Abstract
Lung cancer is a malignant tumor with high morbidity and mortality rates. To date, no suitable molecular diagnostic tool to predict disease recurrence and metastasis has been identified. The current study aimed to evaluate the potential of N-terminal truncated carboxypeptidase E (CPEΔN) to predict the recurrence and metastasis of lung adenocarcinoma. Western blotting revealed the co-expression of CPE and CPEΔN in the surgically collected pathological and pericarcinoma tissues tissues of 62.1% (59/95) lung adenocarcinoma patients. The full length CPE protein was predominantly expressed in pericarcinoma tissues and CPEΔN expression was identified in the pericarcinoma normal tissues of only 5.26% (5/95) patients. The 3-year postoperative recurrence and metastasis rates were significantly higher in patients with positive CPEΔN expression than in patients with negative CPEΔN expression (P=0.009). Furthermore, the overall survival rate of patients with predominant nuclear CPE expression was lower than that of patients with predominant cytoplasmic CPE expression (46.3 vs. 64.7%); however, no statistically significant difference was identified (P=0.125). Thus, the results of the current study indicated that CPEΔN may present a novel molecular biomarker for predicting recurrence and metastasis of lung adenocarcinoma, which may aid with stratifying patients by risk and thus, may facilitate individualized therapy.
Keywords: overall survival, prognosis, N-terminal truncated carboxypeptidase E, lung adenocarcinoma, disease-free survival
Introduction
Lung cancer is a malignant tumor characterized by high morbidity and mortality rates, accounting for ~1.2 million mortalities annually worldwide, which usually result from disease recurrence and metastasis (1). Although diagnostic methods and treatments have markedly improved in recent years, the 5- and 10-year survival rates remain at <15 and <7%, respectively (2). At present, the lack of appropriate molecular diagnostic tools to predict the potential metastasis of lung cancer represents a major clinical obstacle. Therefore, identification of biomarkers that accurately predict future recurrence and metastasis of lung cancer may improve treatment strategies.
Carboxypeptidase E (CPE) is a metal ion-dependent exopeptidase that is predominantly expressed in endocrine and nervous tissues, which converts the prohormones secreted by endocrine or nerve cells, such as adrenocorticotropin/lipotropin (ACTH/LPH), proinsulin, opiomelanocortin and enkephalin, into an active form or into neuropeptides (3–5). Recent studies have demonstrated that abnormal expression of CPE occurs in epithelium-derived cancer tissues, including liver cancer, renal clear cell carcinoma, colorectal cancer, cervical cancer and melanoma (6–14). In 2011, a novel form of CPE, N-terminal truncated carboxypeptidase E (CPEΔN), was identified in hepatocellular carcinoma (HCC) (15,16). Truncated CPE interacts with histone deacetylase (HDAC) 1 and HDAC2 to form a complex, which regulates the expression of metastasis-associated proteins. Furthermore, it was reported that CPEΔN was an independent predictor for recurrence and metastasis of HCC. HCC patients with high CPEΔN expression levels exhibited significantly higher recurrence rates in the 2 years following surgery and lower median survival times than patients with low CPEΔN expression levels (15).
Similar findings have been observed in primary pheochromocytomas/paragangliomas (PHEO/PGL) and colorectal cancer patients (17,18). Previous studies, which have used quantitative polymerase chain reaction to analyze CPEΔN expression, revealed that elevated CPEΔN expression is a statistically significant predictor of poor prognosis (17,18). Since HCC, PHEO/PGL and colorectal cancer are extremely different tumors with distinctive tumor origins, these findings suggest that CPEΔN may be a predictor of metastasis with a broad spectrum.
To evaluate the function of CPEΔN in lung adenocarcinoma, in the present study, CPEΔN expression was analyzed in lung adenocarcinoma tumors by western blot analysis and immunohistochemistry. It has been demonstrated that CPEΔN expression was associated with lymph node metastasis and distant metastasis of lung adenocarcinoma, and that the three-year tumor-free survival rates were significantly lower in patients with CPEΔN expression than in those without CPEΔN expression. The present study aimed to evaluate the potential of CPEΔN as a biomarker for predicting future metastasis of lung adenocarcinoma.
