Abstract
Background
Mitral valve (MV) annular dynamics have been well described in animal models of functional mitral regurgitation (FMR). Despite this little, if any, data exists regarding the dynamic MV annular geometry in humans with FMR. In the current study we hypothesized that three-dimensional (3D) echocardiography, in conjunction with commercially available software, could be used to quantify the dynamic changes in MV annular geometry associated with FMR.
Methods
Intraoperative 3D transesophageal echocardiographic data obtained from 34 patients with FMR and 15 controls undergoing cardiac surgery were dynamically analyzed for differences in mitral annular geometry with TomTec© 4D MV Assessment 2.0 software.
Results
In patients with FMR, the mean mitral annular area (14.6cm2 vs. 9.6cm2), circumference (14.1cm vs. 11.4 cm), anteroposterior (4.0cm vs. 3.0cm) and anterolateral-posteromedial (4.3cm vs. 3.6cm) diameters, tenting volume (6.2mm3 vs. 3.5mm3) and nonplanarity angle (154° ± 15 vs. 136° ± 11) were greater at all points during systole compared to controls (p<0.01). Vertical mitral annular displacement (5.8mm vs. 8.3mm) was reduced in FMR compared to controls (p<0.01).
Conclusions
There are significant differences in dynamic mitral annular geometry between patients with and without FMR. We were able to analyze these changes in a clinically feasible fashion. Ready availability of this information has the potential to aid comprehensive quantification of mitral annular function and possibly assist in both clinical decision-making and annuloplasty ring selection.
Keywords: Mitral regurgitation, echocardiography
INTRODUCTION
Using invasive imaging techniques, three-dimensional (3D) mitral valve (MV) annular dynamics have been well described in large animal models of functional mitral regurgitation (FMR) 1, 2. While the increasing use of real time – 3D echocardiography (RT-3DE) has improved our understanding of static human MV annular geometry significantly over the last decade, little if any data exists on the dynamic 3D changes in annular function in humans with FMR. The knowledge of annular changes during cardiac cycle is based on manual reconstruction of ‘dynamic’ MV models from static images 3-13. Effects of chronic mitral regurgitation (MR) states such as FMR on annular behavior are also extrapolated from annular position at a single point (end-systolic) in the cardiac cycle 5, 14-18.
At present, the echocardiographic assessment of MV in FMR is performed only to quantify regurgitation as a marker of valve dysfunction and exclude stenosis after repair. Ideally, geometric distortion of the mitral annulus incurred from annuloplasty devices should also be objectively quantified and followed. But despite recognition of the prognostic value of mitral annular changes in FMR, technological impediments have precluded their clinical application 19. Demonstration of differences in mitral annular geometry (static and dynamic) between patients with and without FMR in a clinically feasible fashion should be the first step towards achieving this goal. In the current study we hypothesized that RT-3DE, in conjunction with commercially available imaging software, could be used to compare 3D mitral annular geometry during systole in patients with and without FMR.
MATERIAL AND METHODS
Study Population
The data were collected as part of a prospective Institutional Review Board approved protocol with waiver of informed consent. We enrolled 34 consecutive patients with FMR undergoing cardiac surgery. FMR was defined as MR resulting from retraction and mal-coaptation of structurally normal mitral valve leaflets in the presence of global left ventricular dysfunction. In a subset of patients with FMR, localized ischemia-induced wall motion abnormalities (WMA) can be seen to be contributing to MR; in such patients the term ischemic mitral regurgitation (IMR) may also be used. Exclusion criteria included patients with structural abnormalities (flail leaflets, torn chordae) of the mitral apparatus or technically inadequate studies. We also selected 15 controls. These were patients scheduled for cardiac surgery for an unrelated indication and who had normal (>50%) ejection fraction, trace or no mitral regurgitation and absence of any valvular abnormality (Table 1).
TABLE 1.
