Abstract
Paeoniflorn (PF), the principal bioactive component of Paeoniae radix prescribed in traditional Chinese medicine, possesses a wide range of biological effects and exhibits neuroprotective effects in numerous diseases. Previous studies have demonstrated that PF significantly attenuates memory impairment in rats with vascular dementia (VD). In the present study, a bilateral common carotid artery occlusion (BCCAO) rat model was used to explore the underlying mechanisms of PF. The expression levels of neuron-specific enolase (NSE), S100β, B-cell lymphoma 2 (Bcl-2), Bcl-2 associated X protein, cytochrome c and brain-derived neurotrophic factor (BDNF) in the hippocampus were measured by western blot analysis. The results showed that administration of PF for 28 days significantly decreased the expression levels of NSE and S100β, both sensitive markers for brain damage, in vascular dementia (VD) model rats. In addition, PF inhibited the initiation of apoptotic cell death and attenuated the decreased expression levels of BDNF induced by bilateral common carotid artery occlusion. These data confirm the neuroprotective effects of PF on VD and provide a novel insight into the long-term use of PF as a potential treatment in the stages of early cognitive impairment in VD.
Keywords: paeoniflorin, vascular dementia, hippocampus, apoptosis
Introduction
Vascular dementia (VD) is the second most common type of dementia after Alzheimer's disease (1,2). It is predicted that by the year 2040, the number of people with dementia worldwide will reach 81.1 million, with the number of dementia patients in China being the sum of all those in all the developed countries combined (3). The progressive nature of VD leads to an unremitting and largely irreversible deterioration in quality of life, and places a heavy emotional and economic burden on families (4). However, currently there are no drugs licensed for the treatment of vascular cognitive impairment. Thus, there is an urgent requirement to develop therapeutic agents to prevent vascular dementia.
VD is caused by a reduced blood flow to the brain or an impaired vascular system (5). As the population ages and cerebrovascular disease prevalence increases, the occurrence of VD increases as well (6). Etiopathogenic mechanisms causing VD include oxidative stress, cytotoxicity of reactive oxygen species, mitochondrial dysfunction and apoptosis (7,8). The rat bilateral common carotid artery occlusion (BCCAO) model is the most common model used to investigate the effect of chronic cerebral hypoperfusion-induced cognitive dysfunction, and is used to screen drugs with potential therapeutic value for VD (9). Reduced blood flow can result in neuronal energy failure and promote the production of reactive oxygen species, which initiate neuronal apoptosis and lead to the functional deficits typical of VD (10).
Paeoniflorn (PF) (Fig. 1) is the principal bioactive component of Paeoniae radix which has been used for >1,000 years in traditional Chinese medicine (11). In modern pharmacological studies, PF exhibits numerous pharmacological effects such as anti-inflammation, antioxidant, endothelium-dependent vasorelaxation, neuromuscular blocking and cognition enhancement properties (12,13). Xiao et al (14) demonstrated that PF is able to reverse, or alleviate, impairments. such as cerebral infarction, neurological symptoms, tongue protrusion and performance in the water maze, at the chronic stage of transient middle cerebral artery occlusion in rats. Our previous studies have demonstrated that PF significantly attenuates memory impairment in BCCAO model rats (unpublished; Fig. 2) (15); however, the molecular mechanisms underlying the protective effects of PF on VD have not been clearly identified. Therefore, the present study focuses on evaluating the neuroprotective effect of PF and its relation to cell apoptosis in a BCCAO rat model.
Figure 1.

Chemical structure of paeoniflorin.
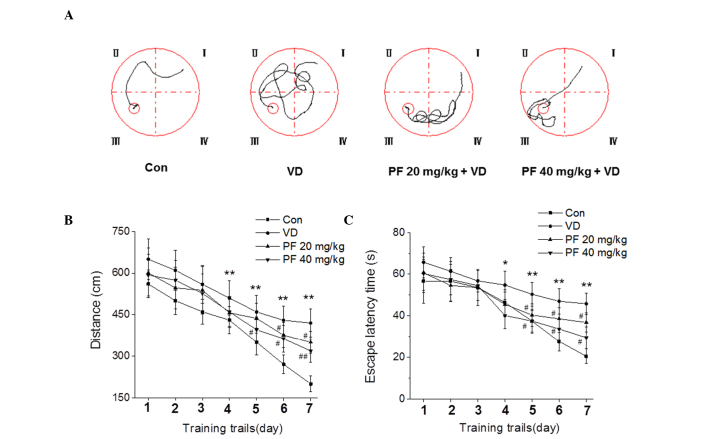
Figure 2.
