Abstract
Studies examining the association between early adversity and longitudinal changes in telomere length within the same individual are rare, yet are likely to provide novel insight into the subsequent lasting effects of negative early experiences. We sought to examine the association between institutional care history and telomere shortening longitudinally across middle childhood and into adolescence. Buccal DNA was collected 2 to 4 times, between the ages of 6 and 15 years, in 79 children enrolled in the Bucharest Early Intervention Project (BEIP), a longitudinal study exploring the impact of early institutional rearing on child health and development. Children with a history of early institutional care (n=50) demonstrated significantly greater telomere shortening across middle childhood and adolescence compared to never institutionalized children (n=29). Among children with a history of institutional care, randomization to high quality foster care was not associated with differential telomere attrition across development. Cross-sectional analysis of children randomized to the care as usual group indicated shorter telomere length was associated with greater percent of the child’s life spent in institutional care up to age 8. These results suggest that early adverse care from severe psychosocial deprivation may be embedded at the molecular genetic level through accelerated telomere shortening.
Keywords: children, longitudinal, early adversity, telomere length
1. Introduction
Telomeres, found at the end of chromosomes, comprise repetitive DNA sequences, proteins, and non-coding RNAs that are conserved across evolution, found in organisms from yeast to humans. In addition to their role in cellular senescence and apoptosis, telomeres also serve as global epigenetic regulators, sensitive to cellular and physiological stressors (Ye et al., 2014). Telomere length (TL) has been proposed as a biological mediator between negative early experiences and later psychopathology and poor health (Blaze et al., 2015). Shorter TL has been associated with environmental stress exposure (e.g., neighborhood-level social environmental risk and prenatal tobacco exposure; Theall et al., 2013a, 2013b) and poor caregiving environments for the child (e.g., physical maltreatment, institutional care, poverty; Drury et al., 2012; Mitchell et al., 2014). High quality parental care may buffer the negative impact of adversity on TL (Asok et al., 2013; Enokido et al., 2014). In adults and adolescents, decreased TL has also been associated with cardiovascular disease (Saliques et al., 2010), diabetes (Willeit et al., 2014), and obesity (Müezzinler et al., 2014), all negative health outcomes linked with experiences of early adversity. Collectively, these findings suggest that TL may link early adversity to negative health outcomes, foreshadow increased health risk, and have salience across the life course.
Though there is mounting evidence that early adversity is associated with TL measured at a single time point, measuring TL trajectory over time may be a more meaningful metric for assessing cellular aging (Chen et al., 2011). A growing, but to date limited, number of studies have examined TL longitudinally. In adult women, greater telomere shortening was found among those who experienced a major life stressor during a one year follow-up period, suggesting a proximal link between stress and changes in TL (Puterman et al., 2014). Even fewer studies of the change in TL in children exist, and only one examined telomere shortening in children in relation to stress. In this study, Shalev and colleagues (2012) found greater TL attrition between ages 5 and 10 years in children who experienced maltreatment during that time period. While this study provides an important extension of cross-sectional results, additional longitudinal studies are needed.
Using data from the Bucharest Early Intervention Project (BEIP), a longitudinal randomized controlled trial of foster-care for children who experienced early psychosocial deprivation, we examined the impact of early and cumulative exposure to institutional caregiving on TL change across middle childhood and into adolescence (age range 6–15 years). We predicted that children exposed to early institutional care (ever institutionalized group: EIG) would demonstrate accelerated telomere shortening compared to never institutionalized children (never institutionalized group: NIG). We further examined whether randomization to high-quality foster care at a mean age of 22 months, moderated TL change, hypothesizing that those randomized to the foster care intervention (foster care group; FCG) would have attenuated telomere attrition compared to the children assigned to the care as usual condition (care as usual group; CAUG). Given our previous cross-sectional findings only within the EIG where shorter TL was associated with increased percent of time in institutional care through 54 months of age (Drury et al., 2012), we examined whether a similar pattern persisted with exposure captured through 8 years of age.
