ABSTRACT
The widespread dissemination of carbapenem-resistant Acinetobacter spp. has created significant therapeutic challenges. At present, rapid molecular diagnostics (RMDs) that can identify this phenotype are not commercially available. Two RMD platforms, PCR combined with electrospray ionization mass spectrometry (PCR/ESI-MS) and molecular beacons (MB), for detecting genes conferring resistance/susceptibility to carbapenems in Acinetobacter spp. were evaluated. An archived collection of 200 clinical Acinetobacter sp. isolates was tested. Predictive values for susceptibility and resistance were estimated as a function of susceptibility prevalence and were based on the absence or presence of beta-lactamase (bla) NDM, VIM, IMP, KPC, and OXA carbapenemase genes (e.g., blaOXA-23, blaOXA-24/40, and blaOXA-58 found in this study) against the reference standard of MIC determinations. According to the interpretation of MICs, 49% (n = 98) of the isolates were carbapenem resistant (as defined by either resistance or intermediate resistance to imipenem). The susceptibility sensitivities (95% confidence interval [CI]) for imipenem were 82% (74%, 89%) and 92% (85%, 97%) for PCR/ESI-MS and MB, respectively. Resistance sensitivities (95% CI) for imipenem were 95% (88%, 98%) and 88% (80%, 94%) for PCR/ESI-MS and MB, respectively. PRIMERS III establishes that RMDs can discriminate between carbapenem resistance and susceptibility in Acinetobacter spp. In the context of a known prevalence of resistance, SPVs and RPVs can inform clinicians regarding the best choice for empiric antimicrobial therapy against this multidrug-resistant pathogen.
KEYWORDS: Acinetobacter, beta-lactams, carbapenemases
INTRODUCTION
Resistance to antibiotics is a major public health threat, and rapid diagnostic platforms are needed to assist clinicians in choosing effective empiric therapy. In the platforms for rapid identification of multidrug-resistant gram-negative bacteria and evaluation of resistance studies (PRIMERS) I and II, analytical strategies were developed and tested to evaluate whether genotypic results obtained by nucleic acid amplification technologies could identify susceptibility and resistance to beta-lactam antibiotics using a carefully chosen panel of susceptible and highly beta-lactam-resistant Enterobacteriaceae (1). The rapid molecular diagnostic (RMD) platforms that formed the testing basis for that investigation were (i) PCR coupled with electrospray ionization mass spectrometry (PCR/ESI-MS); (ii) molecular beacons (MB); (iii) a DNA microarray kit; and (iv) a next-generation sequencing platform.
In PRIMERS I and II, we showed that RMD platforms could help inform empiric beta-lactam therapy against Escherichia coli and Klebsiella pneumoniae. Moreover, our efforts demonstrated that it was possible to transform beta-lactam resistance genotypic data into a practical decision-making tool, which may be useful to clinicians when the prevalence of resistance for a given population is applied.
The next challenge is whether RMD platforms and analytical strategies can be employed against other Gram-negative multidrug-resistant (MDR) pathogens. Acinetobacter spp. are proving to be among the most problematic pathogens facing contemporary clinicians (2). This nefarious status is attributed to difficulties in identifying Acinetobacter spp. to the species level, the increasing panoply of resistance phenotypes that confound treatment decisions, and an emerging understanding of their virulence properties (3, 4). Despite the insights obtained using whole-genome sequencing, vexing questions remain regarding the nosology of syndromes caused by Acinetobacter spp., including the choice of optimal initial and definitive therapies.
Regarding the correct identification of Acinetobacter spp., multiple commercially available microbiological and RMD platforms exist that try to identify species within the genus. Proper species identification is important, as relevant differences exist between the species with regard to treatment decisions, epidemiology, immunogenicity, and most importantly, resistance profiles (e.g., carbapenem resistant [CR] or susceptible [CS]; Acinetobacter baumannii versus Acinetobacter pittii). Currently, clinicians may place more weight on the CR and CS designation than correct species identification, but this should change.
Due to rising antimicrobial resistance, carbapenems are the cornerstone of therapy for the treatment of serious infections due to Acinetobacter spp. Unfortunately, the widespread dissemination of metallo-beta-lactamases (MBLs) and particularly the OXA carbapenemases has created significant therapeutic challenges. In the United States each year, ∼12,000 cases of MDR Acinetobacter infections occur and are associated with at least 500 deaths (http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf#page=59). More than half of these isolates are CR, and the number is growing in other parts of the world. Knowing when to use, or not to use, alternative therapies (colistin, polymyxin B, tigecycline, etc.) is critical to patient care and can be lifesaving.