PF attenuates learning and memory impairment in VD rats in the Morris water maze test. (A) Pathway maps of searching for the hidden platform. (B) The distance moved to reach the hidden platform. (C) The mean escape latency time that rats spent finding the hidden platform. *P<0.05, **P<0.01 vs. the control group; #P<0.05, ##P<0.05 vs. the VD group. n=7. Two-way analysis of variance followed by a Bonferroni multiple group comparison were used. Con, control; VD, vascular dementia; PF, paeoniflorn.
Materials and methods
Animals
Male Sprague Dawley rats (16–18 months old; 300–450 g body weight) were obtained from the Vital River Laboratories (Beijing, China). Animals were housed with ad libitum access to food and water at a temperature of 22±2°C, humidity of 55±5% and a 12-h light/dark cycle. The study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (16). The protocol was approved by the Committee on the Ethics of Animal Experiments of the National Research Institute of Family Planning.
Reagents
PF (purity >98%) was purchased from Shandong Zhongxing Guangrun Biotechnology Co., Ltd. (Nanjing, China). The following primary antibodies were used in western blot analysis: Anti-neuron-specific endolase (NSE) (sc-292097), anti-S100β (sc-28533), anti-brain-derived neurotrophic factor (BDNF) (sc-20981), anti-B-cell lymphoma 2 (Bcl-2) (sc-492), anti-Bcl-2 associated X protein (Bax) (sc-493) and anti-cytochrome c (sc-7159) (all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Study design
PF and saline were administered by oral gavage daily for 28 days. Rats were randomized into the following four groups prior to surgery, according to a computer-generated randomization schedule (n=10 for each group): i) Control group; ii) VD group: VD + saline (2 ml/kg, once daily for 28 days); iii) low dose PF group: VD + PF (20 mg/kg, once daily for 28 days); and iv) high dose PF group: VD + PF (40 mg/kg, once daily for 28 days).
Establishment of vascular dementia
Rats were deeply anesthetized with mebumal sodium (50 mg/kg) by intraperitoneal injection. A midline incision was made to expose the bilateral common carotid arteries. The common carotid arteries were carefully separated from the surrounding tissues, including the vagus nerve, and ligated with 4-0 silk suture (Johnson&Johnson Medical Ltd., Wokingham, UK). The control rats were subjected to the same surgical procedure without occlusion of the arteries. During surgery, rectal temperatures were maintained at 37±0.5°C with a thermostatically controlled warming plate (Harvard Apparatus, Holiston, MA, USA).
Collection and preservation of brain tissues
After 28 days of BCCAO, the rats were anesthetized with 10% chloral hydrate (350 mg/kg, i.p.) and then decapitated. The brain was rapidly removed and dissected on ice to obtain the hippocampus. All brain tissues were stored at −80°C until further biochemical analysis.
