ABSTRACT
Clostridium difficile infection (CDI) is becoming less exclusively a health care-associated CDI (HA-CDI). The incidence of community-associated CDI (CA-CDI) has increased over the past few decades. It has been postulated that asymptomatic toxigenic C. difficile (TCD)-colonized patients may play a role in the transfer of C. difficile between the hospital setting and the community. Thus, to investigate the relatedness of C. difficile across the hospital and community settings, we compared the characteristics of symptomatic and asymptomatic host patients and the pathogens from these patients in these two settings over a 3-year period. Two studies were simultaneously conducted; the first study enrolled symptomatic CDI patients from two tertiary care hospitals and the community in two Australian states, while the second study enrolled asymptomatic TCD-colonized patients from the same tertiary care hospitals. A total of 324 patients (96 with HA-CDI, 152 with CA-CDI, and 76 colonized with TCD) were enrolled. The predominant C. difficile ribotypes isolated in the hospital setting corresponded with those isolated in the community, as it was found that for 79% of the C. difficile isolates from hospitals, an isolate with a matching ribotype was isolated in the community, suggesting that transmission between these two settings is occurring. The toxigenic C. difficile strains causing symptomatic infection were similar to those causing asymptomatic infection, and patients exposed to antimicrobials prior to admission were more likely to develop a symptomatic infection (odds ratio, 2.94; 95% confidence interval, 1.20 to 7.14). Our findings suggest that the development of CDI symptoms in a setting without establishment of hospital epidemics with binary toxin-producing C. difficile strains may be driven mainly by host susceptibility and exposure to antimicrobials, rather than by C. difficile strain characteristics.
KEYWORDS: Clostridium difficile, asymptomatic, community-acquired infections, health care-acquired infection, ribotyping
INTRODUCTION
Over the past 3 decades, the epidemiology of Clostridium difficile infection (CDI) has markedly changed, and several countries have reported a significant increase in the incidence and severity of the disease as well as numerous hospital outbreaks. The changes have been partly attributed to the emergence of specific C. difficile strains (PCR ribotypes 001, 027, and 078) with increased toxin production and in some cases resistance to newer fluoroquinolones (1–3). CDI was previously exclusively considered a health care-associated CDI (HA-CDI) affecting elderly patients with multiple comorbidities and a recent history of antimicrobial exposure. However, patients in the community are now also considered at risk of CDI, and C. difficile strains that are known to be highly pathogenic are now frequently isolated from patients with community-associated CDI (CA-CDI) (1). Severe cases of CA-CDI were reported among populations that were considered at low risk of CDI, including pregnant women and healthy young adults without antimicrobial exposure or contact with health care facilities (4, 5).
Symptoms of CDI can range from mild diarrhea to life-threatening conditions, such as pseudomembranous colitis, and are precipitated by the capacity of some C. difficile strains to produce toxins A and B and binary toxin (CDT). Similar to other infectious diseases, not all patients colonized with toxigenic C. difficile (TCD) strains become symptomatic. Loo et al. found that C. difficile ribotype 027 was the predominant strain isolated from symptomatic patients with HA-CDI, whereas asymptomatic patients were more likely to be colonized with other strains (6). However, it is unclear which host and pathogen features determine whether a patient colonized with C. difficile will remain asymptomatic or develop mild or severe forms of the disease in a setting where non-ribotype 027 strains are endemic. Although cases of C. difficile ribotype 027 infection have been reported in Australia (7, 8), C. difficile ribotype 027 has not yet become established, and the most common ribotypes circulating are 014/020, 056, and 002 (9, 10).
It has also been proposed that asymptomatic TCD-colonized patients act as a source of environmental contamination and may result in the emergence of new CDI cases, particularly in a hospital setting (11, 12). Furthermore, epidemiological studies and a mathematical modeling study have demonstrated that CA-CDI importation into the hospital may play a role in maintaining HA-CDI transmission (13–15).
Despite the growing evidence that HA-CDI, CA-CDI, and asymptomatic TCD colonization are interrelated and all three play a significant role in C. difficile epidemiology, no reported study has previously evaluated these three components of C. difficile at the same time. Therefore, the current study aimed to determine whether these three components are in fact interrelated by comparing the predominant C. difficile ribotypes and the characteristics of symptomatic and asymptomatic patients in the health care setting and in the community over a 3-year period.
RESULTS
Over the 3-year study period, 324 patients (96 with HA-CDI, 152 with CA-CDI, and 76 with asymptomatic TCD colonization) were enrolled. One hundred sixty-five patients (50.9%) were enrolled in Queensland, Australia, while 159 (49.1%) were enrolled in Western Australia.
Characteristics of C. difficile isolates.
Five different toxin profiles were identified among the toxigenic C. difficile strains isolated (Table 1). The proportion of toxin profiles did not significantly differ between C. difficile categories (P = 0.816). The most common toxin profile was toxin A positive (A+), toxin B positive (B+), and CDT negative (CDT−) (n = 293, 83.2%). Toxin A-negative (A−), B+, and CDT-positive (CDT+) C. difficile isolates were recovered only from symptomatic patients (n = 3), while an A−, toxin B-negative (B−), and CDT+ isolate was recovered from only one asymptomatic patient. Nontoxigenic C. difficile strains were isolated from 10 symptomatic patients (7 with HA-CDI, 3 with CA-CDI), most likely due to coinfection with TCD strains that were not isolated.
TABLE 1.