In an attempt to address this dilemma, we employed two RMDs (PCR/ESI-MS and MB) to determine whether the identification of specific genotypes can accurately predict antimicrobial susceptibility (i.e., presence of blaOXA-23, blaOXA-24/40, blaOXA-58, blaNDM, blaKPC, blaVIM, and blaIMP, predicting carbapenem resistance). The unique challenge in choosing to study Acinetobacter spp. with these two platforms rests upon the observation that multiple resistance determinants can result in a CR phenotype (e.g., OXA carbapenemases, metallo-beta-lactamases, KPCs, etc.). Previously, we found that results from PCR/ESI-MS and MB are very informative, and RMDs can contribute significantly to the decision to use empiric carbapenem therapy among Enterobacteriaceae. The current study, PRIMERS III, significantly adds to the knowledge obtained from PRIMERS I and II and further establishes the interpretative power of RMDs coupled with the application of unique analytical methods (1). By detecting specific bla genes conferring resistance to carbapenems, clinicians can have confidence in choosing alternative empiric therapies (colistin, tigecycline, etc.) in ∼90% of cases.
RESULTS
Antimicrobial susceptibility testing (AST) and genetic analysis.
In Table 1 we summarized the MICs determined for the 200 Acinetobacter sp. isolates. In this collection of isolates, >90% of Acinetobacter spp. that were CS were also susceptible to ampicillin-sulbactam, amikacin, minocycline, colistin, polymyxin B, and tigecycline; 80 to 89% were susceptible to ciprofloxacin, piperacillin-tazobactam, gentamicin, cefepime, ceftazidime, and tetracycline. When faced with the CS phenotype, most clinicians would favor the use of ampicillin-sulbactam over that of colistin or polymyxin B due to a lower risk of renal toxicity (5).
TABLE 1.
Susceptibility of CS and CR to antimicrobial agents
| Antimicrobial agent (susceptible breakpoint, mg/liter) | % Susceptibilitya (no. susceptible/total no.) |
|
|---|---|---|
| CR (n = 98) | CS (n = 102) | |
| Imipenem (≤4) | 0 (0/98) | 100 (102/102) |
| Doripenem (≤2) | 0 (0/98) | 97.1 (99/102) |
| Meropenem (≤4) | 0 (0/98) | 98.0 (100/102) |
| Ciprofloxacin (≤1) | 0 (0/98) | 81.4 (83/102) |
| Piperacillin-tazobactam (≤16/4) | 1.0 (1/98) | 85.3 (87/102) |
| Gentamicin (≤4) | 2.0 (2/98) | 83.3 (85/102) |
| Cefepime (≤8) | 5.1 (5/98) | 86.3 (88/102) |
| Ceftazidime (≤8) | 6.1 (6/98) | 88.2 (90/102) |
| Tetracycline (≤4) | 10.2 (10/98) | 82.4 (84/102) |
| Ampicillin-sulbactam (≤8/4) | 19.4 (19/98) | 94.1 (96/102) |
| Amikacin (≤16) | 19.4 (19/98) | 94.1 (96/102) |
| Minocycline (≤4) | 37.8 (37/98) | 96.1 (98/102) |
| Colistin (≤2) | 89.8 (88/98) | 96.1 (98/102) |
| Polymyxin B (≤2) | 89.8 (88/98) | 98.0 (100/102) |
| Tigecycline (≤2) | 97.0 (95/98) | 100 (102/102) |
CR, carbapenem resistant; CS, carbapenem susceptible.
In Table 2, the CR patterns of the 200 isolates are detailed. By AST, 98 isolates were resistant to imipenem (MIC of >4 mg/liter), 100 were resistant to meropenem (MIC of >4 mg/liter), and 101 were resistant to doripenem (MIC of >2 mg/liter). Acinetobacter spp. are intrinsically resistant to ertapenem, so this carbapenem was not included in the analysis.
TABLE 2.
Carbapenem phenotypic profile of 200 isolates studied
| No. of isolates | Susceptibility toa: |
||
|---|---|---|---|
| Doripenem | Imipenem | Meropenem | |
| 98 | S | S | S |
| 1 | S | S | R |
| 2 | R | S | S |
| 1 | R | S | R |
| 98 | R | R | R |
| Total | 99 S, 101 R | 102 S, 98 R | 100 S, 100 R |
S, susceptible; R, resistant.
Notably, 98 isolates were resistant to all three carbapenems that are employed in treatment (doripenem, imipenem, and meropenem). From this comparison, we chose imipenem as the reference compound; therefore, we included 98 CR isolates in this analysis. We note that differences in the actual number that are susceptible or resistant may be due to (i) the ability of different carbapenems to penetrate the outer membrane of Acinetobacter spp. and (ii) the activities/potencies of each carbapenem versus the carbapenemase harbored by the strain.