2. Methods
2.1 Participants
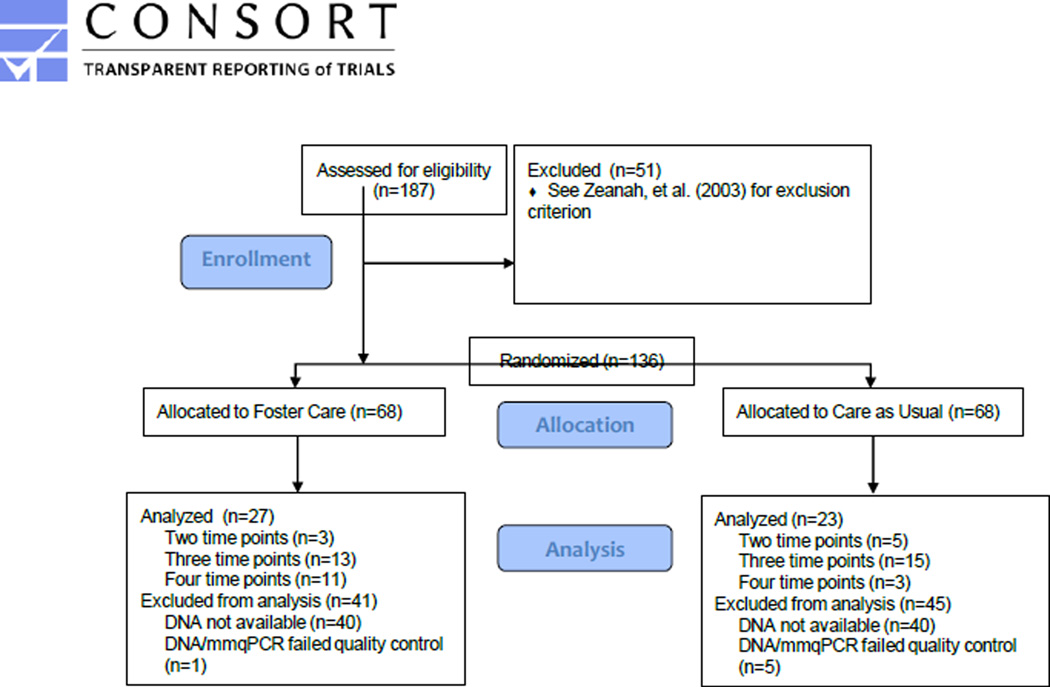
Participants were children enrolled in the BEIP (Zeanah et al., 2003), a longitudinal randomized controlled trial of foster care compared to care as usual for children in Romanian institutions, described in detail elsewhere (Nelson et al., 2007; Zeanah et al., 2009). 136 children, between 6 and 31 months of age, residing in six institutions in Romania were initially enrolled, and following baseline assessments, randomly assigned to CAUG (n=68) or FCG (n=68) (Figure 1, CONSORT). The foster care system was created for this project as an intentional alternative to institutional care. The related ethical considerations have been described in detail (Nelson et al., 2014; Zeanah et al., 2012). A reference group of children without any history of institutional rearing were recruited either from birth records from the same maternity hospitals in which the EIG were born or, for later recruitment, from area schools. Following randomization and placement of children in foster care, all subsequent decisions regarding placement were made by the child protection commissions in Romania.
Figure 1.
CONSORT diagram
As mandated by Romanian law, the Commission on Child Protection provided informed consent for each of the child participants. The Institutional Review Boards of Children’s Hospital of Boston, University of Maryland, and Tulane University approved this study.
2.2 Measures
2.2.1 Full-Scale IQ
At the age 12 follow-up assessment, full-scale IQ was obtained via the Wechsler Intelligence Scale for Children (Wechsler, 2004) which was translated into Romanian and administered by trained and reliable Romanian psychologists.
2.2.2 Monochromic multiplex quantitative polymerase chain reaction (MMP-qPCR)
DNA was collected using Isohelix buccal swabs (Cell Projects, Kent, UK) with careful attention to the integrity, purity, and concentration of the DNA. Swabs were collected, air dried, and stored with a desiccator pellet to decrease potential for bacterial growth. Swabs were immediately frozen and stored frozen until extracted. Integrity of the genomic DNA and purity was assessed via 260/280 and 260/230 ratios from nanodrop, QuBit analyses for double stranded DNA concentration, and agarose gel electrophoresis. The average relative buccal-derived TL (bTL) was determined from the telomere repeat copy number to single gene (albumin) copy number (T/S) ratio using an adapted monochrome multiplex quantitative real-time PCR (MMP-qPCR) and a BioRad CFX96 as previously described (Drury et al., 2014a). All samples were performed in triplicate, with a 7-point standard curve (0.0313ng to 2ng) derived from a single pooled control buccal DNA sample, eliminating plate to plate variability as a result of differences in DNA standards. Triplicate plates were repeated with all samples in a different well position on the duplicate plate. All time points from each individual were run on the same plates to further decrease variance due to batch or plate effects.
