Abstract
Few studies have examined the effect of black tea (Camellia sinensis) theaflavins on obesity-related targets. Pancreatic lipase (PL) plays a central role in fat metabolism and is a validated target for weight loss. We compared the inhibitory efficacy of individual theaflavins and explored the underlying mechanism. Theaflavin-3,3′-digallate (TFdiG), theaflavin-3′-gallate, theaflavin-3-gallate, and theaflavin inhibited PL with IC50 of 1.9, 4.2, 3.0, and >10 μmol/L. The presence and location of the galloyl ester moiety were essential for inhibitory potency. TFdiG exhibited mixed inhibition with respect to substrate concentration. In silico modeling showed that theaflavins bind to Asn263 and Asp206, which form a pocket adjacent to the active site, and galloyl-containing theaflavins are then predicted to perturb the protonation of His264. These data provide a putative mechanism to explain the anti-obesity effects of tea.
Keywords: Tea, Camellia sinensis, theaflavins, pancreatic lipase, obesity
1. INTRODUCTION
In the United States, approximately 68% of adults are overweight or obese (Flegal, Carroll, Ogden, & Curtin, 2010; Ogden, Carroll, Kit, & Flegal, 2014). Although current data suggest that rates of obesity are stabilizing, obese and overweight individuals are at increased risk for a number of chronic diseases including diabetes, cardiovascular disease, and some forms of cancer (Noureddin & Rinella, 2015; Oda, 2012; Park, Morley, Kim, Clegg, & Scherer, 2014; Rani, Deep, Singh, Palle, & Yadav, 2016; Thomas, Weedermann, Fuemmeler, Martin, Dhurandhar, Bredlau, et al., 2014).
Tea (Camellia sinensis, Theaceae) is the second most commonly consumed beverage in the world and approximately 80% of the tea consumed worldwide is in the form of black tea (Yang, Maliakal, & Meng, 2002). Theaflavins (Fig. 1) and thearubigins are characteristic polyphenols in black tea and result from the oxidation of tea leaves during the “fermentation” step of tea processing (Harbowy & Balentine, 1997). A typical cup of brewed black tea (2.5 g tea leaves in 250 mL) yields approximately 30% water-extractable solids (Yang, et al., 2002). A survey of 32 black tea brands showed that the levels of theaflavin (TF), theaflavin-3-gallate (TF3G), theaflavin-3′-gallate (TF3′G), and theaflavin-3,3′-digallate (TFdiG) in the water-extractable solids were 0.1 – 5.4 mg/g, 0.2 – 2.1 mg/g, 0.1 – 0.8 mg/g, and 0.4 – 5.8 mg/g, respectively (Friedman, Kim, Lee, Han, Han, Lee, et al., 2005). Epidemiological and laboratory animal model studies have demonstrated the potential efficacy of tea for weight management and obesity prevention (reviewed in (Grove & Lambert, 2010; Sae-tan, Grove, & Lambert, 2011)).
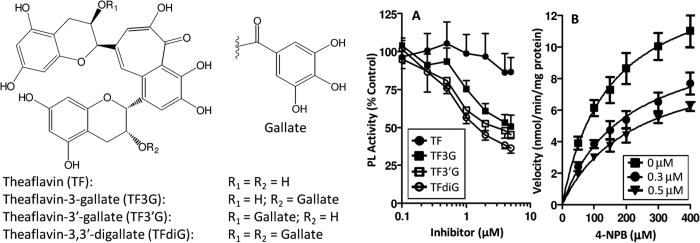
Figure 1.
Inhibition of pancreatic lipase by black tea polyphenols. (A) The dose-dependent inhibitory potency of theaflavin, theaflavin-3-gallate, theaflavin-3′-gallate, and theaflavin-3,3′-digallate against pancreatic lipase in vitro was determined. The data was normalized to a vehicle-control. (B) Kinetic analysis was performed to determine the mode of enzyme inhibition of TFdiG. Incubations were carried out at 37°C for 10 min using 4-NPB as the substrate and porcine pancreatic lipase as the enzyme source. Symbols represent the mean ± SEM of N = 3 – 6 independent experiments.
