ABSTRACT
Currently, there is no agreed method available for broth microdilution susceptibility testing of Haemophilus parasuis, one of the most important bacterial pathogens in pig production. Therefore, the aim of this study was to develop a method that could be easily performed by diagnostic laboratories and that appears suitable for a harmonized susceptibility testing. Growth determinations using one type strain and three field isolates revealed no visible growth of H. parasuis in media which have proven to be suitable for susceptibility testing of fastidious organisms. Therefore, a new medium, cation-adjusted Mueller-Hinton broth (CAMHB) plus NADH and sterile filtered heat-inactivated chicken serum, was developed. The reproducibility of MICs obtained in this medium was evaluated and statistically analyzed, considering a model with two different variables (precondition of five identical MICs and MIC mode accepting a deviation of ±1 dilution step, respectively). No significant differences for both variables were seen between two time points investigated and between results obtained with the recently proposed test medium broth (TMB). Nearly all MICs of quality control strains were in the acceptable range. Subsequently, 47 H. parasuis isolates representing 13 serovars were tested with the newly developed medium and TMB. Statistical analysis of all isolates and 15 antimicrobial agents and antimicrobial combinations showed no significant difference between MICs obtained in supplemented CAMHB and TMB. Because of a simplified implementation in routine diagnostic and a lower chance of interference between medium components and antimicrobial agents, supplemented CAMHB is recommended with an incubation time of 24 h.
KEYWORDS: broth microdilution, susceptibility testing, MICs, Haemophilus parasuis, fastidious organism, TMB, supplemented CAMHB
INTRODUCTION
Haemophilus parasuis is an abundant colonizer of the upper respiratory tract in swine (1). Although in H. parasuis endemic herds, young piglets are usually protected by maternal immunity, several environmental stress factors may lead to disease as well as infections of naive populations. The acute form of disease, Glässer's disease, is characterized by clinical signs of polyarthritis, polyserositis, meningitis, and pneumonia (among others) and can lead to sudden death of animals, especially in weaner pigs (2, 3). At least 15 different H. parasuis serovars have been identified so far, with the most common serovars being 4 and 5 (4, 5).
As antimicrobial agents are used for the treatment of disease, a reliable method for susceptibility testing of the pathogen is essential. H. parasuis is a fastidious organism which requires specific ingredients or supplements to achieve visible growth in broth, and hence, media which are used to test rapidly growing bacteria are not adequate (6, 7). As a consequence, no validated and standardized method is available (7, 8), and many diagnostic laboratories are currently unable to offer susceptibility testing of this very important pathogen in pig production. However, a few studies investigated the susceptibility status of H. parasuis isolates quantitatively by using media such as veterinary fastidious medium (VFM) or Haemophilus test medium (HTM) for broth microdilution susceptibility testing (7, 9–16). VFM is recommended in CLSI standard VET01-A4 for susceptibility testing of Actinobacillus pleuropneumoniae and Histophilus somni (17), while HTM and cation-adjusted Mueller-Hinton broth (CAMHB) supplemented with 2.5% to 5% lysed horse blood (LHB) are CLSI-approved broth media for the testing of fastidious organisms, with HTM being recommended for Haemophilus influenzae and Haemophilus parainfluenzae (18). As it has been seen that VFM is not suitable for all H. parasuis isolates, a broth microdilution susceptibility testing method with test medium broth (TMB) has recently been proposed (7). TMB is a complex medium consisting of 1% (wt/vol) Biosate peptone, 1% (wt/vol) sodium chloride, 0.1% (wt/vol) starch, 0.1% (wt/vol) glucose, and 0.05% (wt/vol) yeast extract, which is supplemented with 0.0025% (vol/vol) NADH, 0.0005% (vol/vol) thiamine HCl, 1% (vol/vol) sterile filtered heat-inactivated chicken serum, and 5% (vol/vol) oleic acid bovine albumin complex. The oleic acid bovine albumin complex is composed of saline with 0.06% (vol/vol) oleic acid and 5% (vol/vol) 0.05 N NaOH, which is finally enriched with 4.75% (vol/vol) bovine serum albumin (7).
Therefore, this study was aimed at (i) comparatively analyzing the suitability of different broth media for susceptibility testing of preferably a wide range of different H. parasuis serovars and (ii) identifying a broth medium for a harmonized routine diagnostic approach. For this, a new medium, CAMHB with a reduced content of supplements, was developed and used for testing of 46 H. parasuis field isolates, including representatives of 13 serovars, in comparison to TMB. Subsequently, statistical analysis was performed to examine the homogeneity of MIC data and to investigate the difference between supplemented CAMHB and TMB. Hence, this study might contribute to establish a standardized broth microdilution susceptibility testing method for H. parasuis.
RESULTS
Growth of H. parasuis in broth media.
To determine the adequacy of the broth media for susceptibility testing, type strain DSM 21448 and three H. parasuis field isolates of serovars 5 (two isolates) and 14 (one isolate) were chosen. The isolates of serovar 5 were included because this serovar is regarded as the most pathogenic H. parasuis serovar in Germany. As well, two of the isolates (no. 24 of serovar 14 and 52 of serovar 5) were selected because they are extremely slow-growing isolates and difficult to culture. The growth curves from two independent experiments revealed that all isolates showed almost consistent growth in VFM and TMB. However, in CAMHB plus 2.5% LHB and HTM, a decrease of the isolates in CFU values over time was detected after only a few hours, except for type strain DSM 21448 (see Fig. S1 to S5 in the supplemental material).
