Abstract
Although previous studies have evaluated the roles of glucocorticoids and lymphocytes in septic shock, the precise mechanism remains unclear. The present study focused on investigating the influence of glucocorticoids on micro (mi)RNA-155 expression levels and the proliferation of T lymphocytes in septic shock. T cells were harvested from in the peripheral blood of patients with septic shock and healthy volunteers and were cultured in vitro. miRNA-155 levels and cell proliferation rates were subsequently analyzed. The proliferation of T cells from patients with septic shock was observed to be significantly lower as compared with that of T cells from healthy volunteers (P<0.05). Furthermore, miRNA-155 levels were significantly higher in the T cells from patients with septic shock, as compared with those from healthy volunteers (P<0.05). Notably, stimulation with dexamethasone increased the proliferation of T lymphocytes from patients with septic shock in a concentration-dependent manner, and markedly reduced miRNA-155 levels. Furthermore, transfection with an anti-miRNA-155 oligodoxynucleotide significantly increased the proliferation of T lymphocytes from patients with septic shock. In conclusion, the results of the present study indicate that glucocorticoids may regulate T-lymphocyte proliferation via the miRNA-155 pathway during septic shock. Therefore, miRNA-155 may be a potential therapeutic target in the treatment of septic shock.
Keywords: septic shock, miRNA-155, lymphocyte, glucocorticoids
Introduction
Sepsis in defined as a systemic inflammatory response to infection (1). The importance of immune function disorder in sepsis and septic shock has previously been demonstrated (2), and previous studies have demonstrated that T cells are pivotal in the pathogenesis of septic complications via suppression of the adaptive immune response (2–4). The role of T cells in the immune response of sepsis is well documented (3,4), and it has previously been demonstrated that septic patients exhibit markedly decreased numbers of total T cells and CD4+ T lymphocytes in their peripheral blood (5). Furthermore, previous studies have demonstrated that the persistent lymphopenia correlates with early and late mortality in sepsis or patients with septic shock (6–8). It has also been demonstrated that the early apoptosis of circulating lymphocytes in septic shock is associated with poor outcomes (7). Therefore, lymphopenia may serve as a biomarker for sepsis-induced immunosuppression and the prevention of lymphopenia may be a potential therapeutic strategy for the treatment of sepsis (8).
Glucocorticoids have anti-inflammatory, anti-allergic and anti-shock properties; therefore, they have been widely used to treat inflammatory and autoimmune diseases, including severe sepsis and septic shock (9). Previous studies have demonstrated that glucocorticoids prevent the release of proinflammatory cytokines via the regulation of lymphocyte function (9,10); therefore, glucocorticoids may significantly improve lymphopenia in septic shock. However, the duration of treatment could differentially affect the patient response to treatment and the mechanisms underlying the role of glucocorticoids on lymphopenia in septic shock remain unclear.
Various studies have investigated the role of glucocorticoids in micro (mi)RNA regulation and have demonstrated that glucocorticoids may affect T-cell function or the apoptosis/proliferation of lymphocytes via miRNA regulation (11,12). As a class of small non-coding RNA molecules, miRNA has been shown to have a critical role in cell proliferation/differentiation, apoptosis, innate immune responses and inflammation, including the regulation of lymphocyte function and sepsis (13,14). Increasing evidence has indicated that miRNA-155 is a critical regulator of inflammation and immune function (15,16). Previous studies have demonstrated that miRNA-155 has an important role in the regulation of various inflammatory and immunological diseases, including Graves' ophthalmopathy, allergic asthma, atherogenesis, multiple sclerosis and organ transplantation (17–21). Moreover, miRNA-155 has been associated with the regulation of immunity and inflammation in infectious diseases, including sepsis (21–23). This is supported by a previous study which demonstrated that glucocorticoids are capable of regulating miRNA-155 expression levels in the livers of septic mice (23). Although previous studies have elucidated that miRNA-155 has a critical role in the proliferation of T cells (24,25), whether glucocorticoids regulate T-cell proliferation or function via miRNA-155 in patients with septic shock remains unclear.
The present study focused on the role of glucocorticoids in the regulation of miRNA-155 and T-cell proliferation in patients with septic shock.