PCR efficiency criteria for both reactions were 90–110%. Coefficients of variations were calculated within each triplicate (CV criteria ≤10%) and between plates (CV criteria ≤6%). Samples with unacceptably high CVs (10% intra- and 6% inter-assay CV) were removed from analysis or repeated (N=6), resulting in a final sample of 79 individuals with multiple time points that each passed quality control metrics on duplicate plates. bTL ratio was derived by the average of the triplicates from both plates. The CV for samples was 2.4%. bTL assays and quality checks were conducted blind to group status.
Individuals contributed anywhere from 2 to 4 data points. Subjects with only one time point of DNA available, or for whom more than one time point did not meet quality control measures on a set of duplicate plates were not included in the final analyses. Mean age at the initial telomere data collection point was 8.07 (SD=0.69), with a range of 6.40 to 9.13. Mean age at the final telomere data collection point was 14.41 (SD=0.70), with a range of 12.94 to 15.61. The mean length of follow-up from the first to last collection was 6.34 (SD=0.44) years, with a range of 4.95 to 7.08 years. It should be noted that initial TL data from the BEIP were previously presented in Drury et al. (2012). The current study directly builds from that first cross-sectional study by including available data in a longitudinal assessment. However, as described above, the present study differs in telomere assaying methodology by using a measurement of T/S ratio with the MMPqPCR that is less susceptible to plate to plate and reference DNA variability, which is particularly useful for longitudinal analyses (Cawthon, 2009).
2.3 Data analysis
bTL values beyond three standard deviations from the mean were winsorized to the next closest value (n=3; 2 EIG and 1 NIG). Linear mixed models were used to examine bTL at multiple points spanning several years while accounting for the non-independence of observations (repeated measurements nested within individuals). Age of assessment was used as a time-varying independent variable, in which individuals’ bTL was modeled over age. Mixed modeling explores systematic differences in rates of change, and allows for the examination of the effects of key variables (e.g., institutional care history) on differences in bTL trajectory. First, institutional care history (dummy coded: 0=never institutionalized, 1=ever institutionalized) was included as a predictor of bTL trajectory. Covariates included sex, ethnicity (Romanian vs. other), full-scale IQ at age 12 as IQ has been found to correlate with TL (Harris et al., 2012) and group differences in IQ exist within the BEIP study (Nelson et al., 2007), age of initial bTL assessment, and initial bTL. We then tested for potential sex effects by adding interaction terms with group and age. Intent-to-treat (ITT) effects of the high quality foster care intervention were examined. The above model was replicated, within the EIG only, with intervention group coded as 0=CAUG or 1=FCG. Given that the history of institutional care and ITT groups were planned analyses, we did not conduct a p-value correction for multiple comparisons. For all longitudinal models, maximum likelihood estimation in Mixed Models in SPSS (version 20) was used, specifying fixed intercept and an autoregressive covariance structure to account for correlation between multiple waves of assessment. This procedure allows for missing data and calculates degrees of freedom using the Satterthwaite method, which can be fractional. For the cross-sectional analysis, ordinary least squares linear regression, controlling for age, sex, and ethnicity, was used to examine the association between percent time spent in institutional care among EIG children at age 8 with the TL data collection point assessed at the closest time point to that age.
3. Results
Demographics are presented in Table 1. NIG youth were significantly more likely to be of Romanian ethnicity, be older at the initial DNA collection, have less time between DNA collection time points, and have higher IQs. No significant differences were present between the CAUG and FCG.
Table 1.
Demographics based on institutional care history group (N=79) and intervention group (N=50) status.
| All participants | Institutionalized youth only | |||||
|---|---|---|---|---|---|---|
| Ever institutionalized (n=50) |
Never institutionalized (n=29) |
t or χ2 | Care as usual (n=23) |
Foster care (n=27) |
t or χ2 |
|
| Sex (% Male) | 50% | 45% | 0.20 | 52% | 48% | 0.08 |
| Ethnicity (% Romanian) |
52% | 86% | 9.39** | 52% | 52% | 0.001 |
| Initial Age (years) |
7.88 (0.76) | 8.38 (0.40) | − 3.78*** |
7.93 (0.85) |
7.85 (0.69) |
0.36 |
| Final Age (years) |
14.39 (0.75) | 14.42 (0.63) | −0.27 | 14.42 (0.78) |
14.36 (0.74) |
0.26 |
| Mean Follow- up Length (years) |
6.50 (0.32) | 6.05 (0.47) | 4.58*** | 6.49 (0.32) |
6.51 (0.32) |
−0.24 |
| Full-scale IQ | 68.06 (17.33) | 94.76 (12.03) | − 8.05*** |
66.70 (12.95) |
69.22 (20.52) |
−0.53 |
Note. Mean (Standard Deviation).
p<0.05.
p<0.01.
p<0.001.