In addition, visible growth of 28 H. parasuis field isolates of various serovars was assessed in microtiter plates with and without antibiotic coating, and in agreement with results from growth determination, no growth was detected in any of the wells when HTM and CAMHB plus 2.5% LHB were used. With VFM, visible growth could be detected only for seven isolates, although the H. parasuis type strain and all three field isolates showed a sufficient increase in CFU/ml during growth curve determination. However, MIC endpoints could be determined in VFM only for the type strain. In contrast to these findings, visible growth of all tested H. parasuis isolates was detected in microtiter plate wells while testing TMB.
Supplemented CAMHB for susceptibility testing of H. parasuis.
CAMHB enriched with the four supplements used in TMB led to visible growth of H. parasuis isolates tested in 96-well plates. The amount of growth in the wells was comparable to the amount of growth in microtiter panels with TMB medium (Table 1). Using only three supplements with CAMHB, tests revealed that NADH was essential for the growth of all H. parasuis isolates and omitting one of the supplements thiamine, sterile filtered inactivated chicken serum, and O-A complex showed no difference in visible growth. To further reduce the number of supplements, it was evaluated if growth of the H. parasuis isolates varied when only two supplements were added to CAMHB. Results of these experiments showed that supplementation of sterile filtered inactivated chicken serum or O-A complex is necessary to obtain visible growth of H. parasuis isolates in CAMHB (Table 1). As O-A complex consists of two different components (oleic acid and bovine serum albumin) and therefore is a complex supplement to prepare, sterile filtered inactivated chicken serum together with NADH was chosen to supplement CAMHB for broth microdilution susceptibility testing of H. parasuis isolates. Finally, the recipe for the supplemented CAMHB was CAMHB plus 0.0025% (vol/vol) NADH and 1% (vol/vol) sterile filtered heat-inactivated chicken serum.
TABLE 1.
Supplementation of CAMHB and determination of visible growth of 15 H. parasuis isolates
| CAMHB supplementa |
Visible growth in microtiter platesb | |||
|---|---|---|---|---|
| NADH (0.0025%) | Heat-inactivated chicken serum (1%) | Thiamine (0.0005%) | O-A complex (5%) | |
| + | + | + | + | +++ |
| + | + | + | − | +++ |
| + | + | − | + | +++ |
| + | − | + | + | +++ |
| − | + | + | + | − |
| + | + | − | − | +++ |
| + | − | − | + | +++ |
| + | − | + | − | − |
| − | + | + | − | − |
| − | + | − | + | − |
| − | − | + | + | − |
| + | − | − | − | − |
| − | − | − | − | − |
+, supplement was added to CAMHB; −, supplement was not added to CAMHB.
The following scheme was used to grade the visible growth of H. parasuis: +++, excellent growth (size of buttons is comparable to the size of buttons with TMB); ++, fair growth; +, poor growth; −, no growth.
Subsequently, colony count determinations during culturing of type strain DSM 21448 and three H. parasuis field isolates in supplemented CAMHB were performed, and adequate growth of all isolates could be detected (see Fig. S6 in the supplemental material).
Control of different batches and producers.
MICs of type strain DSM 21448 and three field isolates using two different batches and another two producers of NADH and chicken serum showed comparable results after 24 h of incubation, with a maximum deviation of one dilution step.
Test ranges of QC strains.
To investigate the influence of test media on MICs of quality control (QC) strains, broth microdilution susceptibility testing of four QC strains was performed using TMB and supplemented CAMHB, although QC ranges have been established in unsupplemented CAMHB or in VFM (Actinobacillus pleuropneumoniae). Threefold-repeated susceptibility testing revealed that MICs from 261 out of 270 QC strain-antimicrobial agent combinations were within acceptable ranges in both media (17). The only exceptions for TMB were the combinations A. pleuropneumoniae with gentamicin and A. pleuropneumoniae with the antibiotics gentamicin and penicillin in supplemented CAMHB in all three repetitions.
Homogeneity of H. parasuis MICs.
To assess the homogeneity of MICs obtained with supplemented CAMHB and TMB with two different incubation times, the H. parasuis type strain and the two field isolates included in the growth curve determinations were used. As the third H. parasuis field isolate (no. 52) showed visible growth only after 48 h in supplemented CAMHB, this isolate was omitted from this analysis. The MIC determinations were repeated in five independent experiments. A panel of 21 antimicrobials and antimicrobial combinations was included in the repeated tests, and two different incubation times, 16 to 20 h and 24 h, were analyzed. Results from the repeated tests are shown in Tables 2 and 3. The multifactorial logistic regression analysis with the 11 antibiotics fulfilling statistical requirements revealed that no significant influence of the different incubation times on MICs obtained in both media was observed, either for the evaluation of data by exact MIC agreement (P = 0.5165) or by essential MIC agreement accepting a deviation of ±1 dilution step (P = 0.1913). For the exact MIC agreement, the percentage of homogeneous MICs was 25.8% after 16 to 20 and 30.3% after 24 h of incubation. The percentages were 92.4% after 16 to 20 h and 97.0% after 24 h of incubation for the essential MIC agreement. When comparing the homogeneity of MICs (i.e., all five MICs were identical) obtained in the two media, supplemented CAMHB showed 27.3% homogeneous MICs, while TMB gave a figure of 28.8%, with the difference not being significant (P = 0.8282). For the MIC mode accepting a deviation of ±1 dilution step, 90.9% of MICs showed homogeneous MICs for supplemented CAMHB, while 98.5% of the MICs were homogeneous for TMB (P = 0.0567). No significant influence on the homogeneity of MICs was seen for the exact (P = 0.5584) and the essential MIC agreement (P = 0.9954) for the antimicrobial agents. In contrast, the bacterial strains had a significant influence on the homogeneity of MICs for the exact MIC agreement (P = 0.0005), whereas no significant influence was seen for the essential MIC agreement (P = 0.4757).