Materials and methods
Patients
Adult patients were recruited from the Intensive Care Unit at the First Hospital of Jilin University (Jilin, China) between October 2012 and May 2014. Ethical approval was obtained from the Medical Ethics Committee of the First Hospital of Jilin University and informed consent was obtained from either the patients or the patients' families. Septic shock was diagnosed based on criteria outlined in the International Guidelines for Management of Severe Sepsis and Septic Shock: 2012 (26). The diagnosis of sepsis was made on the basis of an identifiable or suspected infection site and evidence of systemic inflammatory response syndrome manifested by at least two of the following criteria: i) Body temperature, >38 or <36°C; ii) respiratory rate, >20 breaths/min; iii) heart rate, >90 beats/min; iv) white blood cell count, >12,000/mm3 or <4,000/mm3. Septic shock was defined as sepsis-induced hypotension persisting despite adequate fluid resuscitation. Hypotension was defined as systolic blood pressure (SBP) <90 mmHg, mean arterial pressure <70 mmHg or a SBP reduction of >40 mmHg or standard deviation below normal for the patient's age (26).
A total of 21 patients undergoing septic shock were enrolled in the septic shock group, and 25 healthy volunteers were included in the control group. Exclusion criteria included: i) Patients without informed consent; ii) patients <18 years old; iii) patients undergoing continuous renal replacement therapy prior to sampling; iv) patients receiving immunosuppressive therapy; and v) patients infected with viruses, including Mycobacterium tuberculosis. Blood samples collected by venous puncture were stored in BD Vacutainer tubes supplemented with lithium heparin (BD Biosciences, New Jersey, NY, USA) prior to steroid therapy. Blood samples from patients were collected within 24 h after the onset of septic shock. Demographic characteristics were also collected, and are shown in Table I.
Table I.
Demographic characteristics of all subjects.
| Characteristics | Control (n=25) | Septic shock (n=21) | P-value |
|---|---|---|---|
| Age, years | 65.2±13.4 | 67.2±13.4 | >0.05 |
| Gender, female/male | 16/9 | 13/8 | >0.05 |
| Leukocytes, 109/l | 5.8±1.4 | 14.6±5.5 | <0.001 |
| Lymphocytes, 109/l | 1.1±0.3 | 0.3±0.1 | <0.001 |
| Hs-CRP, mg/l | 2.3±0.7 | 12.6±3.1 | <0.001 |
| Infection site, n (%) | |||
| Lung | – | 9 (42.9) | |
| Abdomen | – | 5 (23.8) | |
| Blood | – | 3 (14.3) | |
| Burn | – | 2 (9.5) | |
| Others | – | 2 (9.5) |
Data are presented as mean ± standard deviation. Hs-CRP, high-sensitivity C-reactive protein.
Isolation of T cells
Total T cells were isolated from peripheral blood samples by negative selection using a Rosette Sep kit (15022; Stem Cell Technologies, Vancouver, CA, USA). T cells were purified using the MACS Pan T Cell Isolation kit II (130-092-881; Miltenyi Biotec, Auburn, CA, USA), according to the manufacturer's protocol. CD4+ T cell purity was ≥96%, as determined by flow cytometry (Attune NxT, Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and antibiotics (100 U/ml penicillin and 0.1 mg/ml streptomycin).