Materials and methods
Patients
A total of 95 lung adenocarcinoma patients who underwent radical resection between January 2010 and June 2011 in Liaoning Cancer Hospital and Institute (Shenyang, Liaoning, China) were recruited for the current study. The patient cohort included 50 female and 45 male patients, with a mean age of 58.7 years (range, 35–75 years). Of the 95 patients, 34, 30, 27 and 4 patients were diagnosed with clinical stage I, II, III and IV disease, respectively. None of the patients had received any treatment prior to surgery. Patients at clinical stages II, III and IV received adjuvant chemotherapy (docetaxel and cisplatin or docetaxel and nedaplatin regimens) and/or immunotherapy after surgery. Follow-up interviews were conducted every 3 months. Of the 100 patients initially included, 3 were lost to follow-up and 2 were without adequate protein samples for analysis. Therefore, 95 samples were included in the final analysis. The date of recurrence/identification of metastasis, data regarding metastasis-affected organs and the date of patient morality were recorded. The study was approved by the Ethics Committee of Liaoning Cancer Hospital and Institute, and informed consent was obtained from all participants.
Reagents
Monoclonal mouse anti-human CPE antibody was purchased from BD Biosciences (Franklin Lakes, NJ, USA; cat. no. 610762). Total protein isolation kit (catalog no. WLA019) was purchased from Wanlei Bio (Shenyang, China) and a lung adenocarcinoma protein array (150-µm spots) was purchased from Shanghai Xinchao Biotechnology (Shanghai, China).
Western blot analysis
Adenocarcinoma and pericarcinoma tissues (obtained >5 cm away from the primary tumor) were maintained in a sterile cryopreservation tube and stored in liquid nitrogen. All stored samples were subject to western blot analysis within 1.5 years of collection. Western blot analysis was performed to analyze the expression of CPE and CPEΔN in adenocarcinoma and pericarcinoma tissues, as previously described (19). Expression levels of CPEΔN were quantified by grayscale scanning (CanoScanLiDE120; Canon, Inc., Tokyo, Japan) and analyzed with Gelpro32 (Media Cybernetics, Rockville, MD, USA). No CPEΔN expression was defined as score ‘0’; positive expression was defined as score ‘1’ (grayscale ratio of CPEΔN; and actin, 0.5–1) and strong expression was defined as score ‘2’ (grayscale ratio of CPEΔN and actin, 1.0–1.5).
Immunohistochemistry
The immunohistochemistry assay was performed, as previously described (20). The expression levels and intracellular localization of CPE and CPEΔN were determined by immunohistochemistry using a 150-µm spot lung adenocarcinoma protein array. Based on immunohistochemical analysis of patients with positive CPE expression, patients were classified into two groups, nuclear CPE expression and cytoplasmic CPE expression, to allow the comparison of overall survival rates between the groups.
Statistical analysis
Data was analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The association between CPEΔN expression and T stage, lymph node metastasis and distant metastasis was analyzed by χ2 test. Disease-free survival and overall survival curves were established using the Kaplan-Meier method and intergroup comparisons were analyzed using the log-rank test. Multivariate analyses of prognostic factors were performed using the Cox regression model. P<0.05 was considered to indicate a statistically significant difference.
Results
CPEΔN expression is higher in lung adenocarcinoma tumor tissues than non-tumor tissues
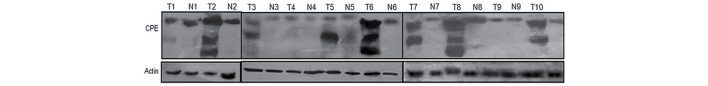
CPEΔN expression in 95 tumor and non-tumor tissues was analyzed by western blotting. A total of 5 samples were excluded due to minimal total amount of protein. The results revealed that the co-expression rate of CPE and CPEΔN in lung adenocarcinoma tissue was 62.1% (59/95). Full-length CPE was expressed at similar levels in tumor and pericarcinoma normal tissues from the same patients, and CPEΔN expression was identified in only 5.26% (5/95) of non-tumor tissues (Fig. 1). Furthermore, the χ2 test revealed that CPEΔN expression was closely associated with lymph node metastasis (P=0.026) and distant metastasis (P=0.002), however, no significant association was identified between CPEΔN expression and age (P=0.555), gender (P=0.291) or T stage (P=0.109) (Table I).
Figure 1.
Total proteins were extracted from adenocarcinoma and pericarcinoma tissues collected during surgery in 10 patients. Expression of CPE was detected by western blotting. Actin was used as an internal control. T, adenocarcinoma; N, pericarcinoma; CPE, carboxypeptidase E.
Table I.