TABLE 2.
Homogeneity of MICs obtained with supplemented CAMHB by using two different incubation times
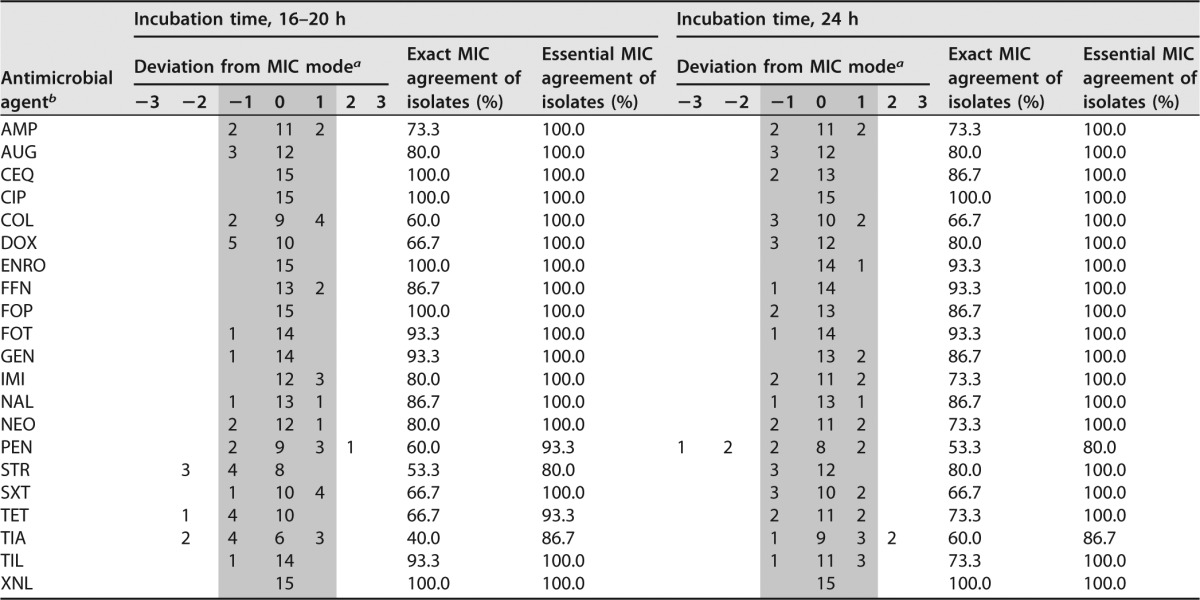
a Data fulfilling the criteria of the essential MIC agreement are in the gray area.
b AMP, ampicillin; AMC, amoxicillin-clavulanic acid; CIP, ciprofloxacin; COL, colistin; CQN, cefquinome; DOX, doxycycline; ENRO, enrofloxacin; FFN, florfenicol; FOP, cefoperazone; FOT, cefotaxime; GEN, gentamicin; IMI, imipenem; MAR, marbofloxacin; NAL, nalidixic acid; NEO, neomycin; PEN, penicillin; STR, streptomycin; SXT, trimethoprim-sulfamethoxazole; TET, tetracycline; TIA, tiamulin; TIL, tilmicosin; XNL, ceftiofur.
TABLE 3.
Homogeneity of MICs obtained with TMB by using two different incubation timesa
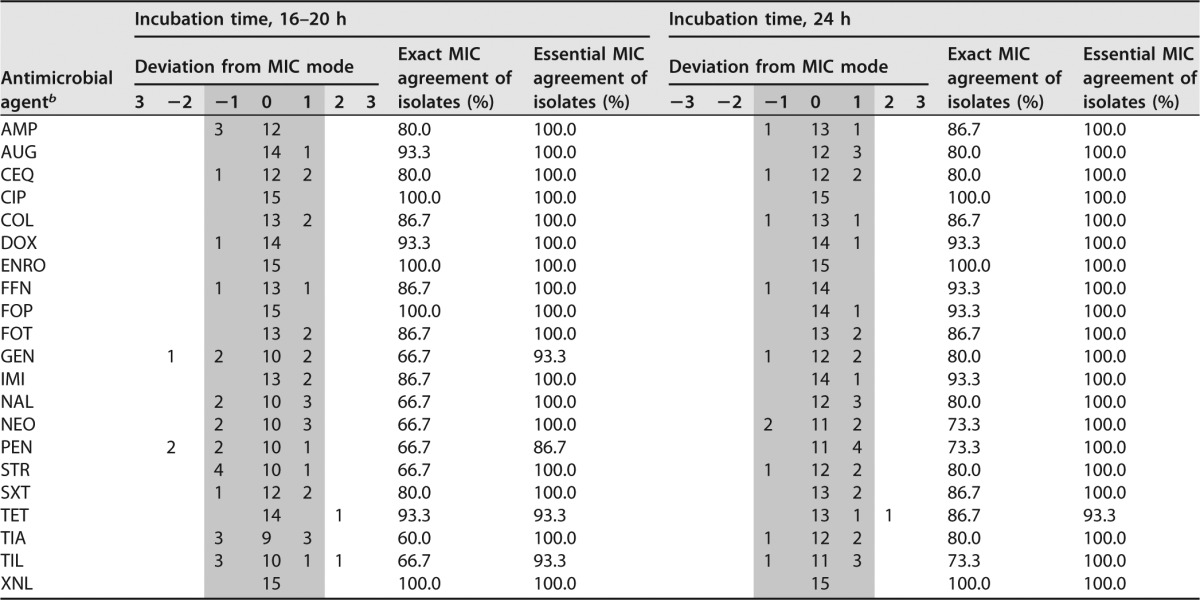
Data fulfilling the criteria of the essential MIC agreement are in the gray area.