Frequency distribution of C. difficile toxin profiles by sourcea
| Toxin profile | No. (%) of patients |
||
|---|---|---|---|
| Symptomatic patientsb |
Asymptomatic patients with TCDc (n = 76) | ||
| HA-CDI (n = 96) | CA-CDI (n = 152) | ||
| A+, B+, CDT+ | 4 (4.2) | 7 (4.6) | 3 (4.0) |
| A+, B+, CDT− | 83 (86.5) | 139 (91.4) | 71 (93.4) |
| A−, B+, CDT+ | 1 (1.0) | 2 (1.3) | 0 (0.0) |
| A−, B+, CDT− | 1 (1.0) | 1 (0.7) | 1 (1.3) |
| A−, B−, CDT+ | 0 (0.0) | 0 (0.0) | 1 (1.3) |
HA, health care associated; CA, community associated; CDI, C. difficile infection; TCDc, toxigenic C. difficile colonization.
Nontoxigenic (A−, B−, CDT−) C. difficile isolates were recovered from seven HA-CDI patients and three CA-CDI patients.
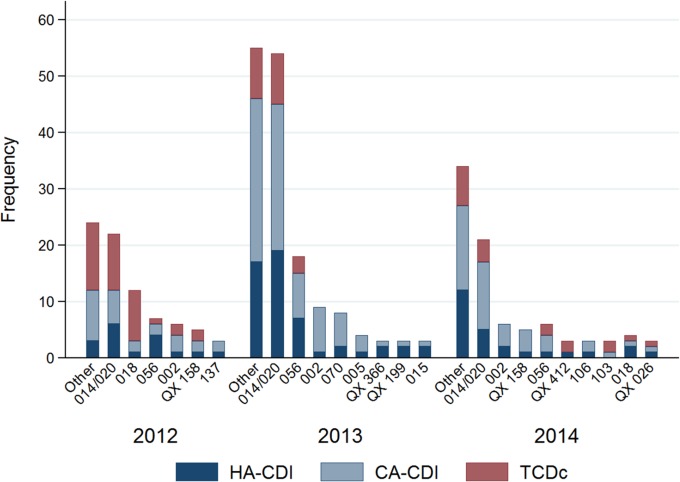
Simpson's indices of diversity were 0.89, 0.89, and 0.88 for HA-CDI, CA-CDI, and asymptomatic TCD colonization, respectively. Although a high diversity of ribotypes (over 90) was identified during the study period, four C. difficile ribotypes (i.e., ribotypes 014/020, 056, 002, and 018) accounted for over 50% of the isolates. C. difficile ribotype 014/020 (n = 97, 29.9%) was the predominant ribotype throughout the 3-year study period among symptomatic patients (both patients with HA-CDI and patients with CA-CDI) and asymptomatic patients (Fig. 1 and the supplemental material). C. difficile ribotype 056 (n = 31, 9.6%) was the second most common ribotype isolated, followed by ribotype 002 (n = 21, 6.5%), which was predominantly found in CA-CDI patients, and ribotype 018 (n = 18, 4.9%), which was mainly found in asymptomatic TCD-colonized patients. Among all study patients, virulent ribotypes C. difficile 244, 078, 251, and 027 in particular were isolated from only four, two, one, and one CDI patients, respectively.
FIG 1.
Distribution of ribotypes by year among symptomatic HA-CDI and CA-CDI patients and asymptomatic toxigenic C. difficile-colonized (TCDc) patients. Ribotypes found at a frequency of less than 3 isolates in a year were grouped into the other category.
The predominant C. difficile ribotypes isolated from symptomatic HA-CDI patients were concordant with the ribotypes identified among asymptomatic TCD-colonized patients; for over 70% of the isolates from symptomatic patients, an isolate with a matching ribotype was isolated from an asymptomatic patient. Likewise, for 79% of the C. difficile isolates from the hospitals, an isolate with a matching ribotype was isolated from the community.
Patients' preadmission characteristics.
The preadmission characteristics of the patients constituting the three C. difficile categories (HA-CDI, CA-CDI, and TCD colonization) are presented in Table 2. The proportion of females significantly differed between the three groups, with a higher proportion of females having CA-CDI (73.7%) than HA-CDI (52.1%) or asymptomatic TCD colonization (52.6%) (P < 0.001). Across the three groups, there was no statistically significant difference in health care exposure in the previous year. With regard to medication exposure in the month prior to enrollment, antimicrobials (P = 0.031) and gastric acid suppressants (P value < 0.001) were more often prescribed to patients that developed HA-CDI than to the other two groups, while laxatives (P < 0.001) were more often prescribed to patients that were asymptomatically colonized. The rates of household exposure to toddlers, elderly people, domestic animals, or livestock did not significantly differ between the groups. Ten percent of the symptomatic patients (HA-CDI patients [10.4%] and CA-CDI patients [10.0%]) reported having an episode of CDI in the past 12 months, whereas none of the asymptomatic TCD-colonized patients reported such an episode (P < 0.001).
TABLE 2.
