics (n = 1707) | 1140 150(13.2) (11.2–15.1) |
122 35 (28.7) (20.7–36.7) |
445 214(48.1) (43.4–52.7) |
<.001 | <.001 | <.001 | <.001 |
| Other races/ethnicities (n = 404) | 245 31 (12.7) (8.5–16.8) |
34 9(26.5) (11.6–41.3) |
125 52 (41.6) (33.0–50.2) |
<.001 | .032 | <.001 | .112 |
Abbreviations: Colo, colonoscopy outreach; FIT, fecal immunochemical test outreach.
Data reported as assigned No., Screened No. (%) (95% CI).
Neoplasia Detection Rates
Rates of CRC identification were 0.4% for FIT outreach, 0.4% for colonoscopy outreach, and 0.2% for usual care (Table 3). Characteristics of patients with CRC detected, including whether CRC was screen-detected by outreach or usual care, are summarized in the eTable in the Supplement. Advanced adenomas were detected among 0.8% of FIT outreach patients, 1.3% of colonoscopy outreach patients, and 0.4% of usual care patients, and 1 or more adenomas of any kind were noted among 4.7% of FIT outreach patients, 9.8% of colonoscopy outreach patients, and 3.1% of usual care patients. Point estimates and P values for neoplasia detection listed in Table 3 should be interpreted with caution, given that these were secondary end points and rare.
Table 3.
Frequency of Neoplasia Detection
| Neoplasia | Patients, No. (%) | P Values | ||||||
|---|---|---|---|---|---|---|---|---|
| Usual Care (n = 3898) |
Colo (n = 479) |
FIT (n = 1593) |
Overall | Usual Care vs Any Outreach | Colo vs FIT | Usual Care vs Colo | Usual Care vs FIT | |
| Any adenoma | ||||||||
| No | 3778 (95.9) | 432 (90.2) | 1518 (95.3) | <.001 | <.001 | <.001 | <.001 | .003 |
| Yes | 120 (3.1) | 47 (9.8) | 75 (4.7) | |||||
| Advanced adenomaa | ||||||||
| No | 3882 (99.6) | 473 (98.7) | 1581 (99.2) | .04 | .025 | .28 | .03 | .11 |
| Yes | 16 (0.4) | 6 (1.3) | 12 (0.8) | |||||
| CRC | ||||||||
| No | 3892 (99.8) | 477 (99.6) | 1587 (99.6) | .21 | NA | NA | NA | NA |
| Yes | 6 (0.2) | 2 (0.4) | 6 (0.4) | |||||
| CRC or advanced adenoma | ||||||||
| No | 3877 (99.5) | 472 (98.5) | 1577 (99.0) | .03 | .014 | .40 | .03 | .06 |
| Yes | 21 (0.5) | 7 (1.5) | 16 (1.0) | |||||
Abbreviations: Colo, colonoscopy outreach; CRC, colorectal cancer; FIT, fecal immunochemical test outreach; NA, not applicable.
Advanced adenoma was defined as an adenoma 1 cm or larger, adenoma with villous or tubulovillous features, and/or adenoma with high-grade dysplasia.
Completion of Follow-up Diagnostic Testing
Eleven of 60 patients with abnormal FIT results did not complete subsequent colonoscopy. Reasons for colonoscopy non-completion included competing health concerns such as need for other elective surgery (n = 5), comorbidities precluding safe colonoscopy (n = 1), failure to respond to follow-up outreach phone calls and mailings (n = 3), refusal to complete colonoscopy (n = 1), and moving out of state (n = 1). It should be noted that 2 of the colonoscopies among patients with abnormal FIT results occurred after the 1-year follow-up period from randomization; neoplasia detection outcomes for these 2 individuals were therefore not included in our summary of these outcomes in Table 3.
No unintended harms occurred as a result of study procedures.
Discussion
This prospective, randomized, comparative effectiveness trial demonstrated that organized mailed outreach efforts substantially increased CRC screening participation among underserved patients. FIT outreach tripled CRC screening rates, and colonoscopy outreach doubled the rates compared with usual care (40.7%, 24.1%, and 12.1%, respectively). The NNI was 3.5 for FIT and 8 for colonoscopy outreach compared with usual care. Significantly higher screening rates were noted for FIT than for colonoscopy outreach. Results were consistent for men, women, whites, blacks, and Hispanics.