Characteristics of cases and controls
| Cases (n= 34) | Controls (n=15) | |
|---|---|---|
| Age (years) | 67.4 (43-88) | 61.8 (32-84) |
| Gender Male Female |
20 (58.8%) 14 (41.2%) |
9 (60.0%) 6 (40.0%) |
| Mitral Regurgitation Grade O 1 2 3 4 |
- - 14 15 5 |
15 - - - - |
| Ejection Fraction < 50% > 50% |
20 14 |
0 15 (100%) |
| Functional Mitral Regurgitation | 34 (100%) | - |
| Ischemic Mitral Regurgitation | 17 (50%) | - |
| Coronary Angiography Yes No |
32 (94%) 2 (6%) |
15 (100%) 0 |
| Coronary Artery Disease | 28 (82%) | 0 |
| Procedure MVR + CABG MVR CABG AVR AVR + CABG PFO Closure |
17 (50%) 9 (26%) 6 (18%) 1 (3%) 1 (3%) - |
- - 5 (33%) 7 (47%) 2 (13%) 1 (7%) |
AVR: aortic valve replacement; CABG: coronary artery bypass graft; MVR: mitral valve replacement; PFO: patent foramen ovale
Intraoperative 3D TEE Examination
After induction of general anesthesia, a comprehensive two-dimensional (2D) transesophageal echocardiography (TEE) examination was performed during the pre-cardiopulmonary bypass period. MR quantification was performed by measuring the vena contracta . Vena contracta is a semi-quantitative method of MR severity assessment. It is based on measurement of the width of the narrowest part of the MR jet in the mid-esophageal long-axis view to clearly identify flow convergence of the MR jet on the left ventricular side. All our cases were patients with moderate or more MR (vena contracta width ≥ 1.5 cm20) and without any evidence of structural disease of the leaflets, papillary muscles or chordae tendinae or a WMA. Image acquisition in 3D was performed with an iE-33 ultrasound system equipped with an X7-2t “matrix” TEE probe (Philips Medical Systems, Andover, MA). Images were acquired with R-wave gating over 4-8 beats during brief periods of apnea and concurrent avoidance of patient or probe movements. In patients with atrial fibrillation and other arrhythmias, the 3D ‘live zoom mode’ was used to acquire an en-face view of the mitral valve. (The 3D live zoom mode displays a magnified 3D image.) A technically adequate image was defined as an en-face left atrial image of the MV, devoid of artifacts. Intraoperative image acquisition was completed in 30 seconds; the data sets were then immediately exported via a universal serial bus (USB) flash drive transfer to a Windows-based workstation for analysis by the TomTec© Imaging Systems ‘Image Arena Browser” (GmbH, Munich, Germany).
Dynamic MV Geometric Analysis
The MV geometric analysis was performed using the TomTec© Image Arena software (GmBH, Munich, Germany) equipped with the 4D MV Assessment 2.0 Program. The feasibility and methodology of intraoperative dynamic geometric analysis has been established previously 21. Briefly, the dynamic MV geometric analysis is performed in a workflow arrangement of seven sequential steps, which are initiated with identification and selection of ‘end-systolic’ and early systolic frames in the data set. Based on the identification of the anatomical landmarks and the frames of interest (End-systolic frame – the last frame before the MV opens) (Early-systolic frame – the last frame before MV starts to close), mitral annulus, coaptation line, leaflets and the aortic valve position are dynamically tracked throughout the systolic phase. This is based on optical flow and pattern recognition of the mitral annulus and leaflets 22-25. At the conclusion of the workflow, both static and dynamic geometric analyses are generated. The time taken for data export and analysis was < 5 minutes. The number of frames encompassing systole varied between 30-50 depending on the patient’s heart rate, depth of imaging and the number of heartbeats over which R wave gating occurred.
Statistical Analysis
Data generated from the static and dynamic analysis of the mitral valve were exported to Microsoft Excel for Mac 2011 (Microsoft Corporation, Redmond, WA), via ‘csv’ (comma separated values) file format. To account for variation in frames per systolic cycle and to normalize for heart rate, time values were averaged to five equal points during the systolic phase. SPSS 18.0 (IBM Inc., Endicott, NY) was used to analyze the data. Baseline demographic data were compared using t-test or Fisher’s Exact, as appropriate. Comparison between MV geometric parameters was made using t-test for single measures, and linear repeated measures analysis for comparisons over time. Pearson’s correlation was used to assess the relationship between vena contracta and different MV geometric parameters throughout systole. Reliability of the echocardiographic evaluation was assessed in a random sample of 9 patients (5 cases, 4 controls) by examining the inter-observer and intra-observer variability for all parameters using Pearson’s correlation. Statistical significance was determined at p≤0.05.