3.1 Telomere shortening across development by institutional care history
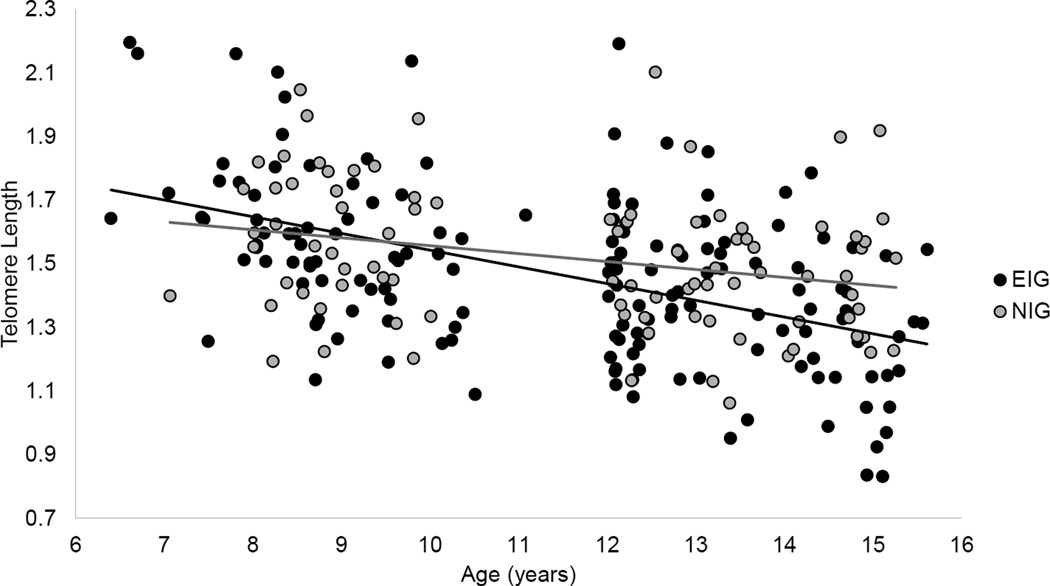
The main effect of institutional care history (i.e., ever vs. never institutionalized) was examined as a predictor of bTL over development, controlling for sex, ethnicity, full-scale IQ, initial age of assessment, and initial bTL (Table 2). Though both groups demonstrated significant telomere attrition across development (mean slope for NIG [−0.06] and EIG [−0.12], ps<0.05), telomere attrition was significantly accelerated in the EIG compared to the NIG (Figure 2). In addition, both full-scale IQ and initial bTL were associated with telomere shortening across development. The interaction of sex and group was not significant (F(1, 236.64)=3.78, p=0.053).
Table 2.
Mixed Model Results for Institutional Care History and Telomere Length Change Across Development.
| Parameter | Estimate | 95% CI | t |
|---|---|---|---|
| Intercept | 0.97 | −0.82, 2.76 | 1.08 |
| Ethnicity (dummy coded) | −0.38 | −0.88, 0.13 | −1.46 |
| Sex (females=1) | 0.23 | −0.21, 0.68 | 1.02 |
| Full-scale IQ at age 12 (centered at mean) | 0.02 | 0.01, 0.04 | 2.79** |
| Initial age of assessment | 0.07 | −0.13, 0.28 | 0.69 |
| Institutional care history (EIG=1) | 1.42 | −0.46, 3.31 | 1.50 |
| Age | −0.06 | −0.12, −0.01 | −2.28* |
| Institutional care history × Initial age | −0.09 | −0.31, 0.13 | −0.80 |
| Institutional care history × FSIQ | −0.002 | −0.01, 0.01 | −0.61 |
| Initial telomere length × Age | 0.03 | 0.02, 0.04 | 7.75*** |
| Ethnicity × Age | 0.04 | −0.01, 0.08 | 1.65 |
| Sex × Age | −0.02 | −0.06, 0.02 | −0.90 |
| Full-scale IQ × Age | −0.002 | −0.003, −0.001 | −2.85** |
| Institutional care history × Age | −0.06 | −0.11, −0.01 | −2.22* |
Note. EIG=ever institutionalized group. FSIQ=full-scale IQ.
p<0.05.
p<0.01.
p<0.001.