Western blot analysis
The isolated hippocampus were homogenized in lysis buffer [10 mM Tris (Tocris Bioscience, Bristol, UK) (pH 7.4), 100 mM NaCl (Sinopharm Chemical Reagent Beijing Co., Ltd., Beijing, China), 1 mM ethylenediamine-N,N,N',N'-tetraacetic acid (Tocris Bioscience), 1 mM ethyleneglycol-bis(2-aminoethyl)-N,N,N',N'-tetraacetic acid (Tocris Bioscience), 1 mM NaF (Sinopharm Chemical Reagent Beijing Co., Ltd.), 20 mM Na4P2O7 (Sinopharm Chemical Reagent Beijing Co., Ltd.), 2 mM Na3VO4 (Sinopharm Chemical Reagent Beijing Co., Ltd.)], 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate (Sinopharm Chemical Reagent Beijing Co., Ltd.), 1% Triton-X 100 (Amresco LLC, Clevelan, OH, USA), 10% glycerol (Sinopharm Chemical Reagent Beijing Co., Ltd.), 1 mM phenylmethylsulfonyl fluoride (made from a 0.3 M stock in dimethylsulfoxide; Tocris Bioscience), 60 µg/ml aprotinin (Santa Cruz Biotechnology Inc.), 10 µg/ml leupeptin (Santa Cruz Biotechnology Inc.), and 1 µg/ml pepstatin (Santa Cruz Biotechnology Inc.)] for 30 min. The soluble fraction was obtained by centrifugation at 3,000 × g for 10 min. Protein concentration was determined using a bicinchoninic acid protein assay (Pulilai, Beijing, China). Equal amounts of protein (40 µg) were boiled at 100°C for 10 min in loading buffer (Fermentas, Beijing, China) and were separated in 8–10% SDS-polyacrylamide gel, and electrotransferred onto a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, USA). The membrane was blocked with 5% non-fat milk in 1X Tris-buffered saline [TBS; 10 mM Tris-HCl (Tocris Bioscience and Sinopharm Chemical Reagent Beijing Co. Ltd.) (pH 8.0), 150 mM NaCl (Sinopharm Chemical Reagent Beijing Co., Ltd.)] and 0.1% Tween-20 (TBST; Santa Cruz Biotechnology Inc.) at 25°C for 1 h and subsequently incubated overnight at 4°C with the following primary antibodies: Anti-NSE (1:500), anti-S100β (1:500), anti-BDNF (1:500), anti-Bcl-2 (1:1,000), anti-Bax (1:1,000) and anti-cytochrome c (1:1,000). The membranes were then washed twice with TBST and probed with the corresponding secondary antibodies conjugated with horseradish peroxidase (sc-2004/sc-2005; HRP; 1:5,000; Santa Cruz Biotechnology Inc.) (anti-mouse/rabbit-HRP was used at a dilution of 1:5,000) at 25°C for 1 h. Following the washes, the blots were developed using an enhanced chemiluminescence system (Amersham ECL plus, GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). The bands were visualized by exposure to X-ray film (X-Omat films, Kodak, Rochester, NY, USA). Anti-β-actin antibody (sc-130656; 1:2,000; Santa Cruz Biotechnology Inc.) was used as a loading control. All samples were analyzed at least in triplicate.
Statistical analysis
The Morris water maze (15) was used to evaluate spatial memory functioning following the treatment. As described in the previous study (17), the water maze apparatus was a circular pool (50 cm height, 150 cm diameter) with a black inner wall, which was filed with water to 28 cm and maintained at 22–25°C. The pool was divided into four quadrants (I–IV) according to four equal distance points on the inner wall. Furthermore, an escape platform painted black (26 cm height, 15 cm diameter) was submerged 2.0 cm under the water surface. The rats were given 4 trials per day for 4 days. The trials began as the rat was placed in the pool facing the side wall at a start position and ended once the animal found the platform. In cases where the rat did not find the platform within 90 sec, it was guided there. Following a period of 20 sec on the platform, the rat was immediately placed again in the pool at a different starting position for the next trial. The swimming traces of the rats were recorded by a camera suspended over the center of the pool. The escape latency and swimming distance of the rats were monitored by a computerized tracking system (Chinese Academy of Medical Sciences, Beijing, China).
The Morris water maze was performed with repeated measures and analyzed by two-way analysis of variance followed by a Bonferroni multiple group comparison. Statistical analysis of other data was performed by one-way analysis of variance followed by a post-Tukey test. For all statistical analyses, a standard software package (Statistical Analysis Software, version 10.0; SAS Institute, Cary, NC, USA) was used. All data are presented as the mean ± standard error. P<0.05 was considered to indicate a statistically significant difference.