RESULTS
Baseline Patient and Imaging Characteristics
Data from 34 patients with FMR (cases) and 15 controls were used for analysis in this study. No significant differences were noted in baseline characteristics between the two groups with regards to age, gender, body-mass index and body surface area. Of the forty nine patients, 5 (10%) had MV data sets acquired with ‘live zoom’ imaging, while in the remaining 44 (90%) it was possible to acquire R-wave gated volumetric images. In all patients, we were able to complete MV geometric analysis within 40 seconds of initiating the workflow steps.
Intra and Inter-Observer Variability
Reliability of the assessment comparing intra and inter-observer correlation was 0.92 and 0.83, respectively (p<0.01 for both).
Mitral Annular Dimensions
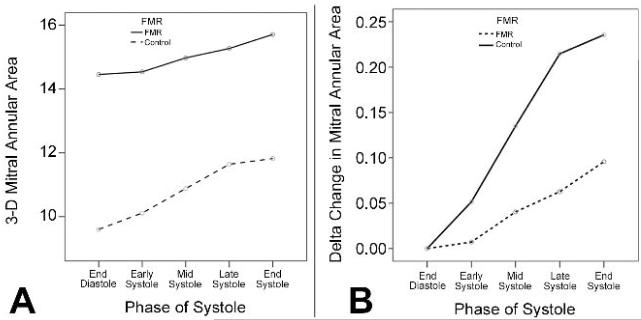
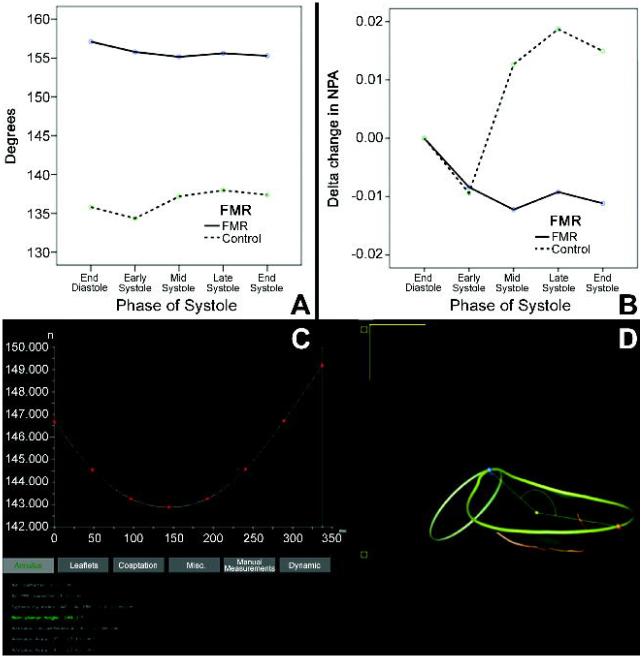
Mean annular dimensions were all significantly enlarged in patients with FMR compared to controls throughout systole (Table 2, Figure 1). Maximum 3D mitral annular area was seen at end-systole in 90% (44/49) of all patients. Parameters of mitral annular shape and left ventricular remodeling such as the anterior and posterior leaflet areas (ALA/PLA), non-planarity angle (NPA), circularity index, and tenting volume were also significantly increased in FMR patients (Table 2, Figure 2).
TABLE 2.
Differences in mitral annular geometric parameters between controls and patients with FMR (cases).
| Annular Dimensions | Controls (n=15) |
Cases (n=34) |
P value |
|---|---|---|---|
| Anteroposterior diameter | 3.0 cm ± 0.1 | 4.0 cm ± 0.14 | <0.01 |
| Anterolateral–Posteromedial diameter |
3.6 cm ± 0.1 | 4.3 cm ± 0.1 | <0.01 |
| Commissural diameter | 3.5 cm ± 0.5 | 4.4 cm ± 0.7 | <0.01 |
| Annular Circumference | 11.4 cm ± 0.5 | 14.1cm ±0.4 | <0.01 |
| 2D Annular Area | 8.7 cm2 ± 0.79 | 14.2 cm2± 0.8 | <0.01 |
| 3D Annular Area | 9.6 cm2± 3 | 14.6 cm2± 5 | <0.01 |
| Anterior Leaflet Area | 6.4 cm2± 2 | 10.0 cm2± 3 | <0.01 |
| Posterior Leaflet Area | 6.1 cm2± 2 | 9.0 cm2± 3 | <0.01 |
| Annular Shape | |||
| Nonplanarity Angle | 136° ± 11 | 155° ± 15 | <0.01 |
| Max Nonplanarity Angle | 140° ± 10 | 158° ± 15 | <0.01 |
| Circularity Index | 0.85 ± 0.02 | 0.93 ± 0.01 | <0.01 |
| Tenting Height | 10.0 mm ± 0.85 | 11.7mm ± 0.77 | 0.13 |
| Tenting Volume | 3.5mm3± 2.0 | 6.2mm3± 3.5 | 0.01 |
| Tenting Volume Fraction | 24% ± 11 | 30% ± 21 | 0.36 |
| Aorto-Mitral Angle | 112° ±14 | 119° ± 15 | 0.12 |
| Annular Excursion | |||
| Maximum Vertical Annular Displacement |
8.3 mm ± 3 | 5.8 mm ± 2 | <0.01 |
| Maximum Vertical Annular Displacement Velocity |
32 mm/s ± 13 | 26 mm/s ± 8 | 0.05 |
| Maximum Vertical Annular Acceleration |
26 mm/s2± 15 | 18 mm/s2± 12 | 0.04 |
Values are described as mean ± standard deviation.