Patients' characteristics and health care, medication, and environmental exposure prior to enrollmenta
| Characteristic | Symptomatic patients |
Asymptomatic patients with TCDc (n = 76) |
P value |
|||
|---|---|---|---|---|---|---|
| HA-CDI (n = 96) | CA-CDI (n = 152) | HA-CDI vs CA-CDI | HA-CDI vs TCDc | HA-CDI vs CA-CDI vs TCDc | ||
| No. (%) of female patients | 50 (52.1) | 112 (73.7) | 40 (52.6) | <0.001 | 0.943 | <0.001 |
| Median (IQR) age (yr) | 61.7 (49.2–75.0) | 66.4 (49.1–75.4) | 66.2 (54.8–76.8) | 0.765 | 0.317 | 0.607 |
| Health care exposure 12 mo prior to enrollment | ||||||
| No. (%) of patients admitted to a hospital | 62 (69.7) | 105 (69.1) | 47 (64.4) | 0.924 | 0.476 | 0.729 |
| Median (SD) no. of admissions | 2.1 (2.2) | 1.5 (1.6) | 2.0 (2.6) | 0.128 | 0.328 | 0.323 |
| Median (IQR) LOS in the last admission | 7 (4–16) | 6 (3–10) | 6 (3–9) | 0.191 | 0.140 | 0.215 |
| No. (%) of patients with medication exposure 30 days prior to enrollment | ||||||
| Antimicrobials | 83 (86.5) | 117 (77.0) | 51 (69.9) | 0.066 | 0.008 | 0.031 |
| Gastric acid suppressants | 52 (54.7) | 34 (22.4) | 29 (40.3) | <0.001 | 0.64 | <0.001 |
| Laxatives | 28 (29.2) | 17 (14.2) | 29 (51.8) | 0.007 | 0.005 | <0.001 |
| No. (%) of patients with the following household exposure prior to enrollment: | ||||||
| People <2 yr old | 3 (3.1) | 6 (4.0) | 4 (5.3) | 1.000 | 0.365 | 0.817 |
| People >65 yr old | 24 (25.3) | 52 (34.2) | 22 (29.3) | 0.138 | 0.553 | 0.322 |
| Cats | 21 (21.9) | 23 (15.1) | 12 (15.8) | 0.176 | 0.314 | 0.363 |
| Dogs | 30 (31.3) | 63 (41.5) | 28 (36.8) | 0.106 | 0.441 | 0.269 |
| Livestock | 8 (8.3) | 15 (9.9) | 7 (9.2) | 0.685 | 0.840 | 0.921 |
| No. (%) of patients with the following smoking status: | ||||||
| Current | 8 (8.3) | 10 (6.6) | 7 (9.3) | 0.604 | 0.819 | 0.740 |
| Ever | 52 (54.2) | 61 (40.1) | 43 (58.1) | 0.031 | 0.608 | 0.016 |
| No. (%) of patients with history of CDI in past year | 10 (10.4) | 15 (10.0) | 0 (0.0) | 0.916 | 0.003 | <0.001 |
HA, health care associated; CA, community associated; CDI, C. difficile infection; TCDc, toxigenic C. difficile colonization; IQR, interquartile range; LOS, length of stay.
Characteristics during admission and prior to specimen collection.
The reason for admission and the procedures, comorbidities, and medication exposure that occurred during admission are described in Table 3. More patients with HA-CDI (11.5%) than asymptomatic TCD-colonized patients (1.4%) underwent a colonoscopy (P = 0.006); however, more asymptomatic TCD-colonized patients than HA-CDI patients required mechanical ventilation (P = 0.006) and underwent orthopedic (P < 0.001) and neurological (P < 0.001) interventions. Significantly lower proportions of patients with HA-CDI than asymptomatic TCD-colonized patients presented with chronic obstructive pulmonary disease (COPD) (P = 0.026) and neurological disorders (P = 0.042). Conversely, a higher proportion of patients with HA-CDI (16.7%) than asymptomatic colonized patients (4.1%) had inflammatory bowel disease (P = 0.008). In terms of medication exposure during the hospital admission, HA-CDI patients (74.0%) and TCD-colonized patients (77.6%) were equally exposed to antimicrobials (P = 0.578). However, penicillins and β-lactamase inhibitors (P = 0.010) were more often prescribed to patients who went on to develop HA-CDI than asymptomatic TCD-colonized patients. HA-CDI patients were more likely than asymptomatic TCD-colonized patients to have had chemotherapy (P = 0.019) and antidiarrheal medication (P = 0.019), while the latter group of patients was more commonly exposed to laxatives (P = 0.029).
TABLE 3.