In Table 1, we also summarize the phenotypic profile of CR Acinetobacter spp.; clearly these isolates are very drug resistant. The only agents with notable activity against CR strains of Acinetobacter spp. were colistin (89.8% susceptible), polymyxin B (89.8% susceptible), and tigecycline (97.0% susceptible). Ampicillin-sulbactam (19.4% susceptible), amikacin (19.4% susceptible), and minocycline (37.8% susceptible) had activity against some but not all CR Acinetobacter sp. isolates. In particular, the low level of susceptibility of this CR collection to minocycline was different from previous reports suggesting susceptibility rates of ∼60% (6). Further investigations are in progress to determine the genetic basis of this observation.
Table 3 summarizes the number and identity of carbapenemase genes that were detected. It is important to keep in mind that all A. baumannii isolates possess a naturally occurring oxacillinase (blaOXA-51-like) gene, which can affect CS. However, CR is dependent upon its level of expression and the gene variant (7, 8). The predominant genes present in our analysis that are recognized to produce a CR phenotype were blaOXA-23 and blaOXA-24/40. MBLs (blaNDM, blaVIM, and blaIMP) were absent from this collection, and at this time it is uncommon in the United States to detect MBLs in Acinetobacter spp.
TABLE 3.
Genotypic profile of 200 isolates studied via PCR ESI-MS/MB
| No. of isolatesa | blaOXA-23 | blaOXA-24/40 | blaOXA-58 |
|---|---|---|---|
| 89/106 | − | − | − |
| 79/82 | + | − | − |
| 24/9 | − | + | − |
| 3/1 | − | − | + |
| 5/2 | + | + | − |
Isolate count for PCR ESI-MS/MB.
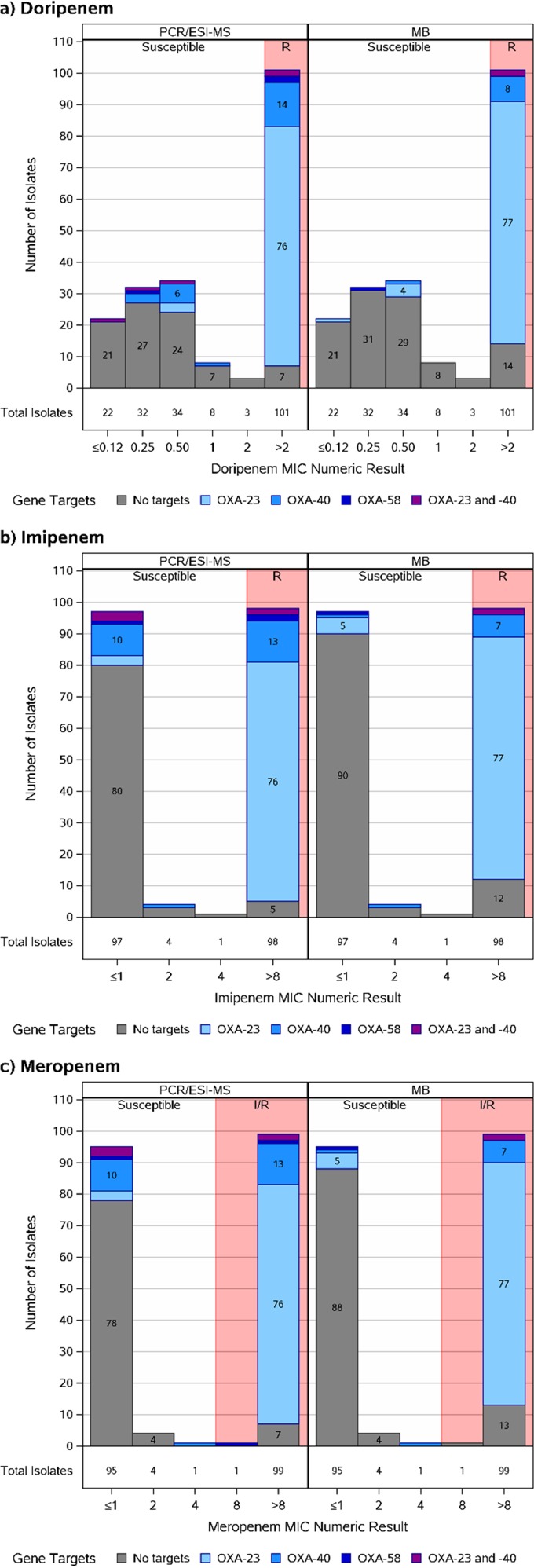
Figure 1 summarizes the distribution of carbapenem MICs versus target (gene) identification. Both platforms identify target genes in Acinetobacter sp. strains within the susceptible and resistant ranges. However, most target gene identification occurred within the resistant Acinetobacter sp. population. Interestingly, very few isolates that demonstrate MICs near the breakpoint possessed bla carbapenemase genes.
FIG 1.
Distribution of Acinetobacter sp. carbapenem MICs versus target (gene) identification for PCR/ESI-MS and MB platforms. All gene targets were examined; only identified targets are presented. Abbreviations: I/R, intermediate/resistant; R, resistant.