Transfection with anti-miRNA-155 oligonucleotides (ODNs)
Antisense ODNs against human miRNA-155 were used as the anti-miRNA-155 inhibitor (HmiR-AN0221-SN-10), and negative control (NC) ODNs (CmiR-AN0001-SN) were also used as for comparison (both Genecopoeia Inc., Guangzhou, China). Cells at 40–60% confluence were transfected. Individual ODNs were mixed with Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol and were subsequently administered to the cells. The efficiency of ODN transfection was assessed by monitoring the uptake of siRNA labeled with 6-carboxyfluorescein. Furthermore, the transfection efficiency for each siRNA was >90% and no significant difference existed between the two types of siRNA.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Cells were seeded into 6-well plates at a concentration of 5×104 cells/well. Following treatment, total RNA was extracted from cells using TRIzol reagent (15596-018; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The RNA sample was treated with 10 U of DNase I in a volume of 50 ml (04716728001; Roche Diagnostics, Basel, Switzerland) at 37°C for 20 min. First-strand cDNA was synthesized using a reverse transcription kit (4368814; Thermo Fisher Scientific, Inc.). qPCR was performed in order to analyze miRNA-155 mRNA expression levels using a TaqMan Universal Master Mix II kit (4440040; Thermo Fisher Scientific, Inc.). β-actin was used as the reference gene. The abundance of miRNA-155 expression in each sample was determined relative to the abundance of the β-actin reference gene. The qPCR data were analyzed and expressed as the relative miRNA levels of the cycle threshold value, which was subsequently converted to the fold change with the 2−ΔΔCT method (27). The primer sequences of miR-155 were as follows: Sense, 5′ TTAATGCTAATCGTGATAG,-3′ and antisense 5′-ACCTGAGAGTAGACCAGA-3′.
Immunofluorescence staining
Cells were seeded into 24-well plates at a concentration of 1×104 cells/well. Following treatment, cells were fixed with 95% alcohol for 5 min and permeabilized with 0.5% Triton X-100 for 5 min. Subsequently, cells were incubated with 5% bovine serum albumin for 0.5 h to block non-specific binding. Then cells were incubated with antibodies against proliferating cell nuclear antigen (rabbit polyclonal antibody PCNA; 1:100; sc-25280, Santa Cruz Biotechnology, Inc.) for 2 h at room temperature. Following washing, cells were incubated with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G secondary antibodies (sc-65561, Santa Cruz Biotechnology, Inc.,) prepared in phosphate-buffered saline (PBS) for 1 h at room temperature. Negative controls were conducted with PBS instead of the primary antibodies. Sections were examined using a fluorescence microscope (BX53, Olympus Corporation, Tokyo, Japan), and the results were expressed as the percentage of positive cells.
Cell proliferation
In order to investigate the rate of proliferation, T cells from patients and controls were stimulated with 10 µg/ml phytohemagglutinin (PHA; Sigma-Aldrich, St. Louis, MO, USA), and in a separate experiment, T cells from patients with septic shock were stimulated with 10, 50 or 100 nM dexamethasone (DXM; D1756, Sigma-Aldrich). In both experiments, the duration of stimulation was 48 h. Cell proliferation was evaluated using cell count and methyl thiazolyl tetrazolium (MTT) assays (Sigma-Aldrich).
For the cell count assay, cells were plated into 6-well plates at a concentration of 5×104 cells/well. Following stimulation, cells were collected by trypsin (C0201, Beyotime Institute of Biotechnology, Haimen, China) digestion and total cell numbers were calculated using a hemocytometer (Countess Automated Cell Counter, Thermo Fisher Scientific, Inc.) following trypan blue (ST798, Beyotime Institute of Biotechnology) exclusion.
For the MTT assay, cells were seeded into 96-well plates at a concentration of 5×103 cells/well. Following treatment, 20 µl MTT (ST316, Beyotime Institute of Biotechnology) was added to the wells and the plates were incubated for 4 h. The supernatant was subsequently removed and the plate was incubated with 150 µl/well dimethyl sulfoxide (DMSO; Sigma-Aldrich) at room temperature for 10 min on a swing bed. Cells were quantified by spectrophotometry at 490 nm using an absorbance microplate reader (ELx800; BioTek Instruments, Inc., Winooski, VT, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation and were analyzed by t-test or one-way analysis of variance, followed by q-test using SPSS software, version 12.0 (SPSS, Inc., Chicago, IL, USA). For all tests, P<0.05 was considered to indicate a statistically significant difference.