Results
PF prevents NSE and S100β activation in the hippocampus
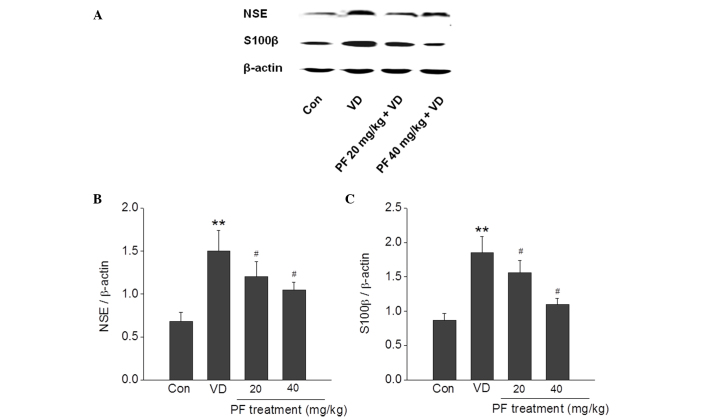
A substantial increase in the expression of NSE and S100β activity following VD has been reported. The results of the present study demonstrated that NSE and S100β were significantly increased in the VD group compared with the control group. PF (20 and 40 mg/kg) significantly reversed this increase in expression of NSE and S100β in the VD group (P<0.05) (Fig. 3).
Figure 3.
Effects of PF on the protein expression levels of NSE and S100β in the hippocampus of VD rats. (A) Representative images of western blotting showing that PF decreased the expression of NSE and S100β. Quantitative analysis of (B) NSE and (C) S100β expression. **P<0.01 vs. the control group; #P<0.05 vs. the VD group. n=4. One-way analysis of variance followed by a post Tukey test were used. Con, control; VD, vascular dementia; PF, paeoniflorn.
PF regulates apoptosis-related protein expression in the hippocampus
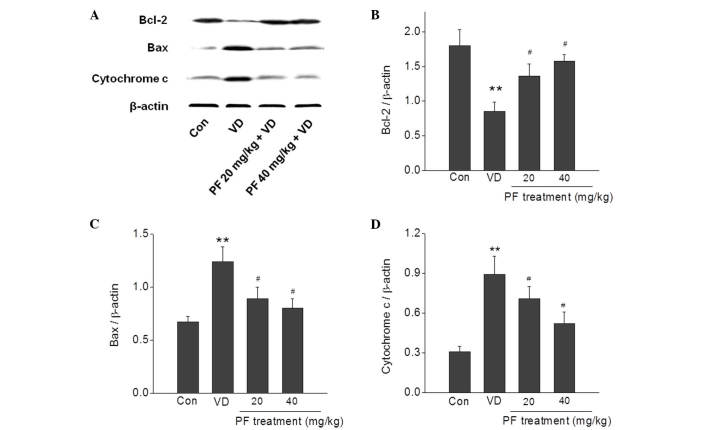
The effects of PF treatment on VD-induced apoptosis-related proteins were examined. The expression levels of Bax and cytochrome c were significantly increased in the VD group compared with the control group (P<0.01), and this was significantly reversed by treatment with PF (20 and 40 mg/kg; P<0.05). In addition, the expression level of Bcl-2 was significantly decreased in the VD group compared with the control group (P<0.01), and treatment with PF (20 and 40 mg/kg) significantly reversed this decrease in expression level (P<0.05) (Fig. 4).
Figure 4.
Effects of PF on the expression levels of apoptosis-related proteins in the hippocampus of VD rats. (A) Representative images of western blotting showing that PF decreased Bax and cytochrome c expression levels, and increased Bcl-2 expression levels, in the hippocampus of VD rats. Quantitative analysis of (B) Bcl-2, (C) Bax and (D) cytochrome c expression. **P<0.01 vs. the control group; #P<0.05 vs. the VD group. n=4. One-way analysis of variance followed by a post Tukey test were used. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; Con, control; VD, vascular dementia; PF, paeoniflorn.
PF upregulates BDNF expression levels in the hippocampus
BDNF protein expression levels in the hippocampus were quantified using western blotting (Fig. 5). The results demonstrated that the BDNF expression level in the VD group was significantly reduced compared with the control group. Treatment with PF (20 and 40 mg/kg) significantly reversed this decrease in BDNF expression level (P<0.05).
Figure 5.