FMR: functional mitral regurgitation
FIGURE 1.
In patients with functional mitral regurgitation, mitral annular area was larger (Panel A) and experienced a smaller delta change (Panel B), compared with controls. These differences were seen throughout systole.
FMR: functional mitral regurgitation
FIGURE 2.
Compared with controls, NPA is much greater in patients with functional mitral regurgitation, indicating a flatter and a less saddle-shaped mitral annulus (Panel A). Also, the delta change in NPA over systole is much lower in patients with functional mitral regurgitation (Panel B). A screenshot from TomTec© 4D MV Assessment 2.0 demonstrates the initial decrease in the saddle shape of the mitral annulus followed by an increase towards end systole (Panel C).
FMR: functional mitral regurgitation
Dynamic Change in MV Geometry
Significant differences were noted in vertical annular displacement velocity and acceleration between patients with FMR and controls (Table 2). The delta changes in 3D mitral annular area, ALA, PLA and NPA were significantly reduced in patients with FMR compared to controls (Figures 1-2).
Correlation between vena contracta and MV geometrical parameters
Mean vena contracta width in patients with FMR was 0.737 ± 0.29 and correlated significantly with annular dimensions such as the PLA (r=0.624, p=0.001), mitral annular circumference (r=0.535, p=0.006), 2D annular area (r=0.587, p=0.002), 3D annular area (r=0.580, p=0.001) and antero-posterior diameter (r=0.582, p=0.02), at all points during the systolic cycle. Vena contracta width correlated slightly with tenting volume (r=0.404, p=0.02) and annular displacement velocity (r=0.369, p=0.045). However, parameters of mitral annular non-planarity such as the NPA, tenting height and circularity index and ALA did not show any correlation with the vena contracta.
COMMENT
In this study, we were able to compare dynamic changes in mitral annular geometry between patients with FMR and controls, in a clinically feasible fashion. Our analyses demonstrated significant differences between the two groups in their static and dynamic annular geometry (Table 2). Our results have shown that the annulus in patients with FMR is flatter, more circular and larger in area and undergoes less vertical displacement than that in controls throughout systole. We were also able to dynamically analyze changes in planarity, shape of mitral annulus and leaflet areas, parameters that have not been previously analyzed either at endsystole or dynamically 22, 26, 27. Importantly, the geometric differences in dimensions between the two groups were maintained throughout systole (Figures 1-2) 3, 4, 27, 28. Mitral annular area also progressively increased, peaking at end-systole (Figure 1) 4, 13, 26-28. Even though the baseline mitral annular area in patients with FMR was larger compared with controls, it underwent a much smaller change (Figure 1) 3, 4, 28, 29. As compared to late systole, the NPA decreased during early systole (i.e. the annulus assumes a more saddle shaped configuration) (Figure 2) 13, 30, and a more circular shape in patients with FMR. In patients with FMR, the PLA demonstrated a greater change over systole than the ALA, with a significant correlation with vena contracta (r=0.624, p=0.001). Interestingly, while tenting volume was much higher in patients with FMR, no significant difference was noted in tenting height (Table 2). This finding emphasizes that 3D tenting volume is a better predictor of mitral valvular tenting compared to 2D measures such as tenting height or tenting area 31, 32.