b AMP, ampicillin; AMC, amoxicillin-clavulanic acid; CIP, ciprofloxacin; COL, colistin; CQN, cefquinome; DOX, doxycycline; ENRO, enrofloxacin; FFN, florfenicol; FOP, cefoperazone; FOT, cefotaxime; GEN, gentamicin; IMI, imipenem; NAL, nalidixic acid; NEO, neomycin; PEN, penicillin; STR, streptomycin; SXT, trimethoprim-sulfamethoxazole; TET, tetracycline; TIA, tiamulin; TIL, tilmicosin; XNL, ceftiofur.
Results from MIC determinations of H. parasuis field isolates.
Using both media, supplemented CAMHB and TMB, there was no difficulty in determining visible growth of all 47 H. parasuis isolates in the wells of microtiter plates and reading of MIC endpoints was feasible. Control of inoculum density showed the required inoculum size of 5 × 105 CFU/ml. The MICs of the isolates are presented in Table 4 and in Table S1 in the supplemental material.
TABLE 4.
MIC distribution of 47 H. parasuis isolates after 24 h of incubation in supplemented CAMHB
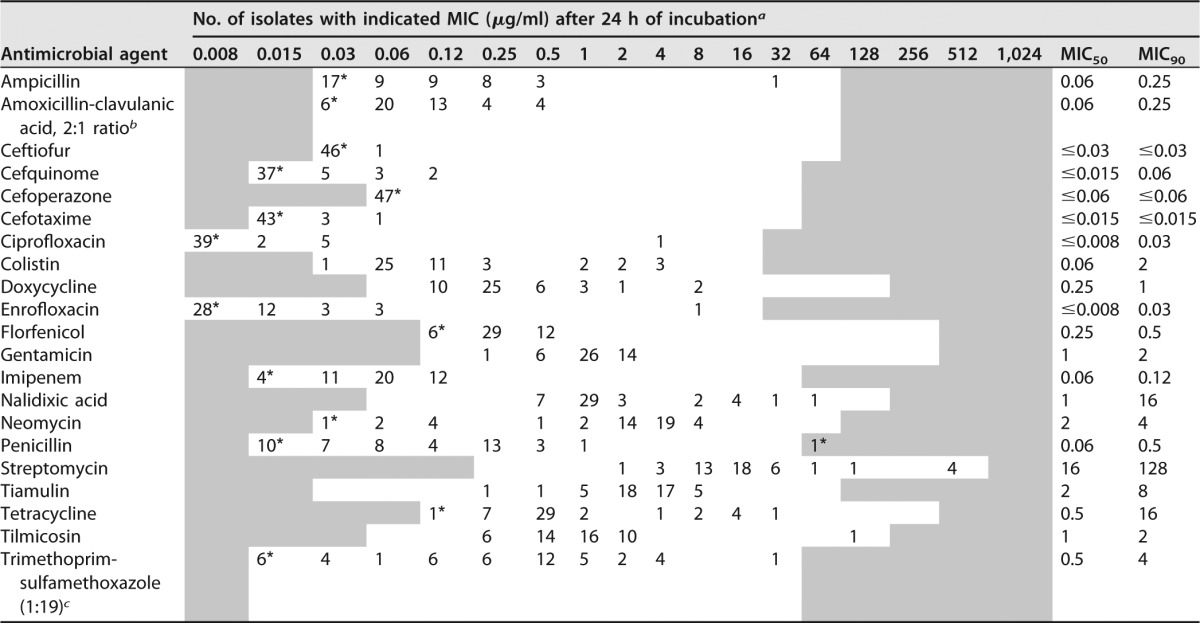
a Asterisked numbers are the number of isolates with MICs equal to or higher or lower than concentrations of the tested range. The tested range of the antimicrobials agents is represented in the white area.
b Data represent the concentration of amoxicillin.
c Data represent the concentration of trimethoprim.
Considering the results from supplemented CAMHB, a bimodal distribution of MICs was detected for the antimicrobial agents ampicillin, ciprofloxacin, colistin, doxycycline, enrofloxacin, nalidixic acid, neomycin, penicillin, streptomycin, tetracycline, and tilmicosin, whereas a unimodal distribution was determined for the remaining antibiotics tested. However, the broadest distribution of MICs was found for the antibiotic combination trimethoprim-sulfamethoxazole. As there are no CLSI-recommended breakpoints for H. parasuis isolates available, a classification of isolates as susceptible or resistant was not possible. However, the majority of isolates proved to have very low MIC50s and MIC90s (equal to or below 0.5 μg/ml) for all β-lactam antimicrobials, and only a couple of isolates showed elevated MICs in comparison to the majority of isolates (Table 4).