Reason for admission and procedures, comorbidities, and medication exposure during admission but prior to specimen collection among patients with HA-CDI and asymptomatic toxigenic C. difficile colonizationa
| Characteristic | No. (%) of patients |
P value | |
|---|---|---|---|
| Symptomatic patients with HA-CDI (n = 96) | Asymptomatic patients with TCDc (n = 76) | ||
| Reason for admission | |||
| New medical/surgical problem | 25 (28.1) | 35 (47.3) | 0.022 |
| Exacerbation of chronic condition | 25 (28.1) | 19 (25.7) | |
| Infection | 31 (34.8) | 12 (16.2) | |
| Elective surgery | 8 (9.0) | 8 (10.8) | |
| Medical procedures | |||
| Insertion of orogastric tubes | 8 (8.3) | 8 (10.8) | 0.680 |
| Gastroscopy | 13 (13.5) | 4 (5.4) | 0.049 |
| Colonoscopy | 11 (11.5) | 1 (1.4) | 0.006 |
| Mechanical ventilationb | 2 (2.1) | 10 (13.5) | 0.006 |
| Surgical procedures | |||
| Orthopedic | 7 (7.3) | 19 (25.0) | <0.001 |
| Abdominal | 12 (12.5) | 6 (7.9) | 0.327 |
| Cardiological/thoracic | 2 (2.1) | 4 (5.3) | 0.238 |
| Neurological | 0 (0.0) | 11 (14.5) | <0.001 |
| Oncological | 5 (5.2) | 0 (0.0) | 0.052 |
| Other surgical procedures | 2 (2.1) | 5 (6.6) | 0.138 |
| Medical conditions | |||
| Cancer | 42 (43.8) | 22 (29.7) | 0.061 |
| Diabetes mellitus | 21 (21.9) | 18 (24.3) | 0.706 |
| Neurological disorder | 17 (17.7) | 23 (31.1) | 0.042 |
| Gastroesophageal reflux disease | 26 (27.1) | 24 (32.4) | 0.448 |
| Chronic obstructive pulmonary disease | 10 (10.4) | 17 (23.0) | 0.026 |
| Chronic kidney disease | 22 (22.9) | 14 (18.9) | 0.527 |
| Congestive heart failure | 11 (11.5) | 12 (16.2) | 0.369 |
| Liver disease | 10 (10.4) | 4 (5.4) | 0.274 |
| Inflammatory bowel disease | 16 (16.7) | 3 (4.1) | 0.008 |
| Diverticulosis | 9 (9.4) | 2 (2.7) | 0.072 |
| Solid organ transplant | 7 (7.3) | 1 (1.4) | 0.069 |
| Medication exposure | |||
| Any antimicrobialc | 71 (74.0) | 59 (77.6) | 0.578 |
| Penicillins and β-lactamase inhibitors | 45 (46.9) | 21 (27.6) | 0.010 |
| Cephalosporins | 29 (30.2) | 34 (44.7) | 0.050 |
| Penicillins | 11 (11.5) | 12 (15.8) | 0.407 |
| Trimethoprim-sulfamethoxazole | 11 (11.5) | 6 (7.9) | 0.437 |
| Carbapenems | 11 (11.5) | 6 (7.9) | 0.437 |
| Ciprofloxacin | 9 (9.4) | 5 (6.6) | 0.354 |
| Aminoglycosides | 8 (8.3) | 5 (6.6) | 0.448 |
| Fluoroquinolonesd | 1 (1.0) | 3 (4.0) | 0.228 |
| Clindamycin | 1 (1.0) | 4 (5.3) | 0.120 |
| Tetracyclines | 1 (1.0) | 0 (0.0) | 0.442 |
| Macrolides | 0 (0.0) | 3 (4.0) | 0.084 |
| Metronidazole | 17 (17.7) | 7 (9.2) | 0.110 |
| Vancomycin | 7 (7.3) | 6 (7.9) | 0.882 |
| Gastric acid-suppressive agents | 59 (61.5) | 41 (54.0) | 0.321 |
| Proton pump inhibitors | 57 (59.4) | 37 (48.7) | 0.162 |
| H2 blocker | 4 (4.2) | 5 (6.6) | 0.480 |
| Laxatives | 28 (29.2) | 34 (45.3) | 0.029 |
| Nonsteroidal anti-inflammatory drugs | 18 (18.8) | 13 (17.1) | 0.780 |
| Glucocorticoids | 35 (36.5) | 18 (23.7) | 0.072 |
| Chemotherapy | 12 (12.5) | 2 (2.6) | 0.019 |
| Antidiarrheal medication | 12 (12.5) | 2 (2.6) | 0.019 |
HA, health care associated; CDI, C. difficile infection; TCDc, toxigenic C. difficile colonization.
Excludes mechanical ventilation during surgical procedures.
Excludes metronidazole and vancomycin.
Ciprofloxacin not included.
Predictors of symptomatic and severe forms of the disease.
The multivariate logistic regression model (Table 4) revealed that patients exposed to antimicrobials within 30 days prior to hospitalization were at a higher risk of developing symptoms (odds ratio [OR], 2.94; 95% confidence interval [CI], 1.20 to 7.14), whereas patients with COPD were at lower risk of developing symptoms of the infection (OR, 0.31; 95% CI, 0.12 to 0.83).
TABLE 4.
Logistic regression for predictors of symptomatic HA-CDI compared to asymptomatic toxigenic C. difficile colonization
| Characteristic | OR (95% CI)a |
|
|---|---|---|
| Univariate model | Multivariate model | |
| Female | 0.98 (0.53–1.79) | 0.92 (0.45–1.85) |
| Age (per decade) | 0.91 (0.76–1.09) | 0.96 (0.78–1.19) |
| Admitted to a hospital in past 12 mo | 1.27 (0.66–2.44) | 1.05 (0.48–2.27) |
| Medication exposure 30 days prior to admission | ||
| Antimicrobials | 2.78 (1.28–5.88) | 2.94 (1.20–7.14) |
| Gastric acid-suppressive agents | 1.79 (0.96–3.33) | 1.67 (0.76–3.57) |
| Medical conditions | ||
| Cancer | 1.85 (0.97–3.45) | 1.15 (0.52–2.50) |
| Diabetes mellitus | 0.87 (0.43–1.79) | 0.72 (0.30–1.69) |
| Neurological disorder | 0.48 (0.23–0.98) | 0.50 (0.21–1.15) |
| Gastroesophageal reflux disease | 0.78 (0.40–1.49) | 0.74 (0.33–1.64) |
| Chronic obstructive pulmonary disease | 0.39 (0.17–0.91) | 0.31 (0.12–0.83) |
| Chronic kidney disease | 1.27 (0.60–2.70) | 1.16 (0.47–2.86) |
| Congestive heart failure | 0.67 (0.28–1.61) | 1.03 (0.35–3.03) |
OR odds ratio; CI, confidence interval. Boldface data indicate statistically significant results.
During the follow-up period, four TCD-colonized patients developed symptomatic CDI. Fifty-three and six patients with HA-CDI and CA-CDI, respectively, had recurrent CDI. Nine deaths were recorded, including three among participants with HA-CDI, two among participants with CA-CDI, and four among participants asymptomatically colonized with TCD. Three patients, all with HA-CDI, were admitted to an intensive care unit (ICU). No colectomies were recorded.