Discrimination summaries and predictive values.
Analyses presented in this section were conducted before discrepancy resolution. A phenotypic/genotypic isolate discrimination summary is provided in Table 4 as a cross-classification of the PCR/ESI-MS and MB results with the MIC results for the 200 isolates. For imipenem, the susceptibility and resistance sensitivities were 0.82 (95% confidence intervals [CI], 0.74, 0.89) and 0.95 (CI, 0.88, 0.98), respectively, for PCR/ESI-MS and were 0.92 (CI, 0.85, 0.97) and 0.88 (CI, 0.80, 0.94), respectively, for MB. Results for meropenem and doripenem were similar (Table 5 and Fig. 2).
TABLE 4.
Phenotypic/genotypic isolate discrimination summary
| Test | No. of isolates resistant or susceptible toa: |
|||||
|---|---|---|---|---|---|---|
| Imipenem |
Meropenem |
Doripenem |
||||
| Resistant | Susceptible | Resistant | Susceptible | Resistant | Susceptible | |
| Total (MIC) | 98 | 102 | 100 | 100 | 101 | 99 |
| PCR ESI/MS | ||||||
| Positive | 93 | 18* | 93 | 18* | 94 | 17* |
| Negative | 5** | 84 | 7** | 82 | 7** | 82 |
| MB | ||||||
| Positive | 86 | 8* | 86 | 8* | 87 | 7* |
| Negative | 12** | 94 | 14** | 92 | 14** | 92 |
*, Overtreatment; **, undertreatment; no asterisk, appropriate treatment.
TABLE 5.
Sensitivities and predictive value summary
| Parameter and antimicrobial agent (n) | Value determined byb: |
|
|---|---|---|
| PCR/ESI-MS | MB | |
| Susceptibility sensitivities | ||
| Doripenem (99) | 0.83 (0.74, 0.90) | 0.93 (0.86, 0.97) |
| Imipenem (102) | 0.82 (0.74, 0.89) | 0.92 (0.85, 0.97) |
| Meropenem (100) | 0.82 (0.73, 0.89) | 0.92 (0.85, 0.96) |
| Resistance sensitivities | ||
| Doripenem (101) | 0.93 (0.86, 0.97) | 0.86 (0.78, 0.92) |
| Imipenem (98) | 0.95 (0.88, 0.98) | 0.88 (0.80, 0.94) |
| Meropenem (100) | 0.93 (0.86, 0.97) | 0.86 (0.78, 0.92) |
| Susceptibility predictive valuesa | ||
| Doripenem | 0.89 (0.82, 0.96) | 0.82 (0.74, 0.89) |
| Imipenem | 0.91 (0.85, 0.98) | 0.83 (0.76, 0.91) |
| Meropenem | 0.89 (0.81, 0.96) | 0.81 (0.74, 0.89) |
| Resistance predictive valuesa | ||
| Doripenem | 0.89 (0.85, 0.93) | 0.95 (0.91, 0.98) |
| Imipenem | 0.89 (0.85, 0.93) | 0.94 (0.91, 0.98) |
| Meropenem | 0.89 (0.84, 0.93) | 0.94 (0.90, 0.98) |
Assuming 40% susceptibility.
Estimates with 95% confidence intervals are presented.
FIG 2.
Estimates of the susceptibility and resistance sensitivities displayed using discrimination summary plots. Results are presented with 95% confidence intervals for PCR/ESI-MS and MB.