The results of our study have important clinical implications for a comprehensive echocardiographic assessment of FMR. Whereas the static annular dimensions at end-systole represent structure, their dynamic nature represents the functional aspect of mitral annulus. Our results show that there are significant changes in annular structure and function in patients with FMR compared to those without. Hence, a case can be made that the current model of echocardiographic interrogation of flow dependent variables, without taking into account the dynamics of function, is far from comprehensive. A comprehensive assessment of mitral annular geometry should take into account the entire spectrum of changes over time 33. Our results also raise the possibility of following the annular function as a marker ventricular reverse remodeling after revascularization therapy (surgical or percutaneous). Therefore, the ability to track the annulus through the cardiac cycle in a clinically feasible fashion is a significant advance from the current paradigm of mitral vale assessment.
Additionally, the demonstration of altered mitral annular dynamics throughout systole calls into question the use of flexible ring annuloplasty in the treatment of FMR. Proponents of flexible ring annuloplasty believe that these devices preserve mitral annular function and provide a more anatomically correct repair 34. The results of this study demonstrate the flaw in this belief and the presumed benefit 35 and support the concept that the function of ring annuloplasty is one of restoration and not preservation of annular geometry. This is the goal of the latest generation of saddle shaped annuloplasty rings, which have been designed to reestablish a more normal systolic human annular and leaflet geometry 36, 37. Data from animal studies has been used to improve annuloplasty ring design but technological limitations have precluded the performance of such analyses for routine clinical use 38. Our performance of these analyses in a timely fashion brings us a step closer to incorporating this information into clinical decision-making and objectively assessing the concept of offering an ‘annular solution to a ventricular problem’ 19.
We acknowledge certain limitations in our study: Firstly, our control group represented patients undergoing TEE for clinical indications and therefore might not represent a normal population. However, given that there was no clinical or echocardiographic evidence of MV disease, we believe it represented an adequate control group. Secondly, our 3D echocardiographic data were collected in real-time and the geometric analyses were performed off-line. However the lag time between data acquisition and export and analysis was less than five minutes and the results were readily available.
In conclusion, mitral annular geometry in patients with FMR is significantly altered throughout systole as compared to those without FMR. It is now clinically feasible to perform dynamic analysis of mitral annular geometry with the ready availability of information. Appreciation of this knowledge has the potential to objectively quantify mitral annular function and possibly follow results of therapy for FMR. In future this may help in design and selection of annuloplasty rings for FMR.
Acknowledgements
This study was supported by the following grant:
Echocardiography to Predict Recurrent Ischemic Mitral Regurgitation after Surgical Mitral Valve Repair (RC Gorman, PI). National Institute of Health. R01-HL 103723
Abbreviations and Acronyms
- 2D
Two-dimensional
- 3D
Three-dimensional
- ALA
Anterior leaflet angle
- CPB
Cardiopulmonary bypass
- CSV
Comma separated values
- FMR
Functional mitral regurgitation
- IMR
Transesophageal echocardiography
- MR
Mitral regurgitation
- MV
Mitral valve
- NPA
Non-planarity angle
- PLA
Posterior leaflet angle
- RT-3DE
Real-time three-dimensional echocardiography
- TEE
Transesophageal echocardiography
- USB
Universal serial bus
- WMA
Wall motion abnormalities
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gorman JH, 3rd, Gupta KB, Streicher JT, et al. Dynamic three-dimensional imaging of the mitral valve and left ventricle by rapid sonomicrometry array localization. The Journal of thoracic and cardiovascular surgery. 1996;112(3):712–26. doi: 10.1016/S0022-5223(96)70056-9. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen TC, Itoh A, Carlhall CJ, et al. The effect of pure mitral regurgitation on mitral annular geometry and three-dimensional saddle shape. The Journal of thoracic and cardiovascular surgery. 2008;136(3):557–65. doi: 10.1016/j.jtcvs.2007.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daimon M, Saracino G, Fukuda S, et al. Dynamic change of mitral annular geometry and motion in ischemic mitral regurgitation assessed by a computerized 3D echo method. Echocardiography. 2010;27(9):1069–77. doi: 10.1111/j.1540-8175.2010.01204.x. [DOI] [PubMed] [Google Scholar]