Correlation between CPEΔN protein expression and clinicopathological features in 95 lung adenocarcinoma patients.
| CPEΔN expression | ||||
|---|---|---|---|---|
| Parameter | Patients, n | (−), n | (+), n | P-value |
| Gender | 0.291 | |||
| Female | 50 | 18 | 32 | |
| Male | 45 | 21 | 24 | |
| Age at diagnosis, years | 0.555 | |||
| ≤55 | 30 | 11 | 19 | |
| >55 | 65 | 28 | 37 | |
| T status | 0.109 | |||
| T1 | 3 | 1 | 2 | |
| T2 | 68 | 31 | 37 | |
| T3 | 20 | 6 | 14 | |
| T4 | 4 | 1 | 3 | |
| N status | 0.026 | |||
| N0 | 45 | 25 | 20 | |
| N1 | 24 | 6 | 18 | |
| N2 | 24 | 8 | 16 | |
| NX | 2 | 0 | 2 | |
| M status | 0.012 | |||
| M0 | 91 | 39 | 52 | |
| M1 | 4 | 1 | 3 | |
| Clinical stage | 0.061 | |||
| I | 34 | 19 | 15 | |
| II | 30 | 11 | 19 | |
| III | 27 | 9 | 18 | |
| IV | 4 | 1 | 3 | |
CPEΔN, N-terminal truncated carboxypeptidase E; (−), negative expression; (+), positive expression; T, tumor; N, node; M, metastasis.
CPEΔN expression is associated with lung adenocarcinoma recurrence and metastasis
Of the 95 patients included in the study, 36 patients exhibited negative CPEΔN expression and 56 patients exhibited positive CPEΔN expression. The negative and positive CPEΔN patient groups were both followed up for 36 months. A disease-free survival Kaplan-Meier curve was established, which revealed that patients of the positive CPEΔN expression group exhibited significantly higher postoperative recurrence and metastasis rates (87.5%) when compared with patients of the negative CPEΔN expression group (69.2%) (P=0.009) (Fig. 2). Subsequently, the 59 patients of the positive CPEΔN expression group were divided into high CPEΔN expression (immunohistochemistry score, 2) and low CPEΔN expression immunohistochemistry score, 1) groups. The 2-year disease-free survival rate of the low CPEΔN expression group (39.3%; 13/33) was significantly higher than that of the high CPEΔN expression group (11.5%; 3/26) (P=0.020; Table II).
Figure 2.
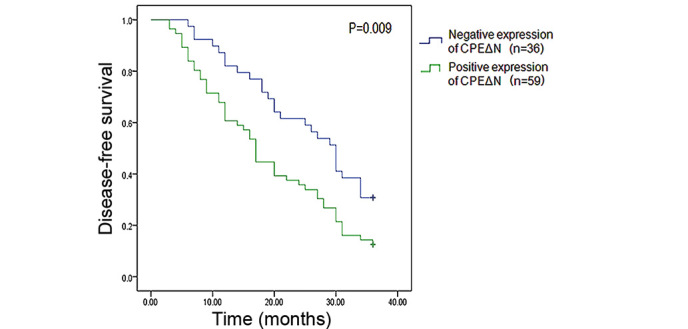
Kaplan-Meier analysis revealed that the 3-year recurrence rate of patients with positive CPEΔN expression (87.5%) was significantly higher than that of patients without CPEΔN expression (69.2%). CPEΔN, N-terminal truncated carboxypeptidase E.
Table II.
Correlation between CPEΔN protein expression and tumor recurrence 2 years after surgery in 59 lung adenocarcinoma patients.
| Tumor recurrence | Patients, n | High CPEΔN expression, n | Low CPEΔN expression, n | P-value |
|---|---|---|---|---|
| Yes | 43 | 23 | 20 | 0.020 |
| No | 16 | 3 | 13 |
CPEΔN, N-terminal truncated carboxypeptidase E.