Although most laboratory studies have focused on the obesity preventive effects of green tea, there is growing evidence that black tea and theaflavins may also be useful for prevention of obesity (Yang, Zhang, Zhang, Huang, & Wang, 2016). Black tea polyphenols have been shown to significantly reduce serum triglyceride concentration in rats after co-administration of a liquid fat test meal and black tea extract compared to control treated rats (Kobayashi, Ichitani, Suzuki, Unno, Sugawara, Yamahira, et al., 2009; Uchiyama, Taniguchi, Saka, Yoshida, & Yajima, 2011). Mice fed a high fat diet supplemented with 5% black tea polyphenol extract had significantly less body weight gain, parametrial adipose tissue mass, and liver lipid content compared to mice fed a high-fat diet (Uchiyama, et al., 2011). These results suggest that black tea polyphenols may modulation dietary triglyceride digestion.
Pancreatic lipase (PL) is an enzyme secreted into the duodenum that plays a key role in the digestion and absorption of fats; in fact, PL may be responsible for cleaving 50-70% of dietary fats (Birari & Bhutani, 2007). Given the importance of PL for lipid digestion, it represents an attractive target for obesity prevention. Orlistat (tetrahydrolipstatin) is a Food and Drug Administration approved PL inhibitor marketed as a weight loss drug (Harrison, Fecht, Brunt, & Neuschwander-Tetri, 2009).
We have previously reported that (−)-epigallocatechin-3-gallate (EGCG), the major polyphenol in green tea, dose-dependently inhibits PL in vitro (IC50 = 7.5 μmol/L); this inhibition was non-competitive with respect to substrate concentration (Grove, Sae-tan, Kennett, & Lambert, 2012). By contrast, (−)-epigallocatechin, which has no galloyl ester, was ineffective. Wang et al., recently reported similar results (Wang, Sun, Dong, Liu, & Liu, 2014). An in silico model for the interaction between EGCG and PL was proposed by the same group, but the authors used a PL dimer as a protein structure (Wu, He, Yao, Zhang, Liu, Wang, et al., 2013). Although the crystal structure of PL was solved using the dimer, this is not the enzymatically active structure of PL, and conclusions regarding molecular interactions based on this structure are spurious. In addition, the authors used an incorrect structure for EGCG.
Less is known about the effects of the theaflavins on PL. Nakai et al. have reported that oolong tea polyphenols, including some theaflavin isomers, inhibit PL in vitro (Nakai, Fukui, Asami, Toyoda-Ono, Iwashita, Shibata, et al., 2005). IC50 values ranged from 0.068 μmol/L (oolongtheanin 3′-O-gallate) to >20 μmol/L ((−)-epigallocatechin). In each of these studies, polyphenols containing a galloyl ester had significantly greater inhibitory potency than nongalloyl containing molecules. The authors provided no information vis-à-vis the enzymatic mechanism of inhibition.
It has been posited that the requirement of the galloyl ester indicates that the ester moiety competes with the esters in triglycerides resulting in inhibition of lipase-mediated cleavage. Although interesting, this mechanism fails to account for 1) the lack of formation of the deesterified polyphenol in the reaction mixture and 2) the non-competitive mode of inhibition reported for EGCG. The objectives of the present study were to compare the PL inhibitory activity of purified black tea theaflavins, to determine the inhibitory kinetics of the most potent theaflavin, and to develop an in silico model to better understand the inhibitory activity of the theaflavins.