DISCUSSION
Previous studies that examined the relationship between C. difficile strains and the development of symptoms were conducted during an outbreak (16) or in settings where binary toxin-producing C. difficile strains were predominant (6); this is the first epidemiological study of C. difficile that was conducted simultaneously in a health care setting and a community setting and that examined symptomatic and asymptomatic patients in a setting without establishment of hospital epidemics with binary toxin-producing C. difficile strains. There was no difference in the ribotype diversity of the isolates across the HA-CDI, CA-CDI, and asymptomatic TCD-colonized patients, reflecting similar pathogen population structures. Furthermore, the most prevalent C. difficile ribotypes were similar across the HA-CDI, CA-CDI, and asymptomatic TCD-colonized patients, suggesting that transmission of C. difficile is occurring between the hospitals and the communities and that asymptomatic TCD-colonized individuals as well as symptomatic patients may be acting as a vehicle of transmission between these two settings.
The finding also suggests that C. difficile ribotypes may not be determinants of the development of symptomatic infection but, rather, that the development of symptoms may be mainly driven by host factors, such as immune state and disruption of the gut microbiome by exposure to antimicrobials or underlying conditions affecting the gastrointestinal tract (17–19). Our findings differ from those of a previous study in which a binary toxin-producing C. difficile strain (i.e., ribotype 027) was more likely than other strains to cause symptomatic disease (6). This difference could be explained by the very low prevalence of C. difficile ribotype 027 and other highly virulent binary toxin-producing strains in Australia, and therefore, our findings may be expected in other settings without hospital epidemics with binary toxin-producing C. difficile strains.
Several meta-analyses have described the risk factors for HA-CDI (20) and CA-CDI (21); however, female sex is not a well-documented risk factor for CA-CDI, and few studies have described this association (22–26). In our study, we found that nearly three-quarters of the CA-CDI cases occurred in women, whereas HA-CDI and asymptomatic cases were equally distributed between the sexes. This observation may be mostly related to behavioral risk factors among women that occur in the community rather than physiological differences between the sexes. Among the behavioral factors occurring in the community that may put females at risk of CDI are higher rates of antimicrobial prescriptions (27, 28), vegetable consumption (29), and contact with children (30).
While there is no conclusive evidence that contaminated food leads to CDI in humans, studies have found that retail vegetables are contaminated with C. difficile strains similar to those affecting humans (31, 32). Likewise, the C. difficile ribotypes frequently isolated in the current study, such as 014/020 and 056, are common ribotypes found in piglets and veal calves, respectively, in Australia (33, 34). Therefore, the possibility of food being a vehicle of C. difficile transmission cannot be ruled out. Although our study did not find an association between the CDI category and contact with toddlers (30), this association needs to be assessed in the context of gender as an effect modifier. Due to the small number of participants that reported living with toddlers, this analysis was not possible.
Another interesting finding was that 10% of symptomatic patients in both settings (hospital and community) but none of the asymptomatic TCD-colonized patients reported having had a CDI in the previous year. While this may be explained by recall bias, given the greater awareness of the disease among the symptomatic patients, this finding may also reflect differences in immune system capacity, with previous infection not offering protection against further infection in these individuals. Those with some degree of immunosuppression might develop symptoms, and those with a fully functioning immune system might not develop symptoms irrespective of the toxigenicity of the C. difficile strains to which the patient had previously been exposed. This hypothesis warrants further investigation that would require measurement and comparison of the serum antibody, proinflammatory cytokine, and chemokine levels of noncolonized, asymptomatic C. difficile-colonized, and symptomatic CDI patients. However, indirect evidence from the current study supports our hypothesis, given that patients with some degree of immunosuppression (patients on chemotherapy) were more likely to develop symptoms.
This study supports reports elsewhere that inflammatory bowel disease is a risk factor for developing CDI (35); however, a finding that requires further investigation is that patients with COPD were less likely to develop symptoms. Wojciechowski and colleagues reported a reduced risk of CDI for patients with a COPD diagnosis and when systemic corticosteroids were used during antimicrobial treatment (36). This was corroborated by the findings of the present study, whereby COPD was statistically significantly associated with a reduced risk of CDI. Wojciechowski and colleagues argued that corticosteroids attenuate the host immune response to C. difficile toxins, thus reducing the toxin-induced cytokine release that is associated with systemic symptoms of CDI (36). Further studies are required to confirm the mechanism behind the association.
There are some limitations to this study. Although a large sample size (n = 342) of patients was enrolled, the small number of significant health outcomes (i.e., deaths, ICU admission) recorded during the follow-up period precluded statistical analyses to elucidate whether HA-CDI was associated with more severe outcomes than CA-CDI. In addition, more discriminatory strain typing methods (e.g., multilocus variable-number tandem-repeat analysis and whole-genome sequencing) are required to conclusively determine specific transmission events between community and hospital CDI cases as well as the role of asymptomatic colonized patients.
In summary, similar C. difficile ribotypes were circulating in the community and hospitals in this study of two Australian states, suggesting the carryover of strains between settings. Furthermore, asymptomatic and symptomatic patients were colonized with similar C. difficile ribotypes, suggesting that in a setting without establishment of hospital epidemics with binary toxin-producing C. difficile strains, the development of symptoms may be primarily driven by host characteristics rather than C. difficile toxigenicity or ribotype. Future epidemiological studies in settings without hospital epidemics with binary toxin-producing C. difficile strains are needed to confirm our findings and determine the role of patient-, antibiotic-, and C. difficile strain-related factors in the development of symptoms.