Patients with predominant nuclear expression of CPE exhibit a lower overall survival rate than those with predominant cytoplasmic CPE expression
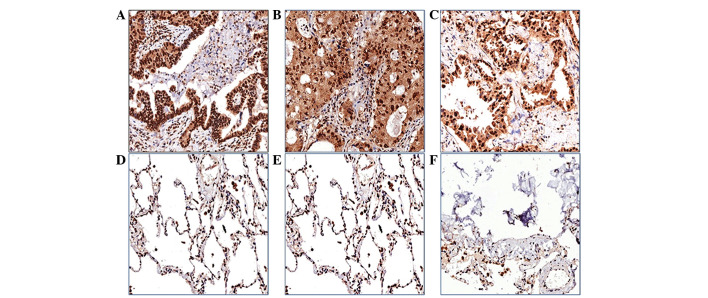
Immunohistochemistry was used to measure CPE expression using a 150-µm spot protein array of lung adenocarcinoma. The expression level of CPE in tumor tissue appeared to be higher than that in the pericarcinoma normal tissues from the same patients, and CPE was predominantly localized in the cell nucleus or cytoplasm (Fig. 3). Patients were classified into two groups, a nuclear CPE expression group (n=41) and a cytoplasmic CPE expression group (n=34), based on where CPE was predominantly expressed in tumor cells. The overall survival rate of patients with nuclear predominant nuclear CPE expression was lower (46.3%) than that of patients with predominant cytoplasmic CPE expression (64.7%), however, the difference was not statistically significant (P=0.125) (Fig. 4).
Figure 3.
CPE expression in (A-C) lung adenocarcinoma and (D-F) pericarcinoma tissue was analyzed by immunohistochemistry (magnification, ×400). CPE expression was significantly higher in lung adenocarcinoma tissues compared with pericarcinoma tissue and CPE was predominantly localized in the cell nucleus or cytoplasm. CPE, carboxypeptidase E.
Figure 4.
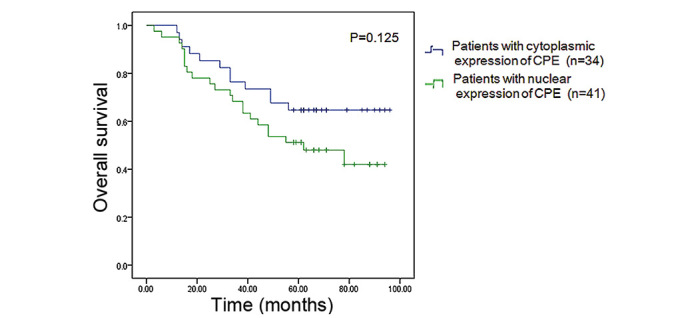
Kaplan-Meier analysis revealed the overall survival rate of patients with cytoplasmic CPE expression (64.7%) was higher than that of patients with nuclear CPE expression (46.3%), however, the difference was not statistically significant (P=0.125). CPE, carboxypeptidase E.
CPEΔN expression is an independent prognostic factor for recurrence and metastasis of lung adenocarcinoma
To determine independent prognostic factors for lung adenocarcinoma, a number of significant variables, including TNM status (stages according to National Comprehensive Cancer Network 2010 guidelines) (21), tumor recurrence, distant metastasis and CPEΔN expression were assessed by multivariate regression analysis. Cox regression analysis revealed that CPEΔN expression was an independent prognostic biomarker for recurrence and metastasis of lung adenocarcinoma (P=0.03; Table III).
Table III.
Multivariate regression analysis of prognostic markers in 95 patients with lung adenocarcinoma.
| Clinicopathological variable | β | χ2 | P-value | HR | 95% CI |
|---|---|---|---|---|---|
| TNM status | 0.424 | 5.195 | 0.023 | 1.527 | 1.061–2.198 |
| Tumor recurrence | 0.342 | 8.104 | 0.004 | 1.407 | 1.112–1.780 |
| Distant metastasis | 1.316 | 5.498 | 0.019 | 3.729 | 1.241–11.204 |
| CPEΔN expression | 0.551 | 4.705 | 0.030 | 1.735 | 1.055–2.854 |
HR, hazard ratio; CI, confidence interval; CPEΔN, N-terminal truncated carboxypeptidase E.
Discussion
CPE is a multifunctional protein that exhibits both enzymatic and non-enzymatic functions. CPE exists in three different forms, which are expressed in various subcellular localizations with distinct functions (18). The first type is released by secretory granules, which results in the cleavage of basic residues to generate mature peptide hormones and neuropeptides. Recently, Skalka et al (22) demonstrated that this secreted form of CPE forms a complex with the Wnt3a ligand and frizzled receptor to negatively regulate the canonical Wnt signaling pathway (22). The second type of CPE exists in the membrane, anchored at the trans-Golgi network, which functions as a sorting receptor for prohormones (23). The third type of CPE is located in both the cytoplasm and nucleus, and is involved in cell signal transduction and transcription regulation. The function of this type of CPE is the most diverse, however, further investigation is required with regard to this protein.