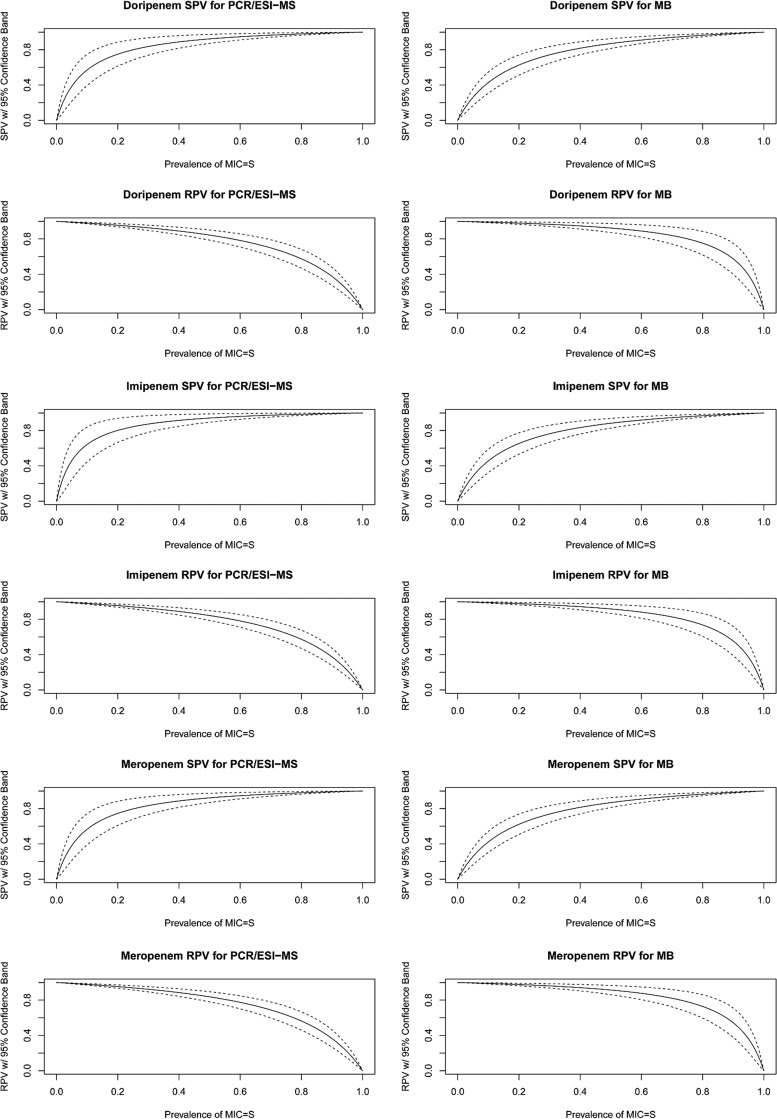
SPVs and RPVs are displayed as a function of the prevalence of susceptibility (Fig. 3). Assuming 40% national imipenem susceptibility (60% resistance), the SPVs were 91% (CI, 85%, 98%) and 83% (CI, 76%, 91%) for PCR/ESI-MS and MB, respectively, while RPVs were 89% (CI, 85%, 93%) and 94% (CI, 91%, 98%) for PCR/ESI-MS and MB, respectively. Results for meropenem and doripenem were similar.
FIG 3.
Susceptibility predictive value (SPV) and resistance predictive value (RPV) plots with 95% confidence bands by drug and platform.
Discrepancy analyses.
Discrepancies were observed due to (i) differences between platform results on a genotypic level; (ii) differences between genotype and predicted CR phenotype; and (iii) differences due to non-baumannii Acinetobacter species identification on the PCR/ESI-MS platform. To resolve these issues, discrepant analysis was performed on 63 isolates using a carbapenemase multiplex PCR as a third identification method, and the results are reported in Table 6. In this manner, we evaluated for false-positive or false-negative genotypes, inconsistencies in CR, and identification of non-A. baumannii species. We found that the number of false positives was higher using PCR/ESI-MS than MB. In contrast, false negatives were more common when testing with MB.
TABLE 6.
Discrepancy resolution
| Carbapenemase gene | No. detected by: |
No. of positive results after discrepancy resolution via carbapenemase multiplex PCRa | |
|---|---|---|---|
| MB | PCR/ESI-MS | ||
| blaOXA-24/40 | 11 (1 false positive, 4 false negatives) | 29 (15 false positives) | 14 |
| blaOXA-23 | 84 (8 false positives, 3 false negatives) | 84 (7 false positives, 2 false negatives) | 79 |
| blaOXA-58 | 1 (1 false positive, 2 false negatives) | 3 (1 false positive) | 2 |
| blaIMP | 0 | 0 | 0 |
| blaKPC | 0 | 0 | 0 |
| blaNDM | 0 | 0 | 0 |
| blaVIM | 0 | 0 | 0 |
Results are from the OXA carbapenemase multiplex PCR on crude lysates to resolve observed discrepancies due to differences between platform results on a genotypic level, differences between genotype and predicted CR phenotype, and examining those isolates in which a non-baumannii species identification was obtained on the PCR/ESI-MS platform. An independent third method, the carbapenemase multiplex PCR, was used to evaluate false-positive or false-negative genotypes and inconsistencies in CR.
DISCUSSION
CR Acinetobacter sp. infections are among the most challenging to treat, as clinicians are often limited to the use of polymyxins and tigecycline as the only effective therapies. Delaying the use of effective therapy against CR Acinetobacter spp. may lead to poor outcomes, whereas the unnecessary use of polymyxin and tigecycline may lead to adverse events associated with these drugs. In PRIMERS I and II, we developed an approach to inform clinicians on how to interpret RMDs that detect beta-lactam resistance in E. coli and K. pneumoniae in areas with different levels of prevalence. In the PRIMERS III study, our goal was to develop a similar approach to guide the best empiric therapy for Acinetobacter sp. infection, as clear guidance in this area is limited.