MATERIALS AND METHODS
Study setting.
Two studies were simultaneously conducted over a 3-year period (2012 to 2014) in two Australian states. The first study examined symptomatic patients with HA-CDI and CA-CDI, whereas the second study examined asymptomatic C. difficile-colonized patients in a health care setting.
The first study enrolled patients in two tertiary care hospitals, The Royal Brisbane and Women's Hospital (RBWH) with 929 beds in Brisbane, Queensland, Australia, and The Sir Charles Gairdner Hospital (SCGH) with 607 beds in Perth, Western Australia. Patients in the community who submitted specimens through their general practitioner (GP) to coordinating laboratories (Sullivan Nicolaides Pathology in Brisbane, Queensland, Australia, and PathWest Laboratory Medicine, Clinipath Laboratories, and Western Diagnostic Pathology in Perth, Western Australia, Australia) were also enrolled. This study used a census design, in which all the stool specimens submitted during the study period to the hospitals and the laboratories by patients 18 year of age or older and experiencing diarrhea were screened for C. difficile. If the specimen was positive for the C. difficile toxin A or B gene, the patient was invited to participate in the study. HA-CDI was defined as health care facility-onset, health care facility-associated CDI constituting the onset of diarrhea 48 h or more after admission to a hospital and as community-onset, health care facility-associated disease constituting the onset of symptoms in a patient who had been discharged from a health care facility within the previous 4 weeks. CA-CDI was defined as community-onset CDI in a patient who had not been admitted to a health care facility in the previous 12 weeks or as health care facility-onset CDI within 48 h or less of admission to the hospital (37).
The second study has been previously described elsewhere (38). In brief, six cross-sectional surveys (two per year) were conducted at RBWH and SCGH. Randomly selected hospitalized patients aged 18 years or older without diarrhea were approached and invited to participate in the study. Patients who were not experiencing diarrhea and who had a toxigenic C. difficile strain (positive for the presence of tcdA, tcdB, and/or the cdtA and cdtB genes) isolated from their stool were considered to have asymptomatic TCD colonization and were included in the current analysis.
The studies received the approval of RBWH (approval no. HREC/11/QRBW/223), the Sir Charles Gairdner Group (approval no. 2011-088), The University of Queensland (approval no. 2011000898), and The University of Western Australia (approval no. RA/4/1/5186) Human Research Ethics Committees. All the participants (or a legal proxy) provided written informed consent for their inclusion in the study. In Western Australia, a waiver of consent was granted when a person was unable to provide consent but the person could be enrolled in the study without any additional risk beyond that associated with their standard care.
Data collection.
A questionnaire with questions regarding the patient's age, sex, occupation, previous hospital admissions, and use of medications in the previous 30 days and his or her cohabitants' ages was administered to all patients from both studies. For hospitalized patients at RBWH and SCGH, medical records were accessed to obtain additional information and to determine the date and the reason for the current admission, comorbidities, as well as the inpatient medications provided and procedures performed prior to specimen collection. Each participant was followed up on a monthly basis for 3 months by examination of the patient's records and a short interview for hospital patients and by a telephone interview for discharged or CA-CDI cases. The follow-up interviews were used to determine the clinical outcomes of the patients (whether they developed symptoms, had a recurrence of CDI, underwent a colectomy, were admitted to an ICU, or died).
Stool specimen collection and processing.
As previously described (38), direct stool specimen culture was performed on ChromID C. difficile agar (bioMérieux). Broth enrichment in Robertson's cooked meat medium followed by ethanol shock and subculture on ChromID C. difficile agar at 48 to 72 h was performed if the direct culture result was negative. Putative C. difficile colonies were subcultured onto prereduced blood agar plates under anaerobic conditions. C. difficile isolates were tested for the presence of toxin genes and were ribotyped by PCR as previously described (39). Strains that did not produce banding patterns matching the pattern for an international ribotype in the reference collection were assigned a local nomenclature (QX type).
Statistical analysis.
The frequency of C. difficile ribotypes was tabulated by year and C. difficile category (HA-CDI, CA-CDI, and asymptomatic TCD colonization) to identify the predominant ribotypes circulating in each category and to examine the changes in ribotype profile over the study period. Simpson's index of diversity was calculated for each category to compare the diversity of ribotypes isolated across the three categories.
Pearson's chi-square test and Fisher's exact test were used to compare categorical variables, whereas the Wilcox-Mann-Whitney U test and Kruskal-Wallis H test were used to compare continuous variables between participant groups. Multivariate logistic regression models were built to identify predictors of symptomatic disease. After adjustment for the age and sex of the patients and known risk factors for CDI (i.e., prior hospital admissions and exposure to antimicrobials and gastric acid-suppressive agents), the inclusion of comorbidities in the regression model was done through a stepwise forward selection by use of the Akaike information criterion as the selection criterion. A significance level cutoff of a P value of 0.05 was used for all analyses. All statistical analyses were conducted using Stata SE, version 14 (Stata Corporation, College Station, TX).
Supplementary Material
ACKNOWLEDGMENTS
We thank all patients, physicians, nurses, laboratories, and hospitals who took part in the study. We also thank Tanya Scheller, Christine Duncan, Suzanne Ditchburn, Welma Van Schalkwyk, Noellene Foster, Sarah MacArthur, and Jessica Macfarlane, who assisted with the enrollment of the patients and data extraction.