In the present study, the association between CPEΔN expression and prognosis of lung adenocarcinoma patients was evaluated. The results revealed that two forms of CPE, full-length CPE and CPEΔN, are co-expressed in the lung adenocarcinoma tissues. CPEΔN expression was identified in the primary tumor tissues of 62.1% (59/95) patients and non-tumor tissues of 5.26% (5/95) patients. Furthermore, multivariate Cox regression analysis demonstrated that CPEΔN expression was closely associated with the occurrence of lymph node and distant metastasis. Patients with positive CPEΔN expression exhibited significantly lower disease-free survival rates than patients with negative CPEΔN expression (87.5 vs. 69.2%, respectively; P=0.009). These findings suggested that CPEΔN expression indicates a higher risk of recurrence and metastasis in primary lung adenocarcinoma.
Western blot analysis requires a large amount of sample tissue, which is a major problem in clinical research. Development of simplified methods for the analysis of CPEΔN or CPE expression is required. In our previous studies (Sun et al, unpublished data), the subcellular localization of CPEΔN was investigated using confocal microscopy, which revealed that CPEΔN was localized to the nucleus in H1299, A549, 95D, H1395 and Calu3 lung cancer cell lines, whereas full-length CPE was predominantly localized in the cytoplasm. Therefore, it was hypothesized that similar findings may be observed in primary lung adenocarcinoma. The hypothesis was tested with tissue microarray. Since one array can simultaneously test 75 samples along with corresponding pericarcinoma tissues, 75 patients with intact overall survival and progression-free survival profiles were selected. Patients were divided into two groups, a nuclear CPE expression group and a cytoplasmic CPE expression group. A difference in overall survival rate was identified between the nuclear and cytoplasmic CPE expression groups (46.3 vs. 64.7%, respectively); however, this was not statistically significant (P=0.125). However, future studies that include more lung adenocarcinoma patients and samples are required to validate the results of the present study.
In conclusion, the results of the present study demonstrated that CPEΔN expression is associated with poor prognosis in lung adenocarcinoma. These findings may improve understanding with regard to the underlying molecular mechanisms of CPE/CPEΔN expression, which promote lung cancer metastasis. Thus, the evaluation of CPEΔN expression status may aid to identify primary lung adenocarcinoma patients that require more intensive treatment. Furthermore, CPEΔN may present a potential target for therapeutic intervention in the future.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 81372287), Liaoning Province Science and Technology Key Project (grant no. 2012225016) and Shenyang City Science and Technology Key Project (grant no. F12-193-9-15).
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Crinò L, Weder W, van Meerbeeck J, Felip E. ESMO Guidelines Working Group: Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v103–v115. doi: 10.1093/annonc/mdq207. (Suppl 5) [DOI] [PubMed] [Google Scholar]
- 3.Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: Genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88:73–83. doi: 10.1016/S0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- 4.Cawley NX, Wetsel WC, Murthy SR, Park JJ, Pacak K, Loh YP. New roles of carboxypeptidase E in endocrine and neural function and cancer. Endocr Rev. 2012;33:216–253. doi: 10.1210/er.2011-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murthy SR, Pacak K, Loh YP. Carboxypeptidase E: Elevated expression correlated with tumor growth and metastasis in pheochromocytomas and other cancers. Cell Mol Neurobiol. 2010;30:1377–1381. doi: 10.1007/s10571-010-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Coleman DL, Eicher EM. Fat (fat) and tubby (tub): Two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered. 1990;81:424–427. doi: 10.1093/oxfordjournals.jhered.a111019. [DOI] [PubMed] [Google Scholar]
- 7.Bachtiary B, Boutros PC, Pintilie M, Shi W, Bastianutto C, Li JH, Schwock J, Zhang W, Penn LZ, Jurisica I, et al. Gene expression profiling in cervical cancer: An exploration of intratumor heterogeneity. Clin Cancer Res. 2006;12:5632–5640. doi: 10.1158/1078-0432.CCR-06-0357. [DOI] [PubMed] [Google Scholar]