Results
Demographic characteristics of the study subjects
The demographic characteristics of subjects in the two groups are shown in Table I. No significant differences in gender or age were detected between the two groups. The levels of white blood cells (leukocytes) and serum high-sensitivity C-reactive protein were significantly higher in the septic shock group, as compared with the control group; whereas the lymphocyte count was significantly lower in the septic shock group, as compared with the control group. In the present study, the primary cause of septic shock was severe pneumonia (n=9). Other causes included peritonitis (n=5), injury (n=2) and burn (n=2).
miRNA-155 levels and T cell proliferation
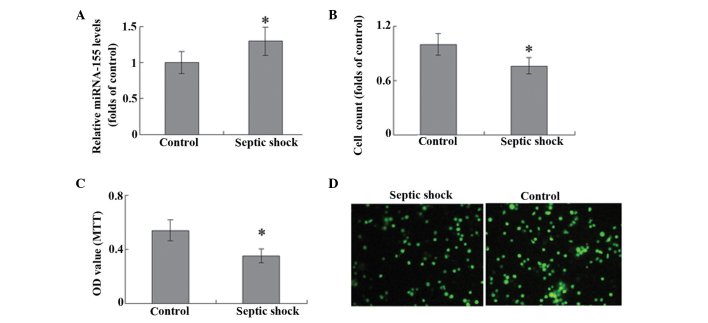
miRNA-155 levels in T cells were significantly higher in the septic shock group, as compared with the control group (P<0.05; Fig. 1A). A cells counting assay was used to evaluate T-cell proliferation, and stimulation with 10 mg/ml PHA was observed to promote the proliferation of T lymphocytes in patients with septic shock and the control group. Notably, the T cells harvested from the peripheral blood of patients with septic shock exhibited significantly weaker proliferative ability, as compared with the controls (P<0.05; Fig. 1B). The results of MTT assay and PCNA staining were consistent with those demonstrated by the cells counts (Fig. 1C and D).
Figure 1.
T cells were isolated from peripheral blood samples and stimulated with 10 µg/ml phytohemagglutinin. (A) miRNA-155 expression levels were detected using reverse transcription-quantitative polymerase chain reaction analysis. (B) Cell proliferation was evaluated using cell counting, (C) methyl thiazolyl tetrazolium (MTT) assay and (D) immunofluorescence staining of proliferating cell nuclear antigen. Magnification, ×100. *P<0.05 vs. the control (n=6). miRNA, microRNA; OD, optical density.
DXM regulates T-cell proliferation and miRNA-155 expression
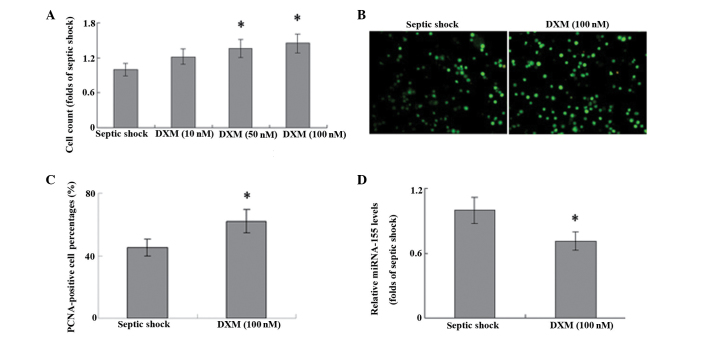
In T cells harvested from patients with septic shock, treatment with DXM significantly increased the proliferation of cells in a concentration-dependent manner, as compared with that in the untreated controls (Fig. 2A; P<0.05). The results of PCNA staining were consistent with these results (Fig. 2B and C), which suggests that DXM treatment may have a role in the proliferation of T cells during septic shock. Furthermore, stimulation with DXM markedly inhibited the expression levels of miRNA-155 in the T cells of patients with septic shock (Fig. 2D).
Figure 2.
T cells were isolated from peripheral blood samples and stimulated with 10, 50 or 100 nM DXM for 48 h. Cell proliferation was evaluated using (A) cell count and (B and C) immunofluorescence staining of PCNA (magnification, ×100). (D) miRNA-155 expression levels were detected using reverse transcription-quantitative polymerase chain reaction analysis. *P<0.05 vs. septic shock (control; n=6). DXM dexamethasone; PCNA, proliferating cell nuclear antigen; miRNA, microRNA.