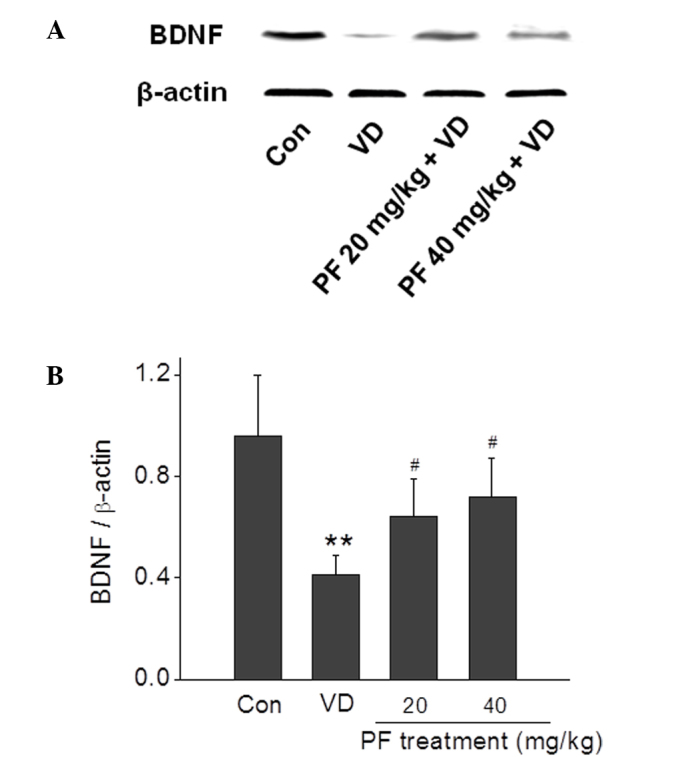
Effects of PF on the protein expression level of BDNF in the hippocampus of VD rats. (A) Representative images of western blotting showing that PF increased BDNF expression levels. (B) Quantitative analysis of BDNF expression. **P<0.01 vs. the control group; #P<0.05 vs. the VD group. n=4. One-way analysis of variance followed by a post Tukey test were used. BDNF, brain-derived neurotrophic factor; Con, control; VD, vascular dementia; PF, paeoniflorn.
Discussion
Cognitive impairment is a key feature of VD. The hippocampus is highly associated with learning and memory, and is one of the brain regions that is most sensitive to hypoxia and reactive oxygen species. Therefore, the hippocampus is thought to be the primary target brain region of BCCAO-induced damage (18). In the present study, the effects of PF on molecular changes in the hippocampus of BCCAO rats was investigated. The results demonstrated that administration of PF for 28 days significantly decreased the expression levels of NSE and S100β, both sensitive markers for brain damage, in VD model rats. In addition, PF inhibited the initiation of apoptotic cell death, and attenuated the decreased expression of BDNF induced by BCCAO damage. These results strongly support the potential therapeutic role of PF in VD.
NSE is a cytoplasmic glycolytic enzyme in neurons, and is passively released into the extracellular space under pathological conditions (19). S100β is a calcium-binding protein primarily expressed and secreted by astrocyte cells in the central nervous system; acute elevation of extracellular S100β has been observed in injury conditions (20). NSE and S100β expression levels have been considered markers of neurodegeneration and are thought to be associated with the severity of the disease (21,22). The changes in NSE and S100β expression levels in the hippocampus of rats were examined in present study. Experimental data showed that the expression levels of NSE and S100β proteins were significantly decreased in PF-treated VD rats compared with VD rats, which indicates that PF treatment can reverse hippocampus impairment.
Apoptosis is a mode of cell death, and has been proposed to explain the cell loss observed in numerous neurological disorders, including VD (23). Bcl-2 and Bax are two primary genes responsible for the regulation of apoptotic cell death, and their individual products possess opposing functions (24). Bcl-2 is functionally characterized as the apoptosis-suppressing factor, whereas Bax is considered as the apoptosis-promoting factor (25). Bcl-2 inhibits cytochrome c release from mitochondria elicited by the pro-apoptotic molecule Bax, resulting in the inhibition of caspase activation and apoptotic death (26,27). In the present study, treatment with PF significantly reduced the expression levels of Bax and cytochrome c, and increased the expression levels of Bcl-2 in the hippocampus of rats with VD. These changes indicate that PF inhibits hippocampal neuron apoptosis in a rat model of VD.