Comparative analysis of MICs.
For simplified interpretability of MICs, an incubation time of 24 h was chosen for comparative analysis. Comparing MIC50s and MIC90s of supplemented CAMHB and TMB, slight differences were detected. Five out of 21 MIC50s were up to two dilution steps higher when using TMB, while it was seen that one MIC50 was one dilution step lower. The MIC90s of cefquinome, florfenicol, streptomycin, and tiamulin differed by one dilution step, with lower values detected while using TMB.
The entire comparison of MICs of 47 isolates and 15 antimicrobial agents fulfilling the inclusion criteria for statistical analysis, independent of MIC50s and MIC90s, showed no significant difference (P = 0.2076) between the two media. However, statistical analysis of individual antimicrobial agents showed significant differences of MICs between the two media for the following antimicrobial agents, which were significantly higher in TMB for the antimicrobials colistin (P = 0.0038), gentamicin (P = 0.0006), and neomycin (P < 0.0001) and significantly lower in TMB for doxycycline (P = 0.0010), florfenicol (P = 0.0003), streptomycin (P = 0.0127), and tiamulin (P = 0.0110).
DISCUSSION
In a recent study, VFM was reported to be unsuitable for broth microdilution susceptibility testing of Australian H. parasuis isolates, and TMB was proposed (7). However, currently there is no standardized method available in the CLSI guidelines for H. parasuis and the usage of different methods can lead to variable MIC results and poor comparability of results (17, 19). Furthermore, due to high losses on pig farms worldwide caused by H. parasuis (20), finding an appropriate and agreed-upon method of susceptibility testing for this important veterinary pathogen is urgently required.
In this study, colony count determinations during culturing in four different media detected growth of H. parasuis field isolates in VFM and TMB, whereas only growth of type strain DSM 21448 could be detected in HTM and CAMHB plus 2.5% LHB. Surprisingly, visible growth in microtiter plate wells was detected in only 25% of the tested H. parasuis field isolates cultured in VFM. The reason why no visible growth in microtiter plates was seen in VFM, although growth could be obtained in growth curves using culture enumeration, is not clear. The absence of visible growth in CAMHB plus 2.5% LHB, HTM, and VFM indicate that all three media are not suitable for broth microdilution susceptibility testing of H. parasuis isolates. In contrast, TMB promotes the growth of all H. parasuis isolates from Germany and even slow-growing isolates, which were difficult to culture on agar plates, showed visible growth in microtiter plate wells. TMB is not commercially available, and preparing the medium in routine diagnostic laboratories is a markedly complex task, as the medium consists of several components and supplements. Additionally, there are issues about batch-to-batch reproducibility and a low content of inhibitors that affect antimicrobial agents that still have to be addressed. If a nonapproved medium is used for broth microdilution susceptibility testing, validation of the medium has to undergo an extensive procedure. One important factor is the applicability of QC strains and their MICs within a specified range. In this study, nearly all MICs of QC strains were in the acceptable range with both media used, although the QC ranges have been established in media other than TMB or supplemented CAMHB, and therefore, it was not expected to have all MICs in the published ranges. It should be noted that the QC strain A. pleuropneumoniae is also a fastidious bacterium, and susceptibility testing in VFM medium according to CLSI document VET01-A4 is recommended (17). This could be the reason for the deviation of a few MICs from the acceptable range of the QC strain A. pleuropneumoniae. However, the results from QC strains indicate that there is no effect of medium components on the activity of antimicrobial agents.
A new medium was established for routine broth microdilution susceptibility testing of H. parasuis, based on CAMHB and enriched with essential supplements (7, 21). CAMHB was used since it is a validated and agreed medium for susceptibility testing with an optimal rate of cations, showing high reproducibility of MIC data and being recommended in CLSI document VET01-A4 for MIC determination of rapidly replicating bacteria. It is also low in inhibitors for sulfonamides, trimethoprim and tetracycline. Since supplementation of CAMHB with 2.5% LHB resulted in the absence of growth of H. parasuis field isolates, the essential supplements (NADH and sterile filtered inactivated chicken serum) from TMB were chosen to enrich CAMHB in the same concentrations as in TMB (7). Importantly, NADH-containing HTM is recommended in CLSI document M07-A9 (18) for susceptibility testing of fastidious organism such as Haemophilus influenzae and is therefore a CLSI-verified supplement.
Statistical analysis revealed that both incubation times tested had no influence on the homogeneity of MICs. Due to the low growth rate of H. parasuis compared to other rapidly replicating bacteria and few isolates showing weak growth after 16 to 20 h of incubation, an incubation time of 24 h is suggested, which might be extended to 48 h for isolates which show no growth after 24 h.
The supplemented CAMHB appears to be a suitable medium for broth microdilution susceptibility testing of the majority of H. parasuis isolates from Germany. As the medium had no significant influence on the homogeneity of the MICs and although TMB seems to support the growth of H. parasuis isolates slightly better, CAMHB provides the advantage of a validated basic broth medium (17). Preparation of this medium is less challenging and sensitive and could be used in routine diagnostic laboratories.