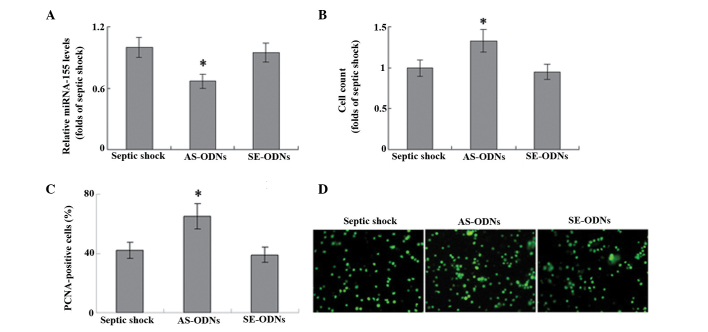
miRNA-155 is associated with T-cell proliferation. In order to explore the role of miRNA-155 in the proliferation of T cells, miRNA-155 expression was knocked down using an ODN against human miRNA-155. The ODN anti-miRNA-155 inhibitor successfully significantly inhibited miRNA-155 mRNA expression levels in T cells (Fig. 3A). In a cell count assay, the anti-miRNA-155 inhibitor significantly increased the proliferation of T cells induced by 10 mg/ml PHA, as compared with that of untransfected cells (P<0.05; Fig. 3B). Furthermore, transfection with this anti-miRNA-155 ODN resulted in a significant increase in the number of PCNA-positive cells in the T lymphocytes of patients with septic shock, as compared with the untransfected control (Fig. 3C; P<0.05). These results suggest that miRNA-155 may be involved in the proliferation of T cells during septic shock.
Figure 3.
Total T cells were isolated from peripheral blood samples and stimulated with 10 ug/ml phytohemagglutinin. ODNs against human miRNA-155 were used as the anti-miRNA-155 inhibitor. (A) miRNA-155 expression levels were detected using reverse transcription-quantitative polymerase chain reaction analysis. Cells proliferation was evaluated using (B) cell count and (C and D) immunofluorescence staining of PCNA. Magnification, ×100. *P<0.05 vs. sseptic shock (control; n=6). AS-ODNs, antisense oligodeoxynucleotides against human miRNA-155; SE-ODNs, sense oligodeoxynucleotides complementary to human miRNA-155; PCNA, proliferating cell nuclear antigen.
Discussion
The results of the present study demonstrate that the miRNA-155 expression levels were increased in the T cells of patients with septic shock. Furthermore, DXM administration successfully inhibited miRNA-155 expression and promoted T cell proliferation, and a small-interfering RNA against miRNA-155 was demonstrated to significantly increase the proliferation of T cells during septic shock. These results suggest that miRNA-155 may be associated with the glucocorticoid-induced proliferation of T cells observed in patients with septic shock.
Lymphocytes have a critical role in the immune response of sepsis (3,4) and it has previously been demonstrated that patients with septic shock exhibit significantly decreased levels of total and CD4+ T lymphocytes in peripheral blood (5). Previous studies have demonstrated that apoptosis of T cells occurs during septic shock; however few have focused on the proliferative ability of T cells in patients with septic shock (5–7). The present study demonstrated that T cells from patients with septic shock exhibited weak proliferative ability under the stimulation of PHA. This suggests that PHA also participates in the persistent lymphopenia in septic shock; however, the importance and mechanism of the balance of proliferation/apoptosis of lymphocytes in patients with sepsis requires further clarification.
miRNAs have been demonstrated to have critical roles in cell proliferation, apoptosis, inflammation and the regulation of lymphocyte function (13–15). Previous studies have outlined the critical role of miRNA-155 in inflammatory and immune diseases (17–21), and the present study demonstrated the important role of miRNA-155 in septic shock. The results of the present study are consistent with previous studies in lipopolysaccharide (LPS)-induced septic mice. In a mice model of sepsis, Wang et al (23) demonstrated that LPS significantly increased miRNA-155 expression levels in liver tissues, in addition to several inflammatory factors. Another study demonstrated that LPS was capable of inducing miRNA-155 expression in the spleens of mice (21). These reports suggest that miRNA-155 has a critical role in sepsis and septic shock. Certain pathways, including glycogen synthase kinase-3β and arginase-2, have been found to be associated with the miRNA-155-mediated regulation of T-cell proliferation in cardiac allograft rejection in a murine transplantation model (14–25). Although other validated target genes of miRNA-155 have also been established (28,29), their role in the proliferation of T lymphocytes during septic shock is yet to be elucidated.