Accumulating evidence has documented the critical role of BDNF, a member of the neurotrophin family, in the regulation of the maintenance, growth and survival of neurons (28). BDNF enhances synaptic transmission and neuronal plasticity in the central nervous system (29), resulting in an increase of learning ability and memory capability (30). BDNF serum concentrations have been reported to correlate with the severity of dementia in patients (31). The results in the current study demonstrated that the neuroprotective effects of PF in VD rats possibly occurred through upregulating the expression of BDNF. BDNF exerts its neuronal protective properties primarily by activating tropomyosin-related kinase B (TrkB) receptors, thus protecting neurons from apoptosis (32). The primary downstream signaling pathways activated by TrkB receptors are phosphatidylinositol 3-kinase/Akt, mitogen-activated protein kinase/Erk or phosphoinositide phospholipase-γ signaling pathways. Further studies are required in order to clarify the downstream signaling pathways.
In conclusion, the results from the present study demonstrate that PF produces a protective effect in VD rats by inhibiting the initiation of apoptotic cell death and attenuating the decreased expression of BDNF induced in the VD model. These results confirm the neuroprotective effects of PF on VD and provide a novel insight into the long-term use of PF as a potential treatment in the early cognitive impairment stages of VD.
Acknowledgements
The present study was supported by the National Key Grant of Basic Research Project (grant no. 2010CB530403) and the Capital Medical University National Key Discipline Project (grant no. CMUNEURO-201403).
References
- 1.Ronnemaa E, Zethelius B, Lannfelt L, Kilander L. Vascular risk factors and dementia: 40-Year follow-up of a population-based cohort. Dement Geriatr Cogn Disord. 2011;31:460–466. doi: 10.1159/000330020. [DOI] [PubMed] [Google Scholar]
- 2.Malouf R, Birks J. Donepezil for vascular cognitive impairment. Cochrane Database Syst Rev CD004395. 2004 doi: 10.1002/14651858.CD004395.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, et al. Global prevalence of dementia: A Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S, Zhou GG, Liu H, et al. Portective effects of p38 MAPK inhibitor SB202190 against hippocampal apoptosis and spatial learning and memory deficits in a rat model of vascular dementia. Biomed Res Int, 2013;2013:215798. doi: 10.1155/2013/215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellinger KA. The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol. 2007;113:349–388. doi: 10.1007/s00401-006-0185-2. [DOI] [PubMed] [Google Scholar]
- 6.Levine DA, Langa KM. Vascular cognitive impairment: Disease mechanisms and therapeutic implications. Neurotherapeutics. 2011;8:361–373. doi: 10.1007/s13311-011-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett S, Grant MM, Aldred S. Oxidative stress in vascular dementia and Alzheimer's disease: A common pathology. J Alzheimers Dis. 2009;17:245–257. doi: 10.3233/JAD-2009-1041. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Zhang HY, Tang XC. Cholinergic deficiency involved in vascular dementia: Possible mechanism and strategy of treatment. Acta Pharmacol Sin. 2009;30:879–888. doi: 10.1038/aps.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong X, Ma M, Fan X, Li M, Liu Q, Liu X, Xu G. Down-regulation of IGF-1/IGF-1R in hippocampus of rats with vascular dementia. Neurosci Lett. 2012;513:20–24. doi: 10.1016/j.neulet.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 10.Kasparova S, Brezova V, Valko M, et al. Study of the oxidative stress in a rat model of chronic brain hypoperfusion. Neurochem Int. 2005;46:601–611. doi: 10.1016/j.neuint.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Nizamutdinoval IT, Jim YC, Kim JS, et al. Paconol and paconiflorin, the main active principles of Paconiaalbiflora, protect the heart from myocardial ischemia/reperfusion injury in rats. Planta Med. 2008;74:14–18. doi: 10.1055/s-2007-993775. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe H. Candidates for cognitive enhancer extracted from medicinal plants: Paeoniflorin and tetramethylpyrazine. Behav Brain Res. 1997;83:135–141. doi: 10.1016/S0166-4328(97)86057-3. [DOI] [PubMed] [Google Scholar]