- 8.Hong Y, Ho KS, Eu KW, Cheah PY. A susceptibility gene set for early onset colorectal cancer that integrates diverse signaling pathways: Implication for tumorigenesis. Clin Cancer Res. 2007;13:1107–1114. doi: 10.1158/1078-0432.CCR-06-1633. [DOI] [PubMed] [Google Scholar]
- 9.Cool DR, Loh YP. Carboxypeptidase E is a sorting receptor for prohormones: Binding and kinetic studies. Mol Cell Endocrinol. 1998;139:7–13. doi: 10.1016/S0303-7207(98)00081-1. [DOI] [PubMed] [Google Scholar]
- 10.Fricker LD, Snyder SH. Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase. J Biol Chem. 1983;258:10950–10955. [PubMed] [Google Scholar]
- 11.Tang SS, Zhang JH, Liu HX, Li HZ. PC2/CPE-mediated pro-protein processing in tumor cells and its differentiated cells or tissues. Mol Cell Endocrinol. 2009;303:43–49. doi: 10.1016/j.mce.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He P, Varticovski L, Bowman ED, Fukuoka J, Welsh JA, Miura K, Jen J, Gabrielson E, Brambilla E, Travis WD, Harris CC. Identification of carboxypeptidase E and gamma-glutamyl hydrolase as biomarkers for pulmonary neuroendocrine tumors by cDNA microarray. Hum Pathol. 2004;35:1196–1209. doi: 10.1016/j.humpath.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Cutcliffe C, Kersey D, Huang CC, Zeng Y, Walterhouse D, Perlman EJ. Renal Tumor Committee of the Children's Oncology Group: Clear cell sarcoma of the kidney: Up-regulation of neural markers with activation of the sonic hedgehog and Akt pathways. Clin Cancer Res. 2005;11:7986–7994. doi: 10.1158/1078-0432.CCR-05-1354. [DOI] [PubMed] [Google Scholar]
- 14.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Lee TK, Murthy SR, Cawley NX, Dhanvantari S, Hewitt SM, Lou H, Lau T, Ma S, Huynh T, Wesley RA, et al. An N-terminal truncated carboxypeptidase E splice isoform induces tumor growth and is a biomarker for predicting future metastasis in human cancers. J Clin Invest. 2011;121:880–892. doi: 10.1172/JCI40433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Mitka M. Researchers discover new biomarker that may improve cancer care strategies. JAMA. 2011;305:1184–1185. doi: 10.1001/jama.2011.315. [DOI] [PubMed] [Google Scholar]
- 17.Zhou K, Liang H, Liu Y, Yang C, Liu P, Jiang X. Overexpression of CPE-ΔN predicts poor prognosis in colorectal cancer patients. Tumour Biol. 2013;34:3691–3699. doi: 10.1007/s13277-013-0952-3. [DOI] [PubMed] [Google Scholar]
- 18.Liang XH, Li LL, Wu GG, Xie YC, Zhang GX, Chen W, Yang HF, Liu QL, Li WH, He WG, et al. Upregulation of CPE promotes cell proliferation and tumorigenicity in colorectal cancer. BMC Cancer. 2013;13:412. doi: 10.1186/1471-2407-13-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Nie J, Hao B, Li L, Xing G, Wang Z, Zhou Y, Sun Q, Li G, Zhang L, He F. Ceap/BLOS2 interacts with BRD7 and selectively inhibits its transcription-suppressing effect on cellular proliferation-associated genes. Cell Signal. 2008;20:1151–1158. doi: 10.1016/j.cellsig.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Tong ZT, Cai MY, Wang XG, Kong LL, Mai SJ, Liu YH, Zhang HB, Liao YJ, Zheng F, Zhu W, et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene. 2012;31:583–594. doi: 10.1038/onc.2011.254. [DOI] [PubMed] [Google Scholar]
- 21.Jazieh AR, Bamefleh H, Demirkazik A, Gaafar RM, Geara FB, Javaid M, Khader J, Khodadad K, Omar W, Saadeddin A, et al. MENA Lung Cancer Regional Guidelines Committee: Modification and implementation of NCCN guidelines on non-small cell lung cancer in the Middle East and North Africa region. J Natl Compr Canc Netw. 2010;8:S16–S21. doi: 10.6004/jnccn.2010.0118. (Suppl 3) [DOI] [PubMed] [Google Scholar]
- 22.Skalka N, Caspi M, Caspi E, Loh YP, Rosin-Arbesfeld R. Carboxypeptidase E: A negative regulator of the canonical Wnt signaling pathway. Oncogene. 2013;32:2836–2847. doi: 10.1038/onc.2012.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Jawahar S, Qian Y, Duong Q, Chan G, Parker A, Meyer JM, Moore KJ, Chayen S, Gross DJ, et al. Missense polymorphism in the human carboxypeptidase E gene alters enzymatic activity. Hum Mutat. 2001;18:120–131. doi: 10.1002/humu.1161. [DOI] [PubMed] [Google Scholar]