Our results, taken together with findings from other randomized studies of outreach interventions, suggest that outreach strategies have potential to significantly improve screening rates for the underserved and merit implementation.23,24,26,27 Two groups have studied effects of outreach with patient navigation, including mailed invitation, telephone follow-up, and patient navigation among underserved primary care patients, reporting increases in screening of 13% to15.5% above usual care.26,27 Lasser et al26 offered choice of colonoscopy or gFOBT, while Percac-Lima et al27 offered colonoscopy alone. These studies using patient navigation differed from our study in the intensity of patient contact; they made more telephone calls to patients and provided more extended support and shared decision making regarding test choice. Interestingly, our less intense approach for facilitating participation resulted in similar rates of colonoscopy screening for patients invited to colonoscopy outreach compared with usual care, but higher rates of screening when FIT was offered.
Impact of mailed invitation to gFOBT among underserved populations has also been studied in randomized trials.23–25 All included mailed outreach to complete an enclosed gFOBT kit with screening information. Increases in screening over usual care of 8% to 29% were observed. The 2 studies that included mailed gFOBT arms with and without telephone outreach found higher rates of screening associated with the addition of telephone outreach.24,25 Our study confirms the effectiveness of mailed outreach with gFOBTs and extends these prior findings by demonstrating that participation using this approach is substantially higher than with outreach invitations to complete colonoscopy.
For underserved populations, our findings raise the possibility that large-scale public health efforts to boost screening may be more successful if noninvasive tests, such as FIT, are offered over colonoscopy. Modeling and cost-effectiveness studies of the relative effectiveness of FIT vs colonoscopy for preventing CRC mortality hinge on 2 factors: participation in initial and repeated FIT and colonoscopy quality— particularly its ability to reduce incidence and mortality from right-sided CRC.5,31 Our study focuses on comparative participation in outreach with FIT vs colonoscopy, which has been understudied overall and to our knowledge has not been reported among underserved populations in the United States. Studies comparing outreach with FIT vs colonoscopy from Spain, Italy, and Australia have reported screening rates of 27.4% to 34.2% for FIT and 17.8% to 26.5% for colonoscopy.28–30 While these studies are similar to ours in demonstrating higher FIT vs colonoscopy participation, differences in study populations due to geography preclude direct comparison to our results. Moreover, studies by Quintero et al28 and Segnan et al,29 FITs were distributed after interested patients attended a clinic visit in response to mailed invitations, and thus the intervention did not take advantage of the potential for increased participation associated with including FIT kits with mailed invitations. Nonetheless, together with our findings, these results suggest that substantial differences in test-specific participation rates exist.28–30 These differences may be clinically significant because modeling studies suggest that if test-participation rates for FIT are substantially superior to those for colonoscopy, population FIT screening could result in equivalent or higher mortality benefit with lower colonoscopy utilization.5,9
Several limitations may be considered when interpreting our results. Results reflect screening participation after 1 round of invitation. It is possible that repeated outreach invitations could lead to higher colonoscopy completion and adenoma detection rates, ultimately resulting in superior comparative effectiveness of colonoscopy vs FIT outreach over time. Alternatively, if repeated FIT participation is substantial, and colonoscopy participation remains substantially lower than for FIT, organized FIT invitation may remain a superior strategy. Additionally, 18% of patients receiving an abnormal FIT result did not complete colonoscopy. This could also affect long-term impact of FIT vs colonoscopy outreach. These concerns can be addressed by future research, including a separate trial that our research group is conducting comparing FIT outreach with colonoscopy outreach over 3 rounds of annual invitation.
Our study was not designed to allow for meaningful statistical comparisons of neoplasia outcomes. Therefore, caution should be taken in interpreting any differences in rates of neoplasia detection across groups, though our rates of neoplasia among patients completing FIT and colonoscopy appear qualitatively similar to those reported in prior studies.28,29 We could not assess differences in CRC mortality. In the United States, this is the subject of a Veterans Affairs Cooperative Group Study expected to enroll 50 000 veterans and require over 12 years of follow-up (clinicaltrials.gov NCT01239082). It should be noted, however, that in the Veterans Affairs trial, randomization occurs after trial consent; it is possible that test-specific differences in screening participation may be smaller among patients who have already consented to be a part of a screening trial.
Although screening might have occurred outside of the health system leading to undercounting of our primary outcome, any such screening would be expected to be non-differential across the randomly assigned groups.