- 4.Little SH, Ben Zekry S, Lawrie GM, et al. Dynamic annular geometry and function in patients with mitral regurgitation: insight from three-dimensional annular tracking. J Am Soc Echocardiogr. 2010;23(8):872–9. doi: 10.1016/j.echo.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi F, Corsi C, Sugeng L, et al. Quantification of mitral apparatus dynamics in functional and ischemic mitral regurgitation using real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. 2008;21(4):347–54. doi: 10.1016/j.echo.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe N, Ogasawara Y, Yamaura Y, et al. Quantitation of mitral valve tenting in ischemic mitral regurgitation by transthoracic real-time three-dimensional echocardiography. Journal of the American College of Cardiology. 2005;45(5):763–9. doi: 10.1016/j.jacc.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 7.Rausch MK, Bothe W, Kvitting JP, et al. In vivo dynamic strains of the ovine anterior mitral valve leaflet. Journal of biomechanics. 2011;44(6):1149–57. doi: 10.1016/j.jbiomech.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jassar AS, Brinster CJ, Vergnat M, et al. Quantitative mitral valve modeling using real-time three-dimensional echocardiography: technique and repeatability. Ann Thorac Surg; 91(1):165–71. doi: 10.1016/j.athoracsur.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergnat M, Jassar AS, Jackson BM, et al. Ischemic mitral regurgitation: a quantitative three-dimensional echocardiographic analysis. Ann Thorac Surg; 91(1):157–64. doi: 10.1016/j.athoracsur.2010.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmood F, Gorman JH, 3rd, Subramaniam B, et al. Changes in mitral valve annular geometry after repair: saddle-shaped versus flat annuloplasty rings. Ann Thorac Surg. 2010;90(4):1212–20. doi: 10.1016/j.athoracsur.2010.03.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahmood F, Karthik S, Subramaniam B, et al. Intraoperative application of geometric three-dimensional mitral valve assessment package: a feasibility study. J Cardiothorac Vasc Anesth. 2008;22(2):292–8. doi: 10.1053/j.jvca.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Mahmood F, Subramaniam B, Gorman JH, 3rd, et al. Three-dimensional echocardiographic assessment of changes in mitral valve geometry after valve repair. Ann Thorac Surg. 2009;88(6):1838–44. doi: 10.1016/j.athoracsur.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grewal J, Suri R, Mankad S, et al. Mitral annular dynamics in myxomatous valve disease: new insights with real-time 3-dimensional echocardiography. Circulation. 2010;121(12):1423–31. doi: 10.1161/CIRCULATIONAHA.109.901181. [DOI] [PubMed] [Google Scholar]
- 14.Parish LM, Jackson BM, Enomoto Y, et al. The dynamic anterior mitral annulus. Ann Thorac Surg. 2004;78(4):1248–55. doi: 10.1016/j.athoracsur.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad RM, Gillinov AM, McCarthy PM, et al. Annular geometry and motion in human ischemic mitral regurgitation: novel assessment with three-dimensional echocardiography and computer reconstruction. Ann Thorac Surg. 2004;78(6):2063–8. doi: 10.1016/j.athoracsur.2004.06.016. discussion 2068. [DOI] [PubMed] [Google Scholar]
- 16.Gorman JH, 3rd, Jackson BM, Enomoto Y, et al. The effect of regional ischemia on mitral valve annular saddle shape. Ann Thorac Surg. 2004;77(2):544–8. doi: 10.1016/S0003-4975(03)01354-7. [DOI] [PubMed] [Google Scholar]
- 17.Ray S. The echocardiographic assessment of functional mitral regurgitation. Eur J Echocardiogr; 11(10):i11–17. doi: 10.1093/ejechocard/jeq121. [DOI] [PubMed] [Google Scholar]
- 18.Silbiger JJ, Bazaz R. Contemporary insights into the functional anatomy of the mitral valve. Am Heart J. 2009;158(6):887–95. doi: 10.1016/j.ahj.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Cheng A, Nguyen TC, Malinowski M, et al. Effects of undersized mitral annuloplasty on regional transmural left ventricular wall strains and wall thickening mechanisms. Circulation. 2006;114(1 Suppl):I600–9. doi: 10.1161/CIRCULATIONAHA.105.001529. [DOI] [PubMed] [Google Scholar]
- 20.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 21.Warraich HJ, Shahul S, Matyal R, Mahmood F. Bench to bedside: Dynamic Mitral Valve Assessment. J Cardiothorac Vasc Anesth. 2011 doi: 10.1053/j.jvca.2011.06.021. in press. [DOI] [PubMed] [Google Scholar]