Using a collection of isolates for which a significant portion was CR, mirroring current Centers for Disease Control and Prevention and World Health Organization estimates of CR prevalence in various areas, MB and PCR/ESI-MS discriminated between CS and CR, thus demonstrating their potential to inform empiric antimicrobial therapy against Acinetobacter spp. In these analyses, clinicians can be confident >85% of the time, using either of these two platforms, that results indicating susceptibility or resistance based on gene detection are accurate, thus contributing to better initial antibiotic treatment decisions regarding Acinetobacter infections. Considering the epidemiological data present in the United States and worldwide, this improvement in clinical decision-making can have profound positive consequences on patient care. Simply put, these methods can be a helpful tool for clinicians with regard to treatment decision-making.
However, RMDs are not perfect. In a setting where the level of CR Acinetobacter spp. is low, there is an increased likelihood for the RMD to inaccurately identify resistance (Fig. 3). This could lead to overprescribing with an associated increase in cost. However, the monetary cost in that circumstance is not great, and clinicians feel it is better to overtreat than undertreat where there is uncertainty that can lead to incorrect treatment decisions. The setting in which rapid identification of resistance determinants would have the greatest impact is in an area of high prevalence of CR Acinetobacter spp. with access to a laboratory that operates continuously.
The complexity of the CR phenotype (e.g., CarO mutations, efflux pumps, uncharacterized OXAs) may help explain misclassified isolates. We found using this heterogeneous collection that the presence of porins, efflux pumps, or different bla genes not included in our selection can confound the interpretation of results. Fortunately, this imprecision is still relatively minor.
We also showed that after CR strains are identified using RMDs, tigecycline and polymyxins (colistin) can be used for effective empiric therapy in ∼90% of cases. Minocycline is the only orally available agent to be considered, but resistance rates were surprisingly high (62%) in this collection. The extremely high rates of amikacin, ampicillin-sulbactam, and gentamicin resistance also merit further molecular analysis. These data highlight the major clinical challenge posed by CR Acinetobacter spp., as isolates are frequently resistant to all agents used to treat CS Acinetobacter spp.
In conclusion, we show that the groundwork established in PRIMERS I and II can be extended to another Gram-negative MDR pathogen, Acinetobacter spp. Compared with conventional susceptibility testing done in clinical microbiology laboratories, RMD platforms that can identify blaOXA carbapenemase genes can have a significant impact on the empiric decision to use specific agents. Our analysis also demonstrates that if CR is found, clinicians can use colistin, polymyxin B, or tigecycline in 85 to 90% of cases and choose a correct empiric treatment provided the prevalence of resistance to those agents is low. However, we cannot say this will result in better outcomes. We also cannot address the complexity of single versus combination chemotherapy. Considerations such as these require more detailed analytical studies on a larger number of isolates with diverse phenotypes.
Unfortunately, as Tables 4 and 6 show, these analyses and RMDs have limitations. First, these analyses assume knowledge of the Acinetobacter spp. Many laboratories still misidentify the species of Acinetobacter. Second, the consequences of false-positive/-negative results loom large, as we have discussed above and in PRIMERS I and II (1). In this testing exercise, PCR/ESI-MS showed a high false-positive rate, particularly for blaOXA-24/40. Therapy that is targeted and effectively addresses the resistance pattern in certain cases is preferable to incorrect therapy, which can result in treatment failure and mortality. Further refinement is needed to help place this approach in the appropriate clinical context. Nevertheless, our results are encouraging and point to the successful introduction of RMDs in clinical practice for the correct diagnosis, effective treatment, and antibiotic stewardship of infections caused by MDR Gram-negative bacteria.
MATERIALS AND METHODS
Antimicrobial susceptibility testing (AST) and isolate selection.
The MICs for each strain were used as a gold standard to define susceptibility or resistance for each beta-lactam antibiotic and other antimicrobial drugs. We assembled a panel of 102 CS and 98 CR Acinetobacter spp. from locations worldwide (9–11). We assessed susceptibility to the following antibiotics by broth microdilution: amikacin, gentamicin, tobramycin, ticarcillin-clavulanate, piperacillin-tazobactam, aztreonam, cefepime, ceftazidime, ciprofloxacin, levofloxacin, imipenem, meropenem, doripenem, tetracycline, minocycline, tigecycline, colistin, polymyxin B, and trimethoprim-sulfamethoxazole using Sensititre GNX2F trays (Thermo Fisher Scientific, Oakwood Village, OH) and ampicillin-sulbactam using MicroScan Neg BP combo panel type 34 trays (Beckman Coulter Inc., Brea, CA). Results were interpreted according to the 2014 Clinical and Laboratory Standards Institute (CLSI) guidelines (12). American Type Culture Collection (ATCC) control strains Pseudomonas aeruginosa 27853 and Escherichia coli 25922 were used as quality controls. Breakpoints for Enterobacteriaceae or P. aeruginosa were used when they were not available for Acinetobacter spp. Intermediate interpretations were considered resistant for analytical purposes.
Analysis of bla genes using RMD platforms.