This research was funded by a National Health and Medical Research Council grant (1006243). L.F.-K. is funded by an Endeavor postgraduate scholarship (3781_2014), an Australian National University higher degree scholarship, and a Fondo para la Innovación, Ciencia y Tecnología scholarship (095-FINCyT-BDE-2014). A.C.A.C. is funded by an Australian National Health and Medical Research Council senior research fellowship (1058878).
The study sponsors had no further role in the study design, data collection, analyses, interpretation of results, writing of the article, or the decision to submit it for publication.
We have no competing interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01779-16.
REFERENCES
- 1.Goorhuis A, Bakker D, Corver J, Debast SB, Harmanus C, Notermans DW, Bergwerff AA, Dekker FW, Kuijper EJ. 2008. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin Infect Dis 47:1162–1170. doi: 10.1086/592257. [DOI] [PubMed] [Google Scholar]
- 2.Borgmann S, Kist M, Jakobiak T, Reil M, Scholz E, von Eichel-Streiber C, Gruber H, Brazier JS, Schulte B. 2008. Increased number of Clostridium difficile infections and prevalence of Clostridium difficile PCR ribotype 001 in southern Germany. Euro Surveill 13(49):pii=19057 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19057. [PubMed] [Google Scholar]
- 3.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, Rene P, Monczak Y, Dascal A. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2005. Severe Clostridium difficile-associated disease in populations previously at low risk—four states, 2005. Morb Mortal Wkly Rep 54:1201–1205. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2008. Surveillance for community-associated Clostridium difficile—Connecticut, 2006. Morb Mortal Wkly Rep 57:340–343. [PubMed] [Google Scholar]
- 6.Loo VG, Bourgault A-M, Poirier L, Lamothe F, Michaud S, Turgeon N, Toye B, Beaudoin A, Frost EH, Gilca R, Brassard P, Dendukuri N, Béliveau C, Oughton M, Brukner I, Dascal A. 2011. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 365:1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 7.Riley TV, Thean S, Hool G, Golledge CL. 2009. First Australian isolation of epidemic Clostridium difficile PCR ribotype 027. Med J Aust 190:706–708. [DOI] [PubMed] [Google Scholar]
- 8.Richards M, Knox J, Elliott B, Mackin K, Lyras D, Waring LJ, Riley TV. 2011. Severe infection with Clostridium difficile PCR ribotype 027 acquired in Melbourne, Australia. Med J Aust 194:369–371. [DOI] [PubMed] [Google Scholar]
- 9.Huber CA, Hall L, Foster NF, Gray M, Allen M, Richardson LJ, Robson J, Vohra R, Schlebusch S, George N, Nimmo GR, Riley TV, Paterson DL. 2014. Surveillance snapshot of Clostridium difficile infection in hospitals across Queensland detects binary toxin producing ribotype UK 244. Commun Dis Intell Q Rep 38:E279–E284. [DOI] [PubMed] [Google Scholar]
- 10.Foster NF, Collins DA, Ditchburn SL, Duncan CN, van Schalkwyk JW, Golledge CL, Keed AB, Riley TV. 2014. Epidemiology of Clostridium difficile infection in two tertiary-care hospitals in Perth, Western Australia: a cross-sectional study. New Microbes New Infect 2:64–71. doi: 10.1002/nmi2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanzas C, Dubberke ER, Lu Z, Reske KA, Grohn YT. 2011. Epidemiological model for Clostridium difficile transmission in healthcare settings. Infect Control Hosp Epidemiol 32:553–561. doi: 10.1086/660013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clabots CR, Johnson S, Olson MM, Peterson LR, Gerding DN. 1992. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis 166:561–567. doi: 10.1093/infdis/166.3.561. [DOI] [PubMed] [Google Scholar]
- 13.Walker AS, Eyre DW, Wyllie DH, Dingle KE, Harding RM, O'Connor L, Griffiths D, Vaughan A, Finney J, Wilcox MH, Crook DW, Peto TE. 2012. Characterisation of Clostridium difficile hospital ward-based transmission using extensive epidemiological data and molecular typing. PLoS Med 9:e1001172. doi: 10.1371/journal.pmed.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yakob L, Riley T, Paterson D, Clements A. 2013. Clostridium difficile exposure as an insidious source of infection in healthcare settings: an epidemiological model. BMC Infect Dis 13:376. doi: 10.1186/1471-2334-13-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curry SR, Muto CA, Schlackman JL, Pasculle AW, Shutt KA, Marsh JW, Harrison LH. 2013. Use of multilocus variable number of tandem repeats analysis genotyping to determine the role of asymptomatic carriers in Clostridium difficile transmission. Clin Infect Dis 57:1094–1102. doi: 10.1093/cid/cit475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. 1990. Nosocomial Clostridium difficile colonisation and disease. Lancet 336:97–100. doi: 10.1016/0140-6736(90)91605-A. [DOI] [PubMed] [Google Scholar]