Figure 2.
Telomere length across development based on institutional care history. Note. Predicted values plotted with fitted trend lines. EIG=ever institutionalized group. NIG=never institutionalized group.
3.2 Telomere shortening across development by intent-to-treat groups
We used the same procedure to examine whether randomization into high-quality foster care was associated with differential rates of bTL shortening within children exposed to institutional care (i.e., FCG vs. CAUG). Using the conservative intent-to-treat approach, and counter to our hypothesis, intervention group was not significantly predictive of the linear age parameter (F(1, 143.52)=0.11, p=0.75) using the same covariates, indicating no differences in rate of telomere shortening. The interaction of sex and ITT group was not significant (F(1, 145.02)=3.80, p=0.053).
3.3 Cross-sectional analyses at the Age 8 Follow-Up
We previously reported that percent time spent in institutional care through 54 months of age was a significant predictor of cross sectional bTL (Drury et al., 2012). Therefore, we tested whether this same relationship persisted when examining the percent time spent in institutional care through eight years of age. Percent time spent in institutional care at age 8 was examined as a cross-sectional predictor of TL among the EIG (mean age of telomere measurement=8.84 years [SD=1.49]). Within the EIG, the association of percent time in institutional care at age 8 was not a significant predictor of TL over and above the effect of age, sex, and ethnicity (β=−0.17, ΔR2=0.03, p=0.26). However, because the CAUG had significantly greater range and variability in the amount of cumulative exposure to institutional care than the FCG at this follow-up (Mean=50.70% [SD=24.22] vs. Mean =22.71% [SD=7.81], Levene’s test for equality of variances F=24.97, p<0.001), the regression was conducted within each group separately. Within the CAUG, the association of percent time in institutional care at age 8 was a significant negative predictor of TL(β=−.54, ΔR2=0.22, p=0.027), over and above the effect of covariates. In contrast, there was no association of percent time in institutional care and TL within the FCG (β=−0.002, ΔR2=0.00, p=0.995). Unlike our previous study, sex did not significantly moderate these results.
Additional analyses examined the number of caregiving disruptions in relation to TL, and found no significant associations within the EIG or within each ITT group (i.e., CAUG and FCG) separately.
4. Discussion
This is the first study to examine the trajectory of TL across middle childhood and into adolescence in relation to early psychosocial deprivation. We found evidence of telomere shortening in both children with and without a history of institutional care. Consistent with our hypothesis and previous studies of both adults exposed to early caregiving adversity (Beach et al., 2014; Enokido et al., 2014; Kananen et al., 2010; Kiecolt-Glaser et al., 2011), and children exposed to maltreatment, family violence, or high family stress (Drury et al., 2014a, 2012; Gotlib et al., 2014; Shalev et al., 2012), we found exposure to early institutional caregiving resulted in accelerated telomere shortening across middle childhood and adolescence compared to children without any exposure to institutional caregiving.
Contrary to our expectations, we found no evidence that the foster care intervention moderated telomere shortening over time among the EIG, despite evidence that the amount of time in institutional care was predictive cross-sectionally (Drury et al., 2012). This result differs from other work indicating that high quality caregiving may buffer telomere shortening (Asok et al., 2013). Children in both the FCG and CAUG had experienced significant placement changes since 54 months of age, when the intervention trial ended. At the 12 year assessment relatively few children in the CAUG still resided in institutions and children from both groups have, unfortunately, experienced multiple placement disruptions. These caregiving disruptions may have diminished our ability to detect direct intervention effects out to later ages. It is also possible that the lack of detectable differences in TL trajectory between the FCG and CAUG indicates that the cellular impact of early institutional rearing is persistent and, to some extent, resistant to placement in high quality caregiving. Within the BEIP, a lack of ITT group effects have been documented for some outcomes (e.g., attention deficit hyperactivity disorder symptoms; Humphreys et al., 2015), suggesting the existence of sensitive periods after which full recovery from psychosocial deprivation may not be feasible. A similar sensitive period for cellular aging also may exist. Research into the effects of stress during specific developmental periods may be helpful for probing the sensitivity of the molecular effects of negative caregiving environments to changes in caregiving.