All patients were uninsured participants in this health system’s medical assistance program for the underserved, which only provides for health care within the health system. We excluded patients not seen by the health system within the 8-month period prior to randomization. Screening was offered free of charge. These issues could limit generalizability, though implementation of the 2010 Affordable Care Act in the United States requires provision of screening without cost-sharing and will increase access to primary care for underserved populations similar to those cared for by the health system under study.32,33
We did not include an intervention arm with choice of FIT or colonoscopy; offering choice may have led to even higher rates of screening and warrants future study.8,34 Delivering organized screening required nearly full-time efforts of a medical assistant and a nurse. Whether these efforts can be resourced and implemented sustainably requires further study. We did not include a cost-effectiveness analysis as part of the current report. Such analyses are important for understanding impact of FIT- vs colonoscopy-based population screening strategies and are ongoing by our research group. We used a combined intervention consisting of mailed outreach invitations to free screening with telephone reminders and thus did not investigate the individual effect of each intervention component. However, at least for mailed invitations and telephone reminders, prior work has consistently shown greater increases in screening participation with both components combined.22,24,25
These limitations are balanced by several study strengths, including a large sample size, enrollment of a diverse population, and use of a waiver of informed consent to avoid selection/volunteer bias.
In conclusion, we found that organized outreach, including mailed invitation and telephone follow-up, was highly effective for boosting CRC screening among the underserved. Furthermore, outreach was almost twice as effective when FIT rather than colonoscopy was offered. Organized strategies for boosting screening among the underserved, particularly with offers to complete noninvasive tests such as FIT, merit further study, including analyses of cost, long-term effectiveness, and implementation.
Supplementary Material
Acknowledgments
Funding/Support: Funding for this research was provided in part by the Cancer Prevention and Research Institute of Texas, grant PP100039 (Dr Gupta); the National Institutes of Health (NIH), grant 1 KL2 RR024983-01, through the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research; and NIH/National Cancer Institute grant 1U54CA163308-01 (Dr Skinner).
Role of the Sponsors: The funding sources had no role in the design, execution, analysis, stopping decision, interpretation, or reporting of this study.
Footnotes
Supplemental content at jamainternalmedicine.com
Author Contributions: Dr Gupta had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Study concept and design: Gupta, Rockey, Carter, Ahn, Kashner, Argenbright, Sugg Skinner. Acquisition of data:Gupta, Hammons, Koch, Valdez.
Analysis and interpretation of data: Gupta, Halm, Rockey, Hammons, Koch, Carter, Valdez, Tong, Ahn, Kashner, Argenbright, Tiro, Geng, Pruitt, Sugg Skinner.
Drafting of the manuscript: Gupta, Rockey.
Critical revision of the manuscript for important intellectual content: Gupta, Halm, Rockey, Hammons, Koch, Carter, Valdez, Tong, Ahn, Kashner, Argenbright, Tiro, Geng, Pruitt, Sugg Skinner.
Statistical analysis: Tong, Ahn, Kashner. Obtained funding: Gupta, Rockey, Sugg Skinner.
Administrative, technical, or material support: Gupta, Rockey, Hammons, Koch, Carter, Valdez, Argenbright, Geng, Sugg Skinner.
Study supervision: Gupta, Halm, Rockey.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We would like to thank the staff at JPS and Moncrief Cancer Institute for invaluable assistance with organizing and delivering outreach activities. Specific thanks to Rohan Clark, MD, Mark Koch, MD, Shilpa Madadi, MD, and Sangameshwar Reddy, MD for performing program-related colonoscopies, and Maria Asprilla, MD, for assisting with precolonoscopy visit assessments. We also thank Adam Loewen, for providing and developing the study and tracking database; Sue Crabtree, for assistance with acquiring administrative data for analysis; Helen Landico, and the Polymedco Corporation for providing FIT kits for distribution and materials for FIT processing; Josephine Fowler, MD, and Karshena Valsin, at the JPS Institutional Review Board for assistance with study review and management.
Additional Information: The full trial protocol is available on request.