2. MATERIALS AND METHODS
2.1 Chemicals
Lipase (type II, from porcine pancreas, specific activity = 400 U/mg), 4-nitrophenyl butyrate (4-NPB), and orlistat were purchased from Sigma Chemical Company (St. Louis, MO). Theaflavin (TF, 98% pure), theaflavin-3-gallate (TF3G, 98% pure), theaflavin-3′-gallate (TF3′G, 98% pure), and theaflavin-3,3′-digallate (TFdiG, 98% pure) were purchased from Quality Phytochemicals LLC (Edison, NJ). All other chemicals were of the highest grade commercially available.
2.2 Pancreatic Lipase Activity
PL was suspended in water (10 mg/mL) and incubated at 37°C for 5 min. The solution was centrifuged for 5 min at 664 x g and the supernatant was then used as the enzyme source for subsequent experiments. For each experiment, the PL supernatant was diluted 1:50 in buffer solution (20 mmol/L Tris-HCl, 1.3 mmol/L CaCl2, 150 mmol/L NaCl, pH = 8.0) and combined with the inhibitor of interest. The reaction was then started by the addition of 4-NPB (226 μmol/L final concentration). After incubation at 37°C for 10 min the absorbance was measured spectrophotometrically at 400 nm. Based on previous work in our laboratory, the reaction rate is linear over the time-frame of the experiment. Absorbance values were normalized to the vehicle control and dose-response curves were prepared.
The mode of enzyme inhibition was determined in a manner similar to that described above with the modification that the inhibitor concentration was held constant and 4-NPB concentration (0 – 500 μM) was varied. The maximum velocity (Vmax) and Michaelis-Menten constant (Km) were determined by fitting the initial velocity as a function of concentration of 4-NPB.
2.3 In Silico Modeling
Initial atomic coordinates for the inhibitors were built using the online PRODRG server (Schuttelkopf & van Aalten, 2004). The inhibitors thus generated were manually moved and rotated into the binding pocket adjacent to the active site pocket of the PL structure (1ETH, colipase-tetra ethylene glycol monooctyl ether complex removed) using the program COOT (Emsley & Cowtan, 2004). The initial model of the complex of inhibitors and PL from COOT was energy minimized using the online YASARA server with flexible peptide bonds (Krieger, Joo, Lee, Lee, Raman, Thompson, et al., 2009). The minimized model was analyzed and figures were generated using the program PyMOL (The PyMOL Molecular Graphics System, Version 1.4.1, Schrödinger, LLC.).
2.4 Statistical Analysis
Non-linear regression analysis was used to fit enzyme inhibition curves and Michaelis-Menten plots. All plots show the mean ± standard error of the mean (SEM). Oneway ANOVA with Tukey's Post-test was used to compare Km and Vmax values. Statistical significance was achieved at p < 0.05 (Granato, Calado, & Jarvis, 2014). All analyses were performed using GraphPad Prism (San Diego, CA).
3. RESULTS AND DISCUSSION
Previous epidemiological and laboratory studies have demonstrated the potential of tea and tea-derived polyphenols in weight management (reviewed in (Grove, et al., 2010; Masterjohn & Bruno, 2012; Sae-tan, Grove, & Lambert, 2011)). Inhibition of fat digestion and absorption has been implicated as a potential mechanism and previous studies have shown that tea polyphenols have inhibitory activity against PL (Grove, et al., 2012; Klaus, Pultz, Thone-Reineke, & Wolfram, 2005; Kobayashi, et al., 2009; Sae-Tan, Grove, Kennett, & Lambert, 2011; Wolfram, Raederstorff, Wang, Teixeira, Elste, & Weber, 2005). In spite of this, black tea theaflavins have been understudied. In particular, the manner in which theaflavins interact with pancreatic lipase and the mechanism of enzyme inhibition remain poorly understood.