Previous studies have demonstrated the critical role of glucocorticoid therapy in the treatment of septic shock; in particular, glucocorticoids may prevent the release of proinflammatory cytokines via the regulation of lymphocyte function (9,10). The results of the present study suggest that glucocorticoids may promote the proliferation of T lymphocytes in patients with septic shock. It is well documented that glucocorticoids are capable of affecting T-cell function and lymphocyte apoptosis/proliferation via miRNAs, such as miR-98 and miR-17 (11,12). The present study indicated that miRNA-155 may be a target for glucocorticoids in T cells. This result is consistent with a previous study investigating a mouse model of sepsis, which demonstrated that treatment with DXM inhibited the expression of miRNA-155 to below baseline levels (23). These results indicate that miRNAs may be an important therapeutic target of glucocorticoids in the regulation of inflammatory and immunological diseases. Notably, it has been suggested that glucocorticoids may bind directly to the B-cell integration cluster gene to repress miRNA-155 expression; however, this mechanism requires further investigation in patients with septic shock (30).
In conclusion, the present study demonstrates the key role of miRNA-155 in the proliferation of T cells in patients with septic shock. Therefore, although the precise molecular mechanism remains unclear, the regulation of miRNA-155 via glucocorticoids may be a novel therapeutic mechanism for the regulation of inflammation and immune response in patients with septic shock.
References
- 1.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 2.Chung CS, Watkins L, Funches A, Lomas-Neira J, Cioffi WG, Ayala A. Deficiency of gammadelta T lymphocytes contributes to mortality and immunosuppression in sepsis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1338–R1343. doi: 10.1152/ajpregu.00283.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wisnoski N, Chung CS, Chen Y, Huang X, Ayala A. The contribution of CD4+ CD25+ T-regulatory-cells to immune suppression in sepsis. Shock. 2007;27:251–257. doi: 10.1097/01.shk.0000239780.33398.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang LN, Yao YM, Sheng ZY. The role of regulatory T cells in the pathogenesis of sepsis and its clinical implication. J Interferon Cytokine Res. 2012;32:341–349. doi: 10.1089/jir.2011.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue S, Suzuki-Utsunomiya K, Okada Y, Taira T, Iida Y, Miura N, Tsuji T, Yamagiwa T, Morita S, Chiba T, et al. Reduction of immunocompetent T cells followed by prolonged lymphopenia in severe sepsis in the elderly. Crit Care Med. 2013;41:810–819. doi: 10.1097/CCM.0b013e318274645f. [DOI] [PubMed] [Google Scholar]
- 6.Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42:383–391. doi: 10.1097/SHK.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Tulzo Y, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, Tattevin P, Thomas R, Fauchet R, Drénou B. Early circulating lymphocytes apoptosis in septic shock is associated with poor outcome. Shock. 2002;18:487–494. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Turrel F, Guignant C, Venet F, Lepape A, Monneret G. Innovative therapeutic strategies for restoring lymphocyte functions in septic patients. Inflamm Allergy Drug Targets. 2008;7:181–186. doi: 10.2174/187152808785748173. [DOI] [PubMed] [Google Scholar]
- 9.Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, Keh D, Kupfer Y, Oppert M, Meduri GU. Corticosteroids in the treatment of severe sepsis and septic shock in adults: A systematic review. JAMA. 2009;301:2362–2375. doi: 10.1001/jama.2009.815. [DOI] [PubMed] [Google Scholar]
- 10.Keh D, Boehnke T, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, Volk HD, Doecke WD, Falke KJ, Gerlach H. Immunologic and hemodynamic effects of ‘low-dose’ hydrocortisone in septic shock: A double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003;167:512–520. doi: 10.1164/rccm.200205-446OC. [DOI] [PubMed] [Google Scholar]