- 17.Walk ST, Micic D, Jain R, Lo ES, Trivedi I, Liu EW, Almassalha LM, Ewing SA, Ring C, Galecki AT, Rogers MA, Washer L, Newton DW, Malani PN, Young VB, Aronoff DM. 2012. Clostridium difficile ribotype does not predict severe infection. Clin Infect Dis 55:1661–1668. doi: 10.1093/cid/cis786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker AS, Eyre DW, Crook DW, Wilcox MH, Peto TEA. 2013. Regarding “Clostridium difficile ribotype does not predict severe infection.” Clin Infect Dis 56:1845–1846. doi: 10.1093/cid/cit098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker AS, Eyre DW, Wyllie DH, Dingle KE, Griffiths D, Shine B, Oakley S, O'Connor L, Finney J, Vaughan A, Crook DW, Wilcox MH, Peto TE. 2013. Relationship between bacterial strain type, host biomarkers, and mortality in Clostridium difficile infection. Clin Infect Dis 56:1589–1600. doi: 10.1093/cid/cit127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slimings C, Riley TV. 2014. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 69:881–891. doi: 10.1093/jac/dkt477. [DOI] [PubMed] [Google Scholar]
- 21.Furuya-Kanamori L, Stone JC, Clark J, McKenzie SJ, Yakob L, Paterson DL, Riley TV, Doi SA, Clements AC. 2015. Comorbidities, exposure to medications, and the risk of community-acquired Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 36:132–141. doi: 10.1017/ice.2014.39. [DOI] [PubMed] [Google Scholar]
- 22.Dial S, Delaney JC, Barkun AN, Suissa S. 2005. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 294:2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 23.Itskowitz MS, Lebovitz PJ. 2003. Non-antibiotic associated pseudomembranous colitis: a case report and review of the literature. Adv Stud Med 3:571–574. [Google Scholar]
- 24.Chen Y, Glass K, Liu B, Riley T, Korda R, Kirk M. 26 October 2016. A population-based longitudinal study of Clostridium difficile infection-related hospitalization in mid-age and older Australians. Epidemiol Infect. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aronsson B, Mollby R, Nord CE. 1982. Clostridium difficile and antibiotic associated diarrhoea in Sweden. Scand J Infect Dis Suppl 35:53–58. [PubMed] [Google Scholar]
- 26.Chitnis AS, Holzbauer SM, Belflower RM, Winston LG, Bamberg WM, Lyons C, Farley MM, Dumyati GK, Wilson LE, Beldavs ZG, Dunn JR, Gould LH, MacCannell DR, Gerding DN, McDonald LC, Lessa FC. 2013. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med 173:1359–1367. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barlam TF, Morgan JR, Wetzler LM, Christiansen CL, Drainoni ML. 2015. Antibiotics for respiratory tract infections: a comparison of prescribing in an outpatient setting. Infect Control Hosp Epidemiol 36:153–159. doi: 10.1017/ice.2014.21. [DOI] [PubMed] [Google Scholar]
- 28.Sun C, Jew S, Dasta SL. 2006. Osteopathic physicians in the United States: antibiotic prescribing practices for patients with nonspecific upper respiratory tract infections. J Am Osteopath Assoc 106:450–455. [PubMed] [Google Scholar]
- 29.Milligan RA, Burke V, Beilin LJ, Dunbar DL, Spencer MJ, Balde E, Gracey MP. 1998. Influence of gender and socio-economic status on dietary patterns and nutrient intakes in 18-year-old Australians. Aust N Z J Public Health 22:485–493. doi: 10.1111/j.1467-842X.1998.tb01419.x. [DOI] [PubMed] [Google Scholar]
- 30.Wilcox MH, Mooney L, Bendall R, Settle CD, Fawley WN. 2008. A case-control study of community-associated Clostridium difficile infection. J Antimicrob Chemother 62:388–396. doi: 10.1093/jac/dkn163. [DOI] [PubMed] [Google Scholar]
- 31.Metcalf DS, Costa MC, Dew WM, Weese JS. 2010. Clostridium difficile in vegetables, Canada. Lett Appl Microbiol 51:600–602. doi: 10.1111/j.1472-765X.2010.02933.x. [DOI] [PubMed] [Google Scholar]
- 32.Bakri MM, Brown DJ, Butcher JP, Sutherland AD. 2009. Clostridium difficile in ready-to-eat salads, Scotland. Emerg Infect Dis 15:817. doi: 10.3201/eid1505.081186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knight DR, Thean S, Putsathit P, Fenwick S, Riley TV. 2013. Cross-sectional study reveals high prevalence of Clostridium difficile non-PCR ribotype 078 strains in Australian veal calves at slaughter. Appl Environ Microbiol 79:2630–2635. doi: 10.1128/AEM.03951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight DR, Squire MM, Riley TV. 2015. Nationwide surveillance study of Clostridium difficile in Australian neonatal pigs shows high prevalence and heterogeneity of PCR ribotypes. Appl Environ Microbiol 81:119–123. doi: 10.1128/AEM.03032-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodemann JF, Dubberke ER, Reske KA, Seo DH, Stone CD. 2007. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol 5:339–344. doi: 10.1016/j.cgh.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 36.Wojciechowski AL, Parameswaran GI, Mattappallil A, Mergenhagen KA. 2014. Corticosteroid use is associated with a reduced incidence of Clostridium difficile-associated diarrhea: a retrospective cohort study. Anaerobe 30:27–29. doi: 10.1016/j.anaerobe.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 38.Furuya-Kanamori L, Clements AC, Foster NF, Huber CA, Hong S, Harris-Brown T, Yakob L, Paterson D, Riley TV. 8 September 2016. Asymptomatic Clostridium difficile colonisation in two Australian tertiary hospitals, 2012-2014: a prospective, repeated cross-sectional study. Clin Microbiol Infect. doi: 10.1016/j.cmi.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Carson KC, Boseiwaqa LV, Thean SK, Foster NF, Riley TV. 2013. Isolation of Clostridium difficile from faecal specimens—a comparison of ChromID C. difficile agar and cycloserine-cefoxitin-fructose agar. J Med Microbiol 62:1423–1427. doi: 10.1099/jmm.0.056515-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.