In the present study, TFdiG was the most potent inhibitor of PL with an IC50 = 1.9 ± 0.2 μmol/L (Fig 1A). By contrast, TF, which contains no galloyl esters, had significantly reduced inhibitory potency (IC50 > 10 μmol/L). Interestingly, although both TF3G and TF3′G contain only one galloyl ester, they had greater potency than might be predicted (IC50 = 4.2 ± 0.1 μmol/L and 3.0 ± 0.2 μmol/L, respectively). We have previously reported that EGCG inhibited PL with an IC50 = 7.5 μmol/L (Grove, et al., 2012). These results might lead to the conclusion that black tea has more significant obesity-preventive activity than green tea, given the greater potency of the gallated theaflavins. This difference has not been observed in vivo, and is likely mitigated by the fact that levels of theaflavins in black tea (2 – 6% of water extractable material) are significantly lower than the levels of catechins in green tea (30 – 42% of water extractable material) (Yang, et al., 2002).
The theaflavins have been reported to have very poor oral bioavailability. Even after oral administration of very high doses of theaflavins (700 mg, equivalent to 30 cups of black tea) to human volunteers, peak plasma and urine levels were 1.0 and 4.2 ng/mL (Mulder, van Platerink, Schuyl, & van Amelsvoort, 2001). Based on these previous results, we expect that most of the theaflavin content in a brewed cup of tea remains in in the lumen of the gastrointestinal tract. Given that a typical brewed cup of black tea (2.5 g tea leaves in 250 mL) contains 40 – 60 mg of theaflavins and assuming the volume of the contents of the human stomach does not exceed 2 – 4 L, the concentration of theaflavins is estimated at 10 – 15 μg/mL in the digesta indicating that the effective concentrations determined here are physiologically-relevant to black tea consumption (Curtis & Barnes, 1994; Yang, et al., 2002). Moreover, we found that the inhibitory effects of the gallated theaflavins were within one order of magnitude of the clinically used PL-inhibitor, orlistat (IC50 = 0.8 μmol/L determined here, data not shown).
We found that the presence of the galloyl moiety is important for inhibitory potency. This is in agreement with previous work on green tea catechins by our laboratory and others (Grove, et al., 2012; Nakai, et al., 2005; Wang, et al., 2014). It has been speculated that the requirement indicates that the galloyl ester competes with the triglyceride substrate for PL-mediated cleavage. If this were the case, then one would expect the mode of inhibition to be competitive with regard to substrate concentration. However, analysis of the Michaelis-Menten plot of PL in the presence of TFdiG (Fig. 1B) showed that the Vmax was significantly decreased by TFdiG (Table 1). A significant increase in Km was also observed. These results suggest that TFdiG inhibits PL in a mixed manner with respect to substrate concentration. These results suggest that the importance of the galloyl ester moiety is more than simply to serve as a competitive ester for cleavage. We have previously reported that green tea catechins inhibit PL in a non-competitive manner with regard to substrate concentration (Grove, et al., 2012). Taken together these data suggest that the theaflavins bind to both the free enzyme as well as the enzyme-substrate complex, and likely at a site other than the substrate-binding site.
Table 1.
Kinetics of TFdiG-mediated inhibition of PL in vitro.1
| Theaflavin-3,3’-digallate (μM) | Vmax (pmol/mg/min) | Km (μM) |
|---|---|---|
| 0 | 15.1 ± 1.0a | 149.3 ± 24.2b |
| 0.3 | 11.2 ± 0.6a,b | 199.6 ± 21.8a,b |
| 0.5 | 9.6 ± 0.4b | 225.9 ± 30.4a |
Values represent the mean ± SEM.
Values in each column with different superscripts are statistically significantly different by One-way ANOVA with Tukey's Post-test (p < 0.05).