- 11.Davis TE, Kis-Toth K, Szanto A, Tsokos GC. Glucocorticoids suppress T cell function by up-regulating microRNA-98. Arthritis Rheum. 2013;65:1882–1890. doi: 10.1002/art.37966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith LK, Shah RR, Cidlowski JA. Glucocorticoids modulate microRNA expression and processing during lymphocyte apoptosis. J Biol Chem. 2010;285:36698–36708. doi: 10.1074/jbc.M110.162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HJ, Zhang PJ, Chen WJ, Jie D, Dan F, Jia YH, Xie LX. Characterization and identification of novel serum microRNAs in sepsis patients with different outcomes. Shock. 2013;39:480–487. doi: 10.1097/SHK.0b013e3182940cb8. [DOI] [PubMed] [Google Scholar]
- 15.Yao R, Ma YL, Liang W, Li HH, Ma ZJ, Yu X, Liao YH. MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS One. 2012;7:e46082. doi: 10.1371/journal.pone.0046082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu R, Huffaker TB, Kagele DA, Runtsch MC, Bake E, Chaudhuri AA, Round JL, O'Connell RM. MicroRNA-155 confers encephalogenic potential to Th17 cells by promoting effectorgene expression. J Immunol. 2013;190:5972–5980. doi: 10.4049/jimmunol.1300351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li K, Du Y, Jiang BL, He JF. Increased microRNA-155 and decreased microRNA-146a may promote ocular inflammation and proliferation in Graves' ophthalmopathy. Med Sci Monit. 2014;20:639–643. doi: 10.12659/MSM.890686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malmhäll C, Alawieh S, Lu Y, Sjöstrand M, Bossios A, Eldh M, Rådinger M. MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. J Allergy Clin Immunol. 2014;133:1429–1438. doi: 10.1016/j.jaci.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Gong J, Li P, Li M, Liu Y, Liang S, Gong J. Knockdown of microRNA-155 in kupffer cells results in immunosuppressive effects and prolongs survival of mouse liver allografts. Transplantation. 2014;97:626–635. doi: 10.1097/TP.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Cheng Y, Cui W, Li M, Li B, Guo L. MicroRNA-155 modulates Th1 and Th17 cell differentiation and is associated with multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014;266:56–63. doi: 10.1016/j.jneuroim.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 22.Piccinini AM, Midwood KS. Endogenous control of immunity against infection: Tenascin-C regulates TLR4-mediated inflammation via microRNA-155. Cell Rep. 2012;2:914–926. doi: 10.1016/j.celrep.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZH, Liang YB, Tang H, Chen ZB, Li ZY, Hu XC, Ma ZF. Dexamethasone down-regulates the expression of microRNA-155 in the livers of septic mice. PLoS One. 2013;8:e80547. doi: 10.1371/journal.pone.0080547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunand-Sauthier I, Irla M, Carnesecchi S, Seguín-Estévez Q, Vejnar CE, Zdobnov EM, Santiago-Raber ML, Reith W. Repression of arginase-2 expression in dendritic cells by microRNA-155 is critical for promoting T cell proliferation. J Immunol. 2014;193:1690–1700. doi: 10.4049/jimmunol.1301913. [DOI] [PubMed] [Google Scholar]
- 25.Feng Z, Xia Y, Zhang M, Zheng J. MicroRNA-155 regulates T cell proliferation through targeting GSK3β in cardiac allograft rejection in a murine transplantation model. Cell Immunol. 2013;281:141–149. doi: 10.1016/j.cellimm.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock. Crit Care Med. 2012;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 27.Liu TE, Wang S, Zhang L, Guo L, Yu Z, Chen C, Zheng J. Growth hormone treatment of premature ovarian failure in a mouse model via stimulation of the Notch-1 signaling pathway. Exp Ther Med. 2016;12:215–221. doi: 10.1124/jpet.116.232660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, Pierre P. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci USA. 2009;106:2735–2740. doi: 10.1073/pnas.0811073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu F, Weidmer A, Liu CG, Volinia S, Croce CM, Lieberman PM. Epstein-Barr virus-induced miR-155 attenuates NF-kappaB signaling and stabilizes latent virus persistence. J Virol. 2008;82:10436–10443. doi: 10.1128/JVI.00752-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Q, Wang X, McBride J, Fewell C, Flemington E. B-cell receptor activation induces BIC/miR-155 expression through a conserved AP-1 element. J Biol Chem. 2008;283:2654–2662. doi: 10.1074/jbc.M708218200. [DOI] [PMC free article] [PubMed] [Google Scholar]