Due to the lack of specific breakpoints for H. parasuis, a classification of the 46 field isolates into the categories susceptible, intermediate, and resistant could not be obtained, and percentages of resistances could therefore not be calculated. Nevertheless, bimodal distributions of MICs were indicative of the presence of nonwild type isolates. In a study by Dayao et al., MIC50s and MIC90s of 97 H. parasuis isolates from Australia were tested in TMB, and it was seen that comparable values with a maximum of one dilution step difference were obtained for ampicillin, florfenicol, penicillin, tetracycline, and trimethoprim-sulfamethoxazole. However, for tilmicosin, the MIC90 was four dilution steps higher than in the present study, indicating a difference in MIC distribution of Australian and German isolates tested (7).
Conclusion.
For broth microdilution susceptibility testing of H. parasuis, supplemented CAMBH and TMB have been shown to be most suitable. The use of both media resulted in reproducible and comparable MICs, with MICs of quality control strains being in the acceptable range for nearly all antimicrobial agents tested. Supplemented CAMHB is recommended for routine diagnostics since it is based on an agreed-upon and validated base medium, which is commercially available. For H. parasuis susceptibility testing, an incubation time of 24 h is recommended. The proposed method enables broth microdilution susceptibility testing of H. parasuis isolates in routine diagnostics and might contribute to establishment of a standardized method for this important veterinary pathogen.
MATERIALS AND METHODS
Bacterial isolates.
H. parasuis type strain DSM 21448 (Leibniz-Institute DSMZ, Braunschweig, Germany) and 46 H. parasuis field isolates were used for the present study. The isolates originated from diseased pigs and were collected at pig farms from different geographic regions in Germany between 2011 and 2013. All field isolates were provided by diagnostic laboratories in Germany and included 25 serotyped isolates of serovars 1 (n = 2), 2 (n = 2), 4 (n = 2), 5 (n = 2), 6 (n = 2), 8 (n = 2), 9 (n = 1), 10 (n = 2), 11 (n = 2), 12 (n = 2), 13 (n = 2), 14 (n = 2), and 15 (n = 2). Isolates were cultured on chocolate agar with 10% defibrinated horse blood in ambient air at an incubation temperature of 35°C ± 2°C for 24 to 48 h. To confirm that the field isolates were H. parasuis, a PCR assay amplifying an 821-bp internal fragment of the 16S small subunit rRNA gene was used as described earlier (20).
Growth curves.
To compile growth curves, type strain DSM 21448 of H. parasuis and three field isolates of serovars 5 (2 isolates) and 14 (1 isolate) were used. Growth curves were performed in four different broth media (HTM, TMB, VFM, and CAMHB plus 2.5% LHB), as these media have been proven suitable for susceptibility testing of fastidious organisms (7, 10, 11, 21–23). In brief, isolates were grown on chocolate agar for 24 to 48 h at 35°C ± 2°C in ambient air, colonies were suspended in 0.9% saline, and the suspension was adjusted to an initial concentration of approximately 103 to 104 CFU/ml. Isolates were cultured in a volume of 5 ml for 48 h at 35°C ± 2°C in ambient air without shaking, and the CFU count per milliliter was determined by culture enumeration every 4 h (up to h 24) and 8 h (up to h 48), respectively. The weighted average was calculated from agar plates containing between 5 and 200 CFU/ml.
Development of a modified broth medium.
As TMB is not commercially available and to provide an easier-to-prepare medium for routine diagnostic laboratories, a new medium based on CAMHB (Oxoid, Wesel, Germany) was developed for broth microdilution susceptibility testing of H. parasuis. For this purpose, which of the supplements of TMB were essential to provide sufficient growth of preferably all H. parasuis isolates and serovars was evaluated. CAMHB was taken as a basis since it is the CLSI-approved medium of choice for susceptibility testing of commonly isolated pathogens. For supplementation of CAMHB, the medium was first tested with all TMB supplements, and then one supplement at a time was removed. A list of all formulations tested is given in Table 1. Supplements were added to CAMHB aseptically after autoclaving, and concentrations of supplements were equal to concentrations used in TMB. As pH measurements showed values between pH 7.2 and 7.4, a pH adjustment of the supplemented CAMHB was not necessary.
The necessity of the supplements was tested against a set of 15 H. parasuis isolates in 96-well microtiter plates, and visible growth in the wells was determined (Table 1). NADH (β-NAD, reduced form, disodium salt) (Armin Baack, Schwerin, Germany, and Sigma-Aldrich, Seelze, Germany) and sterile filtered chicken serum (Bio&Sell, Feucht, Germany, and Pan-Biotech, Aidenbach, Germany) of two different producers were verified by broth microdilution susceptibility testing with type strain DSM 21448 and the three field isolates with 21 antimicrobial agents.
Susceptibility testing.