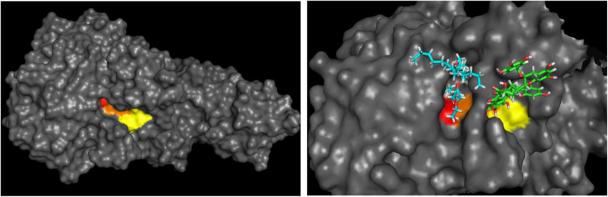
In silico modeling studies were conducted in order to better understand the inhibitory effects of theaflavins against PL. Using the ConSurf Server, we found that two regions on the PL protein surface were highly conserved over 139 unique lipase sequences (Fig. 2) (Ashkenazy, Erez, Martz, Pupko, & Ben-Tal, 2010). One region forms the active site pocket whereas the other conserved region forms a binding pocket adjacent to the active site pocket. Asp206 and Asn263 are two of the three amino acid residues that form the bottom of the second binding pocket—the third amino acid, His264, is also part of the PL catalytic triad (Ser153-His264-Asp177, Fig. 2). We will refer to this second pocket as the “inhibitor-binding pocket.”
Figure 2.
In silico modeling of PL revealed that (A) two conserved binding pockets are seen in pancreatic lipase through in silico modeling. The substrate-binding pocket (shown in red), on the left is composed of Ser153 and Asp177, which form part of the catalytic triad. His264, the remaining amino acid in the catalytic triad (shown in orange), forms the bottom of both the substrate binding pocket as well as the newly identified inhibitor-binding pocket (shown in yellow). Asn263 and Asp206 form the remainder of the inhibitor-binding pocket. (B) Tetra ethylene glycol monooctyl ether is shown binding to the substrate binding pocket (1ETH) and TF3’G is shown binding to the inhibitor binding pocket of PL and interacting with the catalytic triad via the galloyl ring.
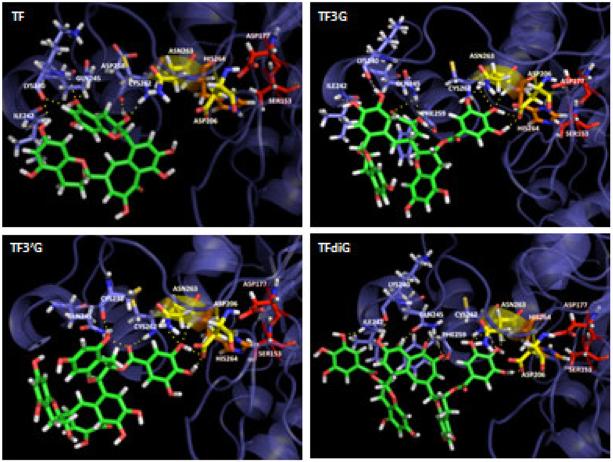
Modeling of the interactions between TF3G, TF3′G, and TFdiG shows that the hydroxyl groups on the galloyl ester form hydrogen bonds with Asn263 and Asp206, part of the inhibitor-binding pocket, and His264, part of the catalytic triad. The carbonyl of the galloyl group in TF3′G and a hydroxyl group on the galloyl ester in TF3G and TFdiG interact with Cys262 of PL (Fig. 3). These bonds stabilize the interactions between the inhibitor and the enzyme and allow the inhibitor to modify the protonation of the key catalytic residue, His264. TF3′G must bind to PL in an alternative conformation to allow the galloyl ester access to the inhibitor pocket (Fig. 3). This predicts a second hydrogen bond which forms between Cys238 and a hydroxyl group on the resorcinol ring. TF interacts with Cys262 but does not have a galloyl group to interact with the bottom of the inhibitor pocket or to interact with His264 and disturb the chemistry of the catalytic site of the enzyme (Fig. 3). This finding explains in part the reduced potency of theaflavin. Overall, these modeling studies support the data derived from the enzymology studies and provide insight into the mechanism by which these compounds inhibit PL.
Figure 3.
The interactions between key amino acids in PL and theaflavin congeners were investigated by in silico modeling. Dashed lines represent hydrogen bonds. Amino acids in the inhibitor pocket are shown in yellow, His264 is shown in orange, and the rest of the catalytic triad is colored red.