To test the replicability of our previous cross-sectional findings within the EIG only, we tested the association between TL and the percentage of a child’s life in institutional care, but in this study we characterized exposure through the age of 8 years and TL from DNA collection closest to this age point. As the experience of institutional care represents a unique stressor, percentage of time in this setting is likely the clearest measure of differential exposure. Consistent with our previous study, we found that greater time spent in institutional care predicted shorter TL at age 8, though only among children in the CAUG. Contrary to prior work from this sample in which boys demonstrated a stronger link between percent of time in institutional care an TL than girls (Drury et al., 2012), we did not find sex differences in this extension. It is possible that this lack of replication is due to differences in sample size, as our method for including only those with usable TL data from multiple assessments reduced the number of EIG children and thus may have been underpowered to detect this effect. Alternatively, the lack of replication may represent changes in chronological age since the end of the formal intervention, as the moderating role of participants’ sex may have diminished in the intervening years.
TL continues to attract considerable interest as a biomarker of cellular allostasis, with multiple studies and meta-analyses documenting associations between shorter TL and age (Frenck et al., 1998), physical activity (Zhu et al., 2011), obesity (Müezzinler et al., 2014), prenatal tobacco exposure (Theall et al., 2013b), neighborhood-level social environmental risk (Theall et al., 2013a), family violence (Drury et al., 2014a), and stress reactivity (Gotlib et al., 2014). While the consistency of findings is compelling, the majority of studies have been cross-sectional. Given the dynamic nature of telomeres, evidence that telomeres serve a more global regulatory function for genomic structure, and that baseline TL is predictive of telomere shortening (Aviv et al., 2009), prospective longitudinal measurements, ideally beginning at birth, are likely to be the most useful for enhancing the understanding of the links between early adversity, TL, and health outcomes.
Limitations to this study exist. All time points of DNA collection were not available on all individuals, as participation, particularly with DNA collection, varied across the course of the study. Further the sample size was limited by our stringent quality control requirements. Specifically, multiple samples from an individual had to met quality control criteria on duplicate plates to include any samples from that individual, as such if only time point passed our within and between plate requirements none of the individual’s time points were included. Although the number of individuals is smaller than our previous cross sectional study, this analysis included 247 observations of overall TL. Further, this is the first study in children of any age to include more than two time points, and previously only a handful of studies in youth have reported two time points. There are limitations to the use of MMP-qPCR methodology for measuring TL, particularly in relation to inter- and intra-assay variability, selection of tissue source, and range of CV values. The range of reported CV values in other studies is between 0.9% to 28% (Aviv et al., 2011). The average CV for triplicates in this study was <3% indicating significant assay consistency. Further, the stringency of our assay design required that each individual had all of their samples run on the same duplicate plates to prevent assay variability from influencing the measurement of TL change. As a result each individual not only had six replicates for each time point but if one time point had replicates that failed to meet quality checks, even if other time points met the requirements, that time point was excluded from analyses. To control for potential tissue confounds related to the DNA standard, all samples were run against a single pooled DNA sample extracted from buccal swabs. While the majority of studies have either not specified or utilized a different source of DNA for analyses, preliminary data in our lab suggest that the source of DNA for the standard curve may impact TL determination using quantitative PCR (Esteves et al., 2016).
Tissue specificity remains a consistent challenge to all studies of TL. Multiple previous studies of TL in children have been reported, including studies utilizing bTL (Asok et al., 2013; Drury et al., 2012), salivary derived TL (Theall et al., 2013a, 2013b) and TL from DNA extracted from peripheral blood (Shalev et al., 2012; Zhu et al., 2011). Our own pilot data suggests a high correlation of TL estimated concurrently from DNA extracted from buccal, blood, and saliva (Esteves et al., 2015), and a recent study reported a high correlation between salivary and blood TL (Mitchell et al., 2014). To date there are no data suggesting that one source of DNA is most accurate for TL. Lastly, in addition to changes in chronological age, pubertal status also changes from middle childhood into adolescence. TL has been associated with estrogen, dehydroepiandrosterone, and testosterone, as such larger studies concurrently examining TL and pubertal stage and TL trajectory are needed (Dismukes et al., 2016; Drury et al., 2014b; Entringer et al., 2015).