- 22.Veronesi F, Corsi C, Caiani EG, et al. Tracking of left ventricular long axis from real-time three-dimensional echocardiography using optical flow techniques. IEEE Trans Inf Technol Biomed. 2006;10(1):174–81. doi: 10.1109/titb.2005.855535. [DOI] [PubMed] [Google Scholar]
- 23.Malpica N, Santos A, Zuluaga MA, et al. Tracking of regions-of-interest in myocardial contrast echocardiography. Ultrasound Med Biol. 2004;30(3):303–9. doi: 10.1016/j.ultrasmedbio.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Caban J, Joshi A, Rheingans P. Texture-based feature tracking for effective time-varying data visualization. IEEE Trans Vis Comput Graph. 2007;13(6):1472–9. doi: 10.1109/TVCG.2007.70599. [DOI] [PubMed] [Google Scholar]
- 25.Foroughi P, Abolmaesumi P, Hashtrudi-Zaad K. Towards real-time registration of 4D ultrasound images. Conf Proc IEEE Eng Med Biol Soc. 2006;1:404–7. doi: 10.1109/IEMBS.2006.260658. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan SR, Bashein G, Sheehan FH, et al. Three-dimensional echocardiographic assessment of annular shape changes in the normal and regurgitant mitral valve. American heart journal. 2000;139(3):378–87. doi: 10.1016/s0002-8703(00)90077-2. [DOI] [PubMed] [Google Scholar]
- 27.Mihalatos DG, Joseph S, Gopal A, et al. Mitral annular remodeling with varying degrees and mechanisms of chronic mitral regurgitation. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2007;20(4):397–404. doi: 10.1016/j.echo.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Daimon M, Gillinov AM, Liddicoat JR, et al. Dynamic change in mitral annular area and motion during percutaneous mitral annuloplasty for ischemic mitral regurgitation: preliminary animal study with real-time 3-dimensional echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2007;20(4):381–8. doi: 10.1016/j.echo.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Sughimoto K, Takahara Y, Mogi K, et al. Annular excursion contributes to efficient cardiac output: a three-dimensional echocardiographic approach. The Journal of heart valve disease. 2010;19(2):244–8. [PubMed] [Google Scholar]
- 30.Mihaljevic T, Lam BK, Rajeswaran J, et al. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. Journal of the American College of Cardiology. 2007;49(22):2191–201. doi: 10.1016/j.jacc.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 31.Song JM, Fukuda S, Kihara T, et al. Value of mitral valve tenting volume determined by real-time three-dimensional echocardiography in patients with functional mitral regurgitation. The American journal of cardiology. 2006;98(8):1088–93. doi: 10.1016/j.amjcard.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 32.Tibayan FA, Wilson A, Lai DT, et al. Tenting volume: three-dimensional assessment of geometric perturbations in functional mitral regurgitation and implications for surgical repair. The Journal of heart valve disease. 2007;16(1):1–7. [PubMed] [Google Scholar]
- 33.Warraich HJ, Shahul S, Matyal R, et al. Bench to bedside: dynamic mitral valve assessment. J Cardiothorac Vasc Anesth. 2011;25(5):863–6. doi: 10.1053/j.jvca.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Cosgrove DM, 3rd, Arcidi JM, Rodriguez L, et al. Initial experience with the Cosgrove-Edwards Annuloplasty System. The Annals of thoracic surgery. 1995;60(3):499–503. doi: 10.1016/0003-4975(95)00458-W. discussion 503-4. [DOI] [PubMed] [Google Scholar]
- 35.Rausch MK, Bothe W, Kvitting JP, et al. Mitral valve annuloplasty: a quantitative clinical and mechanical comparison of different annuloplasty devices. Ann Biomed Eng. 2012;40(3):750–61. doi: 10.1007/s10439-011-0442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez JH, Liou SW, Padala M, et al. A saddle-shaped annulus reduces systolic strain on the central region of the mitral valve anterior leaflet. The Journal of thoracic and cardiovascular surgery. 2007;134(6):1562–8. doi: 10.1016/j.jtcvs.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 37.Vergnat M, Levack MM, Jassar AS, et al. The influence of saddle-shaped annuloplasty on leaflet curvature in patients with ischaemic mitral regurgitation. Eur J Cardiothorac Surg. 2012;42(3):493–9. doi: 10.1093/ejcts/ezs040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rausch MK, Bothe W, Kvitting JP, et al. Mitral valve annuloplasty : a quantitative clinical and mechanical comparison of different annuloplasty devices. Ann Biomed Eng. 2012;40(3):750–61. doi: 10.1007/s10439-011-0442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]