As the number of carbapenemase genes is quite large in Acinetobacter spp., we focused upon the bla genes that are most relevant and prevalent in survey studies worldwide: blaOXA-23, blaOXA-24/40, blaOXA-58, blaNDM, blaKPC, blaVIM, and blaIMP.
PCR/ESI-MS and MB were used to evaluate isolates of Acinetobacter spp. for the presence or absence of the genetic targets (carbapenemase genes) that are known to be associated with CR, as previously described (13, 14). In brief, PCR/ESI-MS is a nucleic acid amplification technology that targets select genes using “smart primers,” determines their exact mass, and then uses algorithms to define the target gene identified (1). The PCR/ESI-MS platform also provides genus- and species-level identification. MBs are fluorescently labeled oligonucleotide hybridization probes that can report the presence of specific nucleic acid targets in heterogeneous solutions, as previously described (1). The platform results were compared with AST results for each carbapenem. The RMD result was considered resistant when any of the targeted genes were detected; the RMD result was considered susceptible when none of the targets were detected.
OXA carbapenemase multiplex PCR for discrepancy resolution.
We applied PCR methods to resolve various discrepancies that arose within the experiments. These discrepancies came about due to (i) differences between platform results, on a genotypic level, (ii) differences between genotype and predicted CR phenotype, and (iii) differences due to non-baumannii Acinetobacter species identification on the PCR/ESI-MS platform (see Table S1 in the supplemental material).
To resolve discrepancies resulting from i and ii above, an OXA carbapenemase multiplex PCR was performed on crude lysates to identify the OXA carbapenemases present (as a third method for discrepancy resolution). This multiplex PCR assay is able to detect multiple OXA carbapenemase genes on the basis of differential PCR product sizes: blaOXA-143 (150 bp), blaOXA-24/40 (264 bp), blaOXA-51 (353 bp), blaOXA-23 (500 bp), blaOXA-58 (600 bp), and blaOXA-235 (700 bp) (15–17). To resolve discrepancies resulting from iii above, select primers were used for more accurate species identification (18–21).
Statistical methods.
Discrimination summary plots were used to display the 95% CI estimates of susceptibility sensitivity, defined as the probability that the platform result is susceptible when the MIC result is susceptible, and of resistance sensitivity, defined as the probability that the platform result is resistant when the MIC result is resistant (1). The primary analysis of the data presented in the manuscript was done prior to discrepancy resolution.
The susceptibility predictive value (SPV) is the probability that a MIC result would indicate susceptibility when the platform result indicates susceptibility, and the resistance predictive value (RPV) is the probability that a MIC result would indicate resistance when the platform result indicates resistance. The SPV and RPV are also functions of the prevalence of susceptibility. Since there are temporal and geographic variations in the prevalence of susceptibility of Acinetobacter spp. to carbapenems, the SPV and RPV were plotted as a function of the prevalence of susceptibility (with 95% confidence bands) to allow for interpretation across the spectrum of prevalence.
The sample size of 200 isolates was chosen based on estimating susceptibility and resistance sensitivities with desirable precision. Roughly half of the isolates were expected to be susceptible and half resistant and thus available for estimating susceptibility/resistance sensitivities. For example, a sample size of 90 isolates produces a two-sided 95% CI with a width of 0.13 when the observed susceptibility/resistance sensitivity is 90%.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award number UM1AI104681. Additional NIH support to R.A.B. includes R01AI100560, R01AI063517, and R01AI072219. This study was also supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, award number 1I01BX001974 to R.A.B. from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development and the Geriatric Research Education and Clinical Center VISN 10 to R.A.B.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Department of Veterans Affairs. S.R.E. and R.A.B. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
T.H., C.M., and R.S. are salaried employees of Ibis Biosciences, Abbott. B.N.K. consults for Pfizer and Abbott. R.A.B. has received grants from Wockhardt, Merck, AstraZeneca, and GlaxoSmithKline. All other authors have no disclosures to report.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01524-16.