In conclusion, children with a history of institutional rearing demonstrated significantly greater telomere shortening across childhood compared to children reared in typical homes. Our findings add to the growing body of research across the life-course linking shorter TL to early adversity, and demonstrate a persistent negative impact of institutional rearing at the cellular level. Taken together, current data suggests the impact of severe psychosocial deprivation across numerous domains (Nelson et al., 2014), and now cellular processes. The lasting impact of early adverse caregiving, such as maltreatment, neglect, or severe psychosocial deprivation, with both physical and psychological difficulties, represents a critical public health concern that spans traditional divisions between physical and mental well-being. Accelerated telomere shortening may be one mechanism by which adversity results in poorer health outcomes for individuals exposed to early adversity (Shalev, 2012). The trajectory of TL change is likely a biological indicator of the lasting impact of early adversity and accelerated TL loss may foreshadow future health risks. Given that the strongest links to TL are found with cardiovascular disease and obesity, children exposed to early institutional care, similar to those exposed to maltreatment and chronic stress, should be carefully assessed not only for neurobehavioral and socio-emotional sequelae but also physical health parameters (Drury, 2015; Shonkoff and Garner, 2012).
Highlights.
-
□
Psychosocial deprivation was associated with accelerated telomere shortening.
-
□
High-quality foster care intervention did not alter telomere shortening.
-
□
For some children, time in institutional care predicted shorter telomere length.
Acknowledgments
Funding was received from R21MH094688-01 and 2K12HD043451-06 (Drury), and R01MH091363 and John D. and Catherine T. MacArthur Foundation (Nelson).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no competing financial interests in relation to the work described.
Contributor Information
Kathryn L. Humphreys, Tulane University School of Medicine.
Kyle Esteves, Tulane University School of Medicine.
Charles H Zeanah, Tulane University School of Medicine.
Nathan A Fox, University of Maryland.
Charles A. Nelson, III, Boston Children’s Hospital/Harvard Medical School and Harvard Graduate School of Education.
Stacy S. Drury, Tulane University School of Medicine.
References
- Asok A, Bernard K, Roth TL, Rosen JB, Dozier M. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Dev. Psychopathol. 2013;25:577–585. doi: 10.1017/S0954579413000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS. Leukocyte telomere dynamics: Longitudinal findings among young adults in the Bogalusa Heart Study. Am. J. Epidemiol. 2009;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39 doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SRH, Lei MK, Brody GH, Yu T, Philibert RA. Nonsupportive parenting affects telomere length in young adulthood among African Americans: Mediation through substance use. J. Fam. Psychol. 2014;28:967–972. doi: 10.1037/fam0000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaze J, Asok A, Roth TL. The long-term impact of adverse caregiving environments on epigenetic modifications and telomeres. Front. Behav. Neurosci. 2015;9 doi: 10.3389/fnbeh.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21–e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, Kark JD, Aviv A. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: Age-dependent telomere shortening is the rule. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2011;66 A:312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismukes AR, Meyer VJ, Shirtcliff EA, Theall KP, Esteves KC, Drury SS. Diurnal and stress-reactive dehydroepiandrosterone levels and telomere length in youth. Endocr. Connect. 2016;5:107–114. doi: 10.1530/EC-16-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS. Unraveling the meaning of telomeres for child psychiatry. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:539–540. doi: 10.1016/j.jaac.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Mabile E, Brett ZH, Esteves K, Jones E, Shirtcliff EA, Theall KP. The association of telomere length with family violence and disruption. Pediatrics. 2014a;134:e128–e137. doi: 10.1542/peds.2013-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Shirtcliff EA, Shachet A, Phan J, Mabile E, Brett ZH, Wren M, Esteves K, Theall KP. Growing up or growing old? Cellular aging linked with testosterone reactivity to stress in youth. Am. J. Med. Sci. 2014b;348:92–100. doi: 10.1097/MAJ.0000000000000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JYY, Fox NA, Zeanah CH, Nelson CA. Telomere length and early severe social deprivation: Linking early adversity and cellular aging. Mol. Psychiatry. 2012;17:719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokido M, Suzuki A, Sadahiro R, Matsumoto Y, Kuwahata F, Takahashi N, Goto K, Otani K. Parental care influences leukocyte telomere length with gender specificity in parents and offsprings. BMC Psychiatry. 2014;14:277. doi: 10.1186/s12888-014-0277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Blackburn EH, Buss C, Simhan HN, Wadhwa PD. Maternal estriol concentrations in early gestation predict infant telomere length. J. Clin. Endocrinol. Metab. 2015;100:267–273. doi: 10.1210/jc.2014-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves K, Schlesinger R, Drury SS. Telomere correlations between multiple tissues in children. 2016 [Google Scholar]