- 13.Tang NY, Liu CH, Hsieh CT, Hsieh CL. The anti-inflammatory effect of paeoniflorin on cerebral infarction induced by ischemia-reperfusion injury in Sprague-Dawley rats. Am J Chin Med. 2010;38:51–64. doi: 10.1142/S0192415X10007786. [DOI] [PubMed] [Google Scholar]
- 14.Xiao L, Wang YZ, Liu J, Luo XT, Ye Y, Zhu XZ. Effects of paeoniflorin on the cerebral infarction, behavioral and cognitive impairments at the chronic stage of transient middle cerebral artery occlusion in rats. Life Sci. 2005;78:413–420. doi: 10.1016/j.lfs.2005.04.069. [DOI] [PubMed] [Google Scholar]
- 15.Zhang LG, Wang LJ, Shen QQ, et al. Paeoniflorin Improves Regional Cerebral Blood Flow and Suppresses Inflammatory Factors in the Hippocampus of Rats with Vascular Dementia. Chin J Integr Med. doi: 10.1007/s11655-015-2124-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council, corp-author. Guide for the Care and Use of Laboratory Animals. 7th. National Academy Press; Washington, D.C.: 1996. pp. 56–66. [Google Scholar]
- 17.D'Hooge R, De Devn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 18.Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Berger RP, Dulani T, Adelson PD, Leventhal JM, Richichi R, Kochanek PM. Identification of inflicted traumatic brain injury in well-appearing infants using serum and cerebrospinal markers: A possible screening tool. Pediatrics. 2006;117:325–332. doi: 10.1542/peds.2005-0711. [DOI] [PubMed] [Google Scholar]
- 20.Gonçalves CA, Leite MC, Nardin P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem. 2008;41:755–763. doi: 10.1016/j.clinbiochem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Mecocci P, Parnetti L, Romano G, Scarelli A, Chionne F, Cecchetti R, Polidori MC, Palumbo B, Cherubini A, Senin U. Serum anti-GFAP and anti-S100 autoantibodies in brain aging, Alzheimer's disease and vascular dementia. J Neuroimmunol. 1995;57:165–170. doi: 10.1016/0165-5728(94)00180-V. [DOI] [PubMed] [Google Scholar]
- 22.Parnetti L, Palumbo B, Cardinali L, Loreti F, Chionne F, Cecchetti R, Senin U. Cerebrospinal fluid neuron-specific enolase in Alzheimer's disease and vascular dementia. Neurosci Lett. 1995;183:43–45. doi: 10.1016/0304-3940(94)11110-5. [DOI] [PubMed] [Google Scholar]
- 23.Sun ZK, Ma XR, Jia YJ, et al. Effects of resveratrol on apoptosis in a rat model of vascular dementia. Exp Ther Med. 2014;7:843–848. doi: 10.3892/etm.2014.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min JJ, Huo XL, Xiang LY, et al. Protective effect of Dl-3n-butylphthalide on learning and memory impairment induced by chronic intermittent hypoxia-hypercapnia exposure. Sci Rep. 2014;4:5555. doi: 10.1038/srep05555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang L, Choi IY, Kim SE, Ko IG, Shin MS, Kim CJ, Kim SH, Jin JJ, Chung JY, Yi JW. Dexmedetomidine ameliorates intracerebral hemorrhage-induced memory impairment by inhibiting apoptosis and enhancing brain-derived neurotrophic factor expression in the rat hippocampus. Int J Mol Med. 2013;31:1047–1056. doi: 10.3892/ijmm.2013.1301. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 27.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 28.Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann N Y Acad Sci. 2005;1053:195–204. doi: 10.1111/j.1749-6632.2005.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 29.Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/S0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, Wittorf A, Soekadar S, Richartz E, Koehler N, Bartels M, et al. BDNF serum and CSF concentrations in Alzheimer's disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387–394. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/S0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]