References
- 1.US Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58(2):241–248. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 7.Walsh JM, Terdiman JP. Colorectal cancer screening: clinical applications. JAMA. 2003;289(10):1297–1302. doi: 10.1001/jama.289.10.1297. [DOI] [PubMed] [Google Scholar]
- 8.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S. Will test-specific adherence predict the best colorectal cancer screening strategy? Ann Intern Med. 2009;150(5):359–360. doi: 10.7326/0003-4819-150-5-200903030-00019. [DOI] [PubMed] [Google Scholar]
- 10.Inadomi JM. Taishotoyama Symposium: barriers to colorectal cancer screening: economics, capacity and adherence. J Gastroenterol Hepatol. 2008;23(Suppl 2):S198–S204. doi: 10.1111/j.1440-1746.2008.05556.x. [DOI] [PubMed] [Google Scholar]
- 11.Lewin ME, Altman S. America’s Health Care Safety Net: Intact but Endangered. Washington, DC: National Academy Press; 2000. Committee on the Changing Market, Managed Care, and the Future Viability of Safety Net Providers. [Google Scholar]
- 12.Lewin ME, Baxter RJ. America’s health care safety net: revisiting the 2000 IOM report. Health Aff (Millwood) 2007;26(5):1490–1494. doi: 10.1377/hlthaff.26.5.1490. [DOI] [PubMed] [Google Scholar]
- 13.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33(1):101–110. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
- 14.Green AR, Peters-Lewis A, Percac-Lima S, et al. Barriers to screening colonoscopy for low-income Latino and white patients in an urban community health center. J Gen Intern Med. 2008;23(6):834–840. doi: 10.1007/s11606-008-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jo AM, Maxwell AE, Wong WK, Bastani R. Colorectal cancer screening among underserved Korean Americans in Los Angeles County. doi: 10.1007/s10903-007-9066-6. http://link.springer.com/article/10.1007/s10903-007-9066-6. Accessed July 3, 2013. [DOI] [PMC free article] [PubMed]
- 16.Levy BT, Nordin T, Sinift S, Rosenbaum M, James PA. Why hasn’t this patient been screened for colon cancer? an Iowa Research Network study. J Am Board Fam Med. 2007;20(5):458–468. doi: 10.3122/jabfm.2007.05.070058. [DOI] [PubMed] [Google Scholar]
- 17.Yarnall KS, Pollak KI, Østbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinwachs D, Allen JD, Barlow WE, et al. NIH state-of-the-science conference statement: enhancing use and quality of colorectal cancer screening. NIH Consens State Sci Statements. 2010;27(1):1–31. [PubMed] [Google Scholar]
- 19.Levy BT, Daly JM, Xu Y, Ely JW. Mailed fecal immunochemical tests plus educational materials to improve colon cancer screening rates in Iowa Research Network (IRENE) practices. J Am Board Fam Med. 2012;25(1):73–82. doi: 10.3122/jabfm.2012.01.110055. [DOI] [PubMed] [Google Scholar]
- 20.Daly JM, Levy BT, Merchant ML, Wilbur J. Mailed fecal-immunochemical test for colon cancer screening. J Community Health. 2010;35(3):235–239. doi: 10.1007/s10900-010-9227-8. [DOI] [PubMed] [Google Scholar]
- 21.Church TR, Yeazel MW, Jones RM, et al. A randomized trial of direct mailing of fecal occult blood tests to increase colorectal cancer screening. J Natl Cancer Inst. 2004;96(10):770–780. doi: 10.1093/jnci/djh134. [DOI] [PubMed] [Google Scholar]
- 22.Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110(9):2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 23.Jean-Jacques M, Kaleba EO, Gatta JL, Gracia G, Ryan ER, Choucair BN. Program to improve colorectal cancer screening in a low-income, racially diverse population: a randomized controlled trial. Ann Fam Med. 2012;10(5):412–417. doi: 10.1370/afm.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coronado GD, Golovaty I, Longton G, Levy L, Jimenez R. Effectiveness of a clinic-based colorectal cancer screening promotion program for underserved Hispanics. Cancer. 2011;117(8):1745–1754. doi: 10.1002/cncr.25730. [DOI] [PubMed] [Google Scholar]
- 25.Walsh JM, Salazar R, Nguyen TT, et al. Healthy colon, healthy life: a novel colorectal cancer screening intervention. Am J Prev Med. 2010;39(1):1–14. doi: 10.1016/j.amepre.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasser KE, Murillo J, Lisboa S, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med. 2011;171(10):906–912. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 27.Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24(2):211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quintero E, Castells A, Bujanda L, et al. COLONPREV Study Investigators Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366(8):697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 29.Segnan N, Senore C, Andreoni B, et al. SCORE3 Working Group-Italy Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology. 2007;132(7):2304–2312. doi: 10.1053/j.gastro.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 30.Multicentre Australian Colorectal-neoplasia Screening (MACS) Group. A comparison of colorectal neoplasia screening tests: a multicentre community-based study of the impact of consumer choice. Med J Aust. 2006;184(11):546–550. doi: 10.5694/j.1326-5377.2006.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 31.Sharaf RN, Ladabaum U. Comparative effectiveness and cost-effectiveness of screening colonoscopy vs. sigmoidoscopy and alternative strategies. Am J Gastroenterol. 2013;108(1):120–132. doi: 10.1038/ajg.2012.380. [DOI] [PubMed] [Google Scholar]
- 32.HR 3590: Patient Protection and Affordable Care Act, 2009. http://thomas.loc.gov/cgi-bin/query/z?c111:H.R.3590. Accessed April 27, 2010, 2010.
- 33.Cassidy A. Health policy brief: preventive services without cost sharing. http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=37. Accessed July 3, 2013.
- 34.Kempe KL, Shetterly SM, France EK, Levin TR. Automated phone and mail population outreach to promote colorectal cancer screening. Am J Manag Care. 2012;18(7):370–378. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