- Frenck RW, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joorman J, Lin J, Wolkowitz OM. Telomere length and cortisol reactivity in children at familial risk for depression. Mol. Psychiatry. 2014;20:615–620. doi: 10.1038/mp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Martin-Ruiz C, von Zglinicki T, Starr JM, Deary IJ. Telomere length and aging biomarkers in 70-year-olds: The Lothian Birth Cohort 1936. Neurobiol. Aging. 2012;33:e3–e8. doi: 10.1016/j.neurobiolaging.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Humphreys KL, Gleason MM, Drury SS, Miron D, Nelson CA, Fox NA, Zeanah CH. Effects of institutional rearing and foster care on psychopathology at age 12 years in Romania: follow-up of an open, randomised controlled trial. The Lancet Psychiatry. 2015;2:625–634. doi: 10.1016/S2215-0366(15)00095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lönnqvist J, Peltonen L, Ripatti S, Hovatta I. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin J-P, Weng N-P, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom. Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, Garfinkel I, Notterman D. Social disadvantage, genetic sensitivity, and children’s telomere length. Proc. Natl. Acad. Sci. U. S. A. 2014;111:5944–5949. doi: 10.1073/pnas.1404293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müezzinler A, Zaineddin AK, Brenner H. Body mass index and leukocyte telomere length in adults: a systematic review and meta-analysis. Obes. Rev. 2014;15:192–201. doi: 10.1111/obr.12126. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Fox NA, Zeanah CH. Romania’s Abandoned Children: Deprivation, Brain Development, and the Struggle for Recovery. Cambridge, MA: Harvard University Press; 2014. [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Puterman E, Lin J, Krauss J, Blackburn EH, Epel ES. Determinants of telomere attrition over 1 year in healthy older women: stress and health behaviors matter. Mol. Psychiatry. 2015;20:529–535. doi: 10.1038/mp.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliques S, Zeller M, Lorin J, Lorgis L, Teyssier JR, Cottin Y, Rochette L, Vergely C. Telomere length and cardiovascular disease. Arch. Cardiovasc. Dis. 2010;103:454–459. doi: 10.1016/j.acvd.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Shalev I. Early life stress and telomere length: Investigating the connection and possible mechanisms: a critical survey of the evidence base, research methodology and basic biology. Bioessays. 2012;34:943–952. doi: 10.1002/bies.201200084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol. Psychiatry. 2012;18:576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Theall KP, Brett ZH, Shirtcliff EA, Dunn EC, Drury SS. Neighborhood disorder and telomeres: Connecting children’s exposure to community level stress and cellular response. Soc. Sci. Med. 2013a;85:50–58. doi: 10.1016/j.socscimed.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theall KP, McKasson S, Mabile E, Dunaway LF, Drury SS. Early hits and long-term consequences: Tracking the lasting impact of prenatal smoke exposure on telomere length in children. Am. J. Public Health. 2013b;103 doi: 10.2105/AJPH.2012.301208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler intelligence scale for children—fourth edition. San Antonio, TX: The Psychological Corporation; 2004. [Google Scholar]
- Willeit P, Raschenberger J, Heydon EE, Tsimikas S, Haun M, Mayr A, Weger S, Witztum JL, Butterworth AS, Willeit J, Kronenberg F, Kiechl S. Leucocyte telomere length and risk of type 2 diabetes mellitus: new prospective cohort study and literature-based meta-analysis. PLoS One. 2014;9:e112483. doi: 10.1371/journal.pone.0112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Renault VM, Jamet K, Gilson E. Transcriptional outcome of telomere signalling. Nat. Rev. Genet. 2014;15:491–503. doi: 10.1038/nrg3743. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. Am. J. Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Fox NA, Nelson CA. The Bucharest Early Intervention Project: case study in the ethics of mental health research. J. Nerv. Ment. Dis. 2012;200:243–247. doi: 10.1097/NMD.0b013e318247d275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Nelson CA, Fox NA, Smyke AT, Marshall P, Parker SW, Koga S. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Dev. Psychopathol. 2003;15:885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wang X, Gutin B, Davis CL, Keeton D, Thomas J, Stallmann-Jorgensen I, Mooken G, Bundy V, Snieder H, Van Der Harst P, Dong Y. Leukocyte telomere length in healthy caucasian and african-american adolescents: Relationships with race, sex, adiposity, adipokines, and physical activity. J. Pediatr. 2011;158:215–220. doi: 10.1016/j.jpeds.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]