For broth microdilution susceptibility testing, customized 50-μl/well microtiter plates (Sensititre, East Grinstead, UK) coated with vacuum-dried antibiotics were used. Testing included 21 antimicrobial agents and antibiotic combinations (Table 5), four of which are currently not licensed for food-producing animals and were included for comparison reasons (cefotaxime, ciprofloxacin, imipenem, and nalidixic acid). Inoculum preparation and density, incubation conditions, and MIC endpoint determinations for H. parasuis followed the recommendations given in CLSI documents M07-A9 and VET01-A4 for rapidly replicating bacteria (17, 18). The direct colony suspension method was used for inoculum preparation. In brief, colonies were selected from a 24-h agar plate, inoculated into 0.9% saline, and adjusted to a turbidity comparable to that of a 0.5 McFarland standard. The saline suspension was diluted 1:200 in broth medium (e.g., CAMHB with 0.0025% NADH and 1% sterile filtered heat-inactivated chicken serum) to yield an inoculum density of approximately 5 × 105 CFU/ml. A volume of 50 μl of this suspension was added to each well of the microtiter plates. Reading of microtiter plates was done visually after 16 to 20 h and 24 h of incubation of plates in an ambient-air incubator. An inoculum density of approximately 5 × 105 CFU/ml was verified by inoculum control as described in document M07-A9. To assess the homogeneity of MICs, broth microdilution susceptibility testing was performed in five replicates. The quality control strains Escherichia coli (ATCC 25922), Enterococcus faecalis (ATCC 29212), Staphylococcus aureus (ATCC 29213), and A. pleuropneumoniae (ATCC 27090) served for quality control procedures along with their CLSI-recommended media (17, 24).
TABLE 5.
Test ranges of antimicrobial agents included in this study
| Antimicrobial agent | Range (μg/ml) |
|---|---|
| Ampicillin | 0.03–64 |
| Amoxicillin-clavulanic acid (2:1 ratio) | 0.03/0.015–64/32 |
| Ceftiofur | 0.03–64 |
| Cefquinome | 0.015–32 |
| Cefoperazone | 0.06–32 |
| Cefotaxime | 0.015–32 |
| Ciprofloxacin | 0.006–16 |
| Colistin | 0.03–16 |
| Doxycycline | 0.06–128 |
| Enrofloxacin | 0.008–16 |
| Florfenicol | 0.12–256 |
| Gentamicin | 0.12–256 |
| Imipenem | 0.015–32 |
| Nalidixic acid | 0.06–128 |
| Neomycin | 0.03–128 |
| Penicillin | 0.015–32 |
| Streptomycin | 0.25–256 |
| Tiamulin | 0.03–64 |
| Tetracycline | 0.12–128 |
| Tilmicosin | 0.06–64 |
| Trimethoprim-sulfamethoxazole (1:19 ratio) | 0.015/0.3–32/608 |
When comparing MICs generated in TMB and supplemented CAMHB, all 47 H. parasuis isolates were used.
Data evaluation and statistical analysis.
The primary aims of this study were the exploration of MICs and the investigation of differences in MICs when using two different media (supplemented CAMHB and TMB). Of secondary interest was a comparison of the stability of MICs after 16 to 20 h and 24 h of incubation.
To investigate whether the stability of the MICs differed between the two incubation times, for each combination of isolate, medium, antibiotic agent and incubation time, MIC data received from five measurements were summarized to a dichotomous outcome. Evaluation was done by taking one model with two variables (precondition of five identical MICs, here referred to as exact MIC agreement, and MIC mode accepting a deviation of ±1 dilution step, referred to as essential MIC agreement, respectively) into consideration. The outcome was set to 1 if preconditions were fulfilled according to the variable and 0 otherwise. Stability of measurements was analyzed based on this outcome with a multifactorial logistic regression model depending on isolate, medium, antibiotic agent, and incubation time. Differences in MICs depending on incubation time were investigated with an analysis of variance (ANOVA) model, modeling the log-transformed MICs depending on the four factors. Due to the distribution of MICs being skewed to the right and a 2-fold dilution series being used, data were log transformed to the base 2. The media were compared separately for antibiotic agents using the two-sided one-sample t test for the differences between the two media. Due to the explorative nature of the experiments, no multiple adjustments were performed and comparison-wise P values were reported. When analyzing the data, antimicrobial agents were omitted from statistical analysis when more than 50% of isolates had a MIC at the lowest concentration tested. Statistical analyses were carried out using SAS software, version 9.3 (SAS Institute, Cary, NC) (25).
Supplementary Material
ACKNOWLEDGMENTS
We thank Vera Nöding, Iris Oltrogge, and Inna Pahl for excellent technical assistance.
This study was financially supported by the BMEL through the DART project (grant number FKZ 2811HS010).
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.01403-16.