In summary, the present study provides mechanistic information regarding the inhibitory effects of theaflavin congeners against PL. These results are explained by the proposed in silico model showing that theaflavins interact with an inhibitor pocket adjacent to the substrate binding domain. Given the relatively high potency of inhibition and the nature of PL (i.e. secreted into the small intestinal lumen), it is likely that these theaflavin congeners play a role in the obesity preventive effects of black tea. This study also provides a picture of the structural requirements and key interactions between PL and theaflavins that may be useful in the identification of other natural inhibitors of this enzyme, and aid in the development of novel synthetic PL inhibitors.
HIGHLIGHTS.
Theaflavins inhibit pancreatic lipase (PL) at physiological concentrations.
Theaflavin-3,3′-digallate (TFdiG) is the most potent inhibitor.
TFdiG inhibits PL in a non-competitive manner with regard to substrate.
An in silico model predicts that TFdiG binds adjacent to the PL active site.
The model predicts thatTFdiG perturbs His264, the key catalytic amino acid.
Acknowledgments
FUNDING
This work was supported by the National Institutes of Health [grant number AT004678] and the United States Department of Agriculture Hatch Project [grant number 4565].
ABBREVIATIONS
- 4-NPB
4-nitrophenyl butyrate
- IC50
median inhibitory concentration
- Km
Michaelis-Menten constant
- PL
pancreatic lipase
- TF
theaflavin
- TF3G
theaflavin-3-gallate
- TF3′G
theaflavin-3′-gallate
- TFdiG
theaflavin-3,3′-digallate
- Vmax
maximum velocity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CHEMICAL COMPOUNDS STUDIED IN THIS ARTICLE:
Theaflavin (PubChem CID: 114777)
Theaflavin-3-gallate (PubChem CID: 169167)
Theaflavin-3′-gallate (PubChem CID: 71307578)
Theaflavin-3,3′-gallate (PubChem CID: 3589471)
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. Consurf 2010: Calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Research. 2010;38:W529–W533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discovery Today. 2007;12(19-20):879–889. doi: 10.1016/j.drudis.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Curtis H, Barnes NS. Invitation to biology. 5th ed. W. H. Freeman Co.; London: 1994. [Google Scholar]
- Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallographica Section D-Biological Crystallography. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among us adults, 1999-2008. Journal of the American Medical Association. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Friedman M, Kim SY, Lee SJ, Han GP, Han JS, Lee KR, Kozukue N. Distribution of catechins, theaflavins, caffeine, and theobromine in 77 teas consumed in the united states. Journal of Food Science. 2005;70(9):C550–C559. [Google Scholar]
- Granato D, Calado VMD, Jarvis B. Observations on the use of statistical methods in food science and technology. Food Research International. 2014;55:137–149. [Google Scholar]
- Grove KA, Lambert JD. Laboratory, epidemiological, and human intervention studies show that tea (camellia sinensis) may be useful in the prevention of obesity. Journal of Nutrition. 2010;140(3):446–453. doi: 10.3945/jn.109.115972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove KA, Sae-tan S, Kennett MJ, Lambert JD. (−)-epigallocatechin-3-gallate inhibits pancreatic lipase and reduces body weight gain in high fat-fed obese mice. Obesity (Silver Spring) 2012;20(11):2311–2313. doi: 10.1038/oby.2011.139. [DOI] [PubMed] [Google Scholar]
- Harbowy ME, Balentine DA. Tea chemistry. Critical Reviews in Plant Sciences. 1997;16(5):415–480. [Google Scholar]
- Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49(1):80–86. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- Klaus S, Pultz S, Thone-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. International Journal of Obesity (Lond) 2005;29(6):615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ichitani M, Suzuki Y, Unno T, Sugawara T, Yamahira T, Kato M, Takihara T, Sagesaka Y, Kakuda T, Ikeda I. Black-tea polyphenols suppress postprandial hypertriacylglycerolemia by suppressing lymphatic transport of dietary fat in rats. Journal of Agricultural and Food Chemistry. 2009;57(15):7131–7136. doi: 10.1021/jf900855v. [DOI] [PubMed] [Google Scholar]