REFERENCES
- 1.Evans SR, Hujer AM, Jiang H, Hujer KM, Hall T, Marzan C, Jacobs MR, Sampath R, Ecker DJ, Manca C, Chavda K, Zhang P, Fernandez H, Chen L, Mediavilla JR, Hill CB, Perez F, Caliendo AM, Fowler VG Jr, Chambers HF, Kreiswirth BN, Bonomo RA. 2016. Rapid molecular diagnostics, antibiotic treatment decisions, and developing approaches to inform empiric therapy: PRIMERS I and II. Clin Infect Dis 62:181–189. doi: 10.1093/cid/civ837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Villoria AM, Valverde-Garduno V. 2016. Antibiotic-resistant Acinetobacter baumannii increasing success remains a challenge as a nosocomial pathogen. J Pathog 2016:7318075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez F, Endimiani A, Bonomo RA. 2008. Why are we afraid of Acinetobacter baumannii? Expert Rev Anti Infect Ther 6:269–271. doi: 10.1586/14787210.6.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karageorgopoulos DE, Falagas ME. 2008. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis 8:751–762. doi: 10.1016/S1473-3099(08)70279-2. [DOI] [PubMed] [Google Scholar]
- 6.Thamlikitkul V, Tiengrim S, Seenama C. 2016. Comparative in vitro activity of minocycline and selected antibiotics against carbapenem-resistant Acinetobacter baumannii from Thailand. Int J Antimicrob Agents 47:101–102. doi: 10.1016/j.ijantimicag.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Figueiredo S, Poirel L, Croize J, Recule C, Nordmann P. 2009. In vivo selection of reduced susceptibility to carbapenems in Acinetobacter baumannii related to ISAba1-mediated overexpression of the natural bla(OXA-66) oxacillinase gene. Antimicrob Agents Chemother 53:2657–2659. doi: 10.1128/AAC.01663-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zander E, Chmielarczyk A, Heczko P, Seifert H, Higgins PG. 2013. Conversion of OXA-66 into OXA-82 in clinical Acinetobacter baumannii isolates and association with altered carbapenem susceptibility. J Antimicrob Chemother 68:308–311. doi: 10.1093/jac/dks382. [DOI] [PubMed] [Google Scholar]
- 9.Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams JM, Donskey CJ, Ecker DJ, Massire C, Eshoo MW, Sampath R, Thomson JM, Rather PN, Craft DW, Fishbain JT, Ewell AJ, Jacobs MR, Paterson DL, Bonomo RA. 2006. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother 50:4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez F, Endimiani A, Ray AJ, Decker BK, Wallace CJ, Hujer KM, Ecker DJ, Adams MD, Toltzis P, Dul MJ, Windau A, Bajaksouzian S, Jacobs MR, Salata RA, Bonomo RA. 2010. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 65:1807–1818. doi: 10.1093/jac/dkq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez F, Hujer AM, Hulten EA, Fishbain J, Hujer KM, Aron D, Thweatt K, Donskey CJ, Bonomo RA. 2010. Antibiotic resistance determinants in Acinetobacter spp and clinical outcomes in patients from a major military treatment facility. Am J Infect Control 38:63–65. doi: 10.1016/j.ajic.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CLSI. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Baldwin CD, Howe GB, Sampath R, Blyn LB, Matthews H, Harpin V, Hall TA, Drader JJ, Hofstadler SA, Eshoo MW, Rudnick K, Studarus K, Moore D, Abbott S, Janda JM, Whitehouse CA. 2009. Usefulness of multilocus polymerase chain reaction followed by electrospray ionization mass spectrometry to identify a diverse panel of bacterial isolates. Diagn Microbiol Infect Dis 63:403–408. doi: 10.1016/j.diagmicrobio.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Mediavilla JR, Endimiani A, Rosenthal ME, Zhao Y, Bonomo RA, Kreiswirth BN. 2011. Multiplex real-time PCR assay for detection and classification of Klebsiella pneumoniae carbapenemase gene (bla KPC) variants. J Clin Microbiol 49:579–585. doi: 10.1128/JCM.01588-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins PG, Lehmann M, Seifert H. 2010. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 35:305. doi: 10.1016/j.ijantimicag.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Higgins PG, Perez-Llarena FJ, Zander E, Fernandez A, Bou G, Seifert H. 2013. OXA-235, a novel class D beta-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother 57:2121–2126. doi: 10.1128/AAC.02413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, Amyes SG, Livermore DM. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 27:351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Higgins PG, Lehmann M, Wisplinghoff H, Seifert H. 2010. gyrB multiplex PCR to differentiate between Acinetobacter calcoaceticus and Acinetobacter genomic species 3. J Clin Microbiol 48:4592–4594. doi: 10.1128/JCM.01765-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins PG, Wisplinghoff H, Krut O, Seifert H. 2007. A PCR-based method to differentiate between Acinetobacter baumannii and Acinetobacter genomic species 13TU. Clin Microbiol Infect 13:1199–1201. doi: 10.1111/j.1469-0691.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 20.Kamolvit W, Higgins PG, Paterson DL, Seifert H. 2014. Multiplex PCR to detect the genes encoding naturally occurring oxacillinases in Acinetobacter spp. J Antimicrob Chemother 69:959–963. doi: 10.1093/jac/dkt480. [DOI] [PubMed] [Google Scholar]
- 21.Zander E, Higgins PG, Fernandez-Gonzalez A, Seifert H. 2013. Detection of intrinsic blaOXA-51-like by multiplex PCR on its own is not reliable for the identification of Acinetobacter baumannii. Int J Med Microbiol 303:88–89. doi: 10.1016/j.ijmm.2012.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.