REFERENCES
- 1.Holtkamp D, Rotto H, Garcia R. 2007. Economic cost of major health challenges in large US swine production systems. Swine News 30:85–90. [Google Scholar]
- 2.Oliveira S, Pijoan C. 2004. Haemophilus parasuis: new trends on diagnosis, epidemiology and control. Vet Microbiol 99:1–12. doi: 10.1016/j.vetmic.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Brockmeier SL, Loving CL, Mullins MA, Register KB, Nicholson TL, Wiseman BS, Baker RB, Kehrli ME. 2013. Virulence, transmission, and heterologous protection of four isolates of Haemophilus parasuis. Clin Vaccine Immunol 20:1466–1472. doi: 10.1128/CVI.00168-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapp-Gabrielson VJ, Gabrielson DA. 1992. Prevalence of Haemophilus parasuis serovars among isolates from swine. Am J Vet Res 53:659–664. [PubMed] [Google Scholar]
- 5.Kielstein P, Rapp-Gabrielson V. 1992. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J Clin Microbiol 30:862–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang JM, Shen HY, Liao M, Ren T, Guo LL, Xu CG, Feng SX, Fan HY, Li JY, Chen JD, Zhang B. 2012. Detection of Haemophilus parasuis isolates from South China by loop-mediated isothermal amplification and isolate characterisation. Onderstepoort J Vet Res 79:E1–E6. [DOI] [PubMed] [Google Scholar]
- 7.Dayao DA, Kienzle M, Gibson JS, Blackall PJ, Turni C. 2014. Use of a proposed antimicrobial susceptibility testing method for Haemophilus parasuis. Vet Microbiol 172:586–589. doi: 10.1016/j.vetmic.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz S, Silley P, Simjee S, Woodford N, van Duijkeren E, Johnson AP, Gaastra W. 2010. Assessing the antimicrobial susceptibility of bacteria obtained from animals. Vet Microbiol 141:1–4. doi: 10.1016/j.vetmic.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Xu X, Zhao Y, Chen P, Zhang X, Chen H, Cai X. 2010. Distribution of antimicrobial resistance among different serovars of Haemophilus parasuis isolates. Vet Microbiol 141:168–173. doi: 10.1016/j.vetmic.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Aarestrup FM, Seyfarth AM, Angen O. 2004. Antimicrobial susceptibility of Haemophilus parasuis and Histophilus somni from pigs and cattle in Denmark. Vet Microbiol 101:143–146. doi: 10.1016/j.vetmic.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 11.de la Fuente AJ, Tucker AW, Navas J, Blanco M, Morris SJ, Gutierrez-Martin CB. 2007. Antimicrobial susceptibility patterns of Haemophilus parasuis from pigs in the United Kingdom and Spain. Vet Microbiol 120:184–191. doi: 10.1016/j.vetmic.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Nedbalcová K, Kučerová Z. 2013. Antimicrobial susceptibility of Pasteurella multocida and Haemophilus parasuis isolates associated with porcine pneumonia. Acta Veterinaria Brno 82:3–7. doi: 10.2754/avb201382010003. [DOI] [Google Scholar]
- 13.Guo L-L, Zhang J-M, Xu C-G, Ren T, Zhang B, Chen J-D, Liao M. 2012. Detection and characterization of β-lactam resistance in Haemophilus parasuis strains from pigs in South China. J Integr Agricul 11:116–121. doi: 10.1016/S1671-2927(12)60789-5. [DOI] [Google Scholar]
- 14.DeRosa DC, Veenhuizen MF, Bade DJ, Shryock TR. 2000. In vitro susceptibility of porcine respiratory pathogens to tilmicosin. J Vet Diagn Invest 12:541–546. doi: 10.1177/104063870001200608. [DOI] [PubMed] [Google Scholar]
- 15.Xu C, Zhang J, Zhao Z, Guo L, Zhang B, Feng S, Zhang L, Liao M. 2011. Antimicrobial susceptibility and PFGE genotyping of Haemophilus parasuis isolates from pigs in South China (2008–2010). J Vet Med Sci 73:1061–1065. doi: 10.1292/jvms.10-0515. [DOI] [PubMed] [Google Scholar]
- 16.San Millan A, Escudero JA, Catalan A, Nieto S, Farelo F, Gibert M, Moreno MA, Dominguez L, Gonzalez-Zorn B. 2007. Beta-lactam resistance in Haemophilus parasuis is mediated by plasmid pB1000 bearing blaROB-1. Antimicrob Agents Chemother 51:2260–2264. doi: 10.1128/AAC.00242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial disc and dilution susceptibility test for bacteria isolated from animals, 4th ed Approved standard VET01-A4. CLSI, Wayne, PA. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 9th ed Approved standard M07-A9. CLSI, Wayne, PA. [Google Scholar]
- 19.Kahlmeter G, Brown DF, Goldstein FW, MacGowan AP, Mouton JW, Osterlund A, Rodloff A, Steinbakk M, Urbaskova P, Vatopoulos A. 2003. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J Antimicrob Chemother 52:145–148. doi: 10.1093/jac/dkg312. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira S, Galina L, Pijoan C. 2001. Development of a PCR test to diagnose Haemophilus parasuis infections. J Vet Diagn Invest 13:495–501. doi: 10.1177/104063870101300607. [DOI] [PubMed] [Google Scholar]
- 21.Blackall PJ. 1988. Antimicrobial drug resistance and the occurrence of plasmids in Haemophilus paragallinarum. Avian Dis 32:742–747. doi: 10.2307/1590993. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen JH, Ferraro MJ. 2000. Antimicrobial susceptibility testing: special needs for fastidious organisms and difficult-to-detect resistance mechanisms. Clin Infect Dis 30:799–808. doi: 10.1086/313788. [DOI] [PubMed] [Google Scholar]
- 23.Barry AL, Pfaller MA, Fuchs PC. 1993. Haemophilus test medium versus Mueller-Hinton broth with lysed horse blood for antimicrobial susceptibility testing of four bacterial species. Eur J Clin Microbiol Infect Dis 12:548–553. doi: 10.1007/BF01970963. [DOI] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals, 3rd ed CLSI supplement VET01S. CLSI, Wayne, PA. [Google Scholar]
- 25.SAS Institute Inc. 2011. SAS/STAT 9.3 user's guide. SAS Institute Inc, Cary, NC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