- Krieger E, Joo K, Lee J, Lee J, Raman S, Thompson J, Tyka M, Baker D, Karplus K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in casp8. Proteins-Structure Function and Bioinformatics. 2009;77:114–122. doi: 10.1002/prot.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterjohn C, Bruno RS. Therapeutic potential of green tea in nonalcoholic fatty liver disease. Nutrition Reviews. 2012;70(1):41–56. doi: 10.1111/j.1753-4887.2011.00440.x. [DOI] [PubMed] [Google Scholar]
- Mulder TPJ, van Platerink CJ, Schuyl PJW, van Amelsvoort JMM. Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. Journal of Chromatography B. 2001;760(2):271–279. doi: 10.1016/s0378-4347(01)00285-7. [DOI] [PubMed] [Google Scholar]
- Nakai M, Fukui Y, Asami S, Toyoda-Ono Y, Iwashita T, Shibata H, Mitsunaga T, Hashimoto F, Kiso Y. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. Journal of Agricultural and Food Chemistry. 2005;53(11):4593–4598. doi: 10.1021/jf047814+. [DOI] [PubMed] [Google Scholar]
- Noureddin M, Rinella ME. Nonalcoholic fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clinical Liver Disease. 2015;19(2):361–379. doi: 10.1016/j.cld.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E. Metabolic syndrome: Its history, mechanisms, and limitations. Acta Diabetologia. 2012;49(2):89–95. doi: 10.1007/s00592-011-0309-6. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011-2012. Journal of the American Medical Association. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nature Reviews Endocrinology. 2014;10(8):455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani V, Deep G, Singh RK, Palle K, Yadav UC. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sciences. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Sae-Tan S, Grove KA, Kennett MJ, Lambert JD. (−)-epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice. Food and Function. 2011;2(2):111–116. doi: 10.1039/C0FO00155D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sae-tan S, Grove KA, Lambert JD. Weight control and prevention of metabolic syndrome by green tea. Pharmacological Research. 2011;64(2):146–154. doi: 10.1016/j.phrs.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuttelkopf AW, van Aalten DMF. Prodrg: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallographica Section D-Biological Crystallography. 2004;60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Weedermann M, Fuemmeler BF, Martin CK, Dhurandhar NV, Bredlau C, Heymsfield SB, Ravussin E, Bouchard C. Dynamic model predicting overweight, obesity, and extreme obesity prevalence trends. Obesity (Silver Spring) 2014;22(2):590–597. doi: 10.1002/oby.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama S, Taniguchi Y, Saka A, Yoshida A, Yajima H. Prevention of diet-induced obesity by dietary black tea polyphenols extract in vitro and in vivo. Nutrition. 2011;27(3):287–292. doi: 10.1016/j.nut.2010.01.019. [DOI] [PubMed] [Google Scholar]
- Wang SH, Sun ZY, Dong SZ, Liu Y, Liu Y. Molecular interactions between (−)-epigallocatechin gallate analogs and pancreatic lipase. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram S, Raederstorff D, Wang Y, Teixeira SR, Elste V, Weber P. Teavigo (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Annals of Nutrition and Metabolism. 2005;49(1):54–63. doi: 10.1159/000084178. [DOI] [PubMed] [Google Scholar]
- Wu XL, He WY, Yao L, Zhang HP, Liu ZG, Wang WP, Ye Y, Cao JJ. Characterization of binding interactions of (−)-epigallocatechin-3-gallate from green tea and lipase. Journal of Agricultural and Food Chemistry. 2013;61(37):8829–8835. doi: 10.1021/jf401779z. [DOI] [PubMed] [Google Scholar]
- Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annual Reviews in Pharmacology and Toxicology. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- Yang CS, Zhang JS, Zhang L, Huang JB, Wang YJ. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Molecular Nutrition and Food Research. 2016;60(1):160–174. doi: 10.1002/mnfr.201500428. [DOI] [PMC free article] [PubMed] [Google Scholar]