Abstract
Introduction
Sepsis and severe sepsis are asociated with high hospital mortality. Little is known about the occurrence of sepsis in general hospital populations. The goal of the present study was to reveal the epidemiology of sepsis in Norwegian hospitals over 1 year.
Methods
Patients admitted to all Norwegian hospitals during 1999 (n = 700,107) were analyzed by searching the database of the Norwegian Patient Registry for markers of sepsis, using International Classification of Diseases (ICD)-10 codes for sepsis and severe infections. In patients with such diagnoses, demographic data, hospital outcome data and ICD-10 codes for organ dysfunction were also retrieved. Sepsis was further classified as primary or secondary, and severe (sepsis with vital organ dysfunction) or nonsevere. The age-adjusted mortality rate, and the sepsis rates for all hospital admissions and in the Norwegian population were calculated.
Results
A total of 6665 patients were classified as having sepsis, and of these 2121 (31.8%) had severe sepsis. The most frequent failing organ system was the circulatory system, and 1562 had septic shock. Mortality increased from 7.1% (in those with no documented organ dysfunction) to 71.8% (in those with three or more organ dysfunctions). The mean mortality was 13.5%, and the mortality of severe sepsis was 27%. The incidence of sepsis was 9.5/1000 hospital admissions and 1.49/1000 inhabitants in 1999.
Conclusion
Sepsis is not uncommon in Norwegian hospitals and is associated with high hospital mortality, which is similar to recent findings from the USA. Awareness of sepsis and its appropriate treatment is mandatory in Norway if we are to reduce mortality from sepsis by 25% in the next 5 years.
Keywords: epidemiology, mortality, Norway, sepsis, severe sepsis
Introduction
Sepsis is an increasing problem in modern medicine. Some explanations for this are an increasing proportion of elderly people in the general population and those admitted to hospitals, more intensive and aggressive treatment of various diseases and injuries, and increased microbial resistance, especially in the hospital environment. Recent reports from the USA suggest that sepsis is a serious national health problem, on the same level as ischaemic heart disease, and that the number of deaths due to severe sepsis is similar to the number of deaths related to ischaemic heart disease [1]. Published estimates of the prevalence of sepsis in European countries are scarce; the present study was conducted to gain national data on the incidence and mortality from sepsis and severe sepsis in Norway.
Methods
The present study used data from the Norwegian Patient Registry (NPR) at Sintef Unimed in Trondheim, Norway. The NPR is a national database in which all Norwegian hospitals must register information on all admissions each year. For each hospital stay, anonymous patient data (age, sex and year of birth), dates for hospital admission and discharge, type of hospital and department, vital status at discharge, International Classification of Diseases (ICD)-10 diagnostic codes [3] (grouped as primary diagnosis and secondary diagnosis) and codes for operative procedures (NOMESCO Classification of Surgical Procedures coding) [4] are registered. The database is not open for general access, but it is possible to request specific searches of the database. The year 1999 was chosen because this was the most recent year at the start of the study (2002) for which complete data were available. All patients (except neonates) with a diagnosis (primary or secondary) of sepsis or severe infection (Table 1) admitted to all Norwegian hospitals were identified. We deliberately excluded codes for neonatal sepsis because this was not the focus of the study. In addition, secondary codes (up to four) were retrieved for all patients identified by the primary search. In this search we also requested data regarding hospital stay (days), type of department, vital status at discharge (dead or alive), and age and sex of the patient. Because intensive care units (ICUs) in Norway are not independent administrative units in the hospital, but have 'technical' beds, the patients continue to be registered as admitted to the original department. Hence, it was not possible to extract data from the registry about stay in the ICU for this cohort of patients.
Table 1.
ICD-10 codes including in this search (primary and secondary diagnosis)
| Code | Diagnosis |
| A26.7 | Erysipelas |
| A39 | Meningococcal infections |
| A40.0 | Streptococcal sepsis |
| A41.0–A41.9 | Other bacterial sepsis |
| A42.7 | Actinomycosis sepsis |
| B37.7 | Candida sepsis |
| T81.4 | Infections after surgical procedures |
ICD, International Classification of Diseases.
The extracted patient data were transferred to a local database (FileMaker, Inc, Pro 6.0, Santa Clara, USA and SPSS Inc, version 11, Chicago, USA), and in that database we conducted a search for patients with secondary diagnostic codes indicating acute organ dysfunction (Table 2). This was performed in order to identify a subgroup of patients with severe sepsis, defined as sepsis with one or more organ dysfunction. The total patient group was then divided into patients with sepsis and severe infection as a primary admission code and those with sepsis and severe infection as a secondary code (originated at the hospital), and into sepsis with and without evidence of acute organ dysfunction. We also analyzed the patient group with regard to incidence and mortality in the Norwegian population (given as events/1000 inhabitants) and in different age groups (10-year cohorts). The number of inhabitants in Norway was 4,461,913 (population average in 1999) and the number of people in the different age cohorts that year was used to calculate age-specific incidence of sepsis [5]. The total number of hospital admissions in Norway was, according to the NPR, 700,107 in 1999 [6].
Table 2.
ICD-10 codes included in the search for organ dysfunction (only secondary codes)
| Organ dysfunction | Code | Diagnosis |
| Circulatory failure | A41.9 | Septic shock |
| I50.9 | Unspecified heart failure | |
| Respiratory failure | J13-18 | Pneumonias |
| J80 | ARDS | |
| J95 | Respiratory failure after procedures | |
| J96.0 | Acute respiratory failure | |
| Renal failure | N17 | Acute renal failure |
| N99.0 | Acute renal failure after treatment | |
| Coagulation failure | D65 | DIC |
| D69 | Purpura and other bleeding instances | |
| Other organ dysfunctions | E86 | Fluid loss |
| E87.2 | Metabolic acidosis | |
| K72 | Acute liver failure |
ARDS, acute respiratory distress syndrome; DIC, disseminated intravascular coagulation; ICD, International Classification of Diseases.
Results
In the primary search group 6665 patients were identified, 3441 of whom were male and 3224 were female. The mean age was 57.9 years. This yields a sepsis incidence of 1.49 cases/1000 inhabitants in the year 1999. In total, 3517 patients (52.8%) had this as their main diagnosis, and 2121 patients (31.8%) had severe sepsis (sepsis with at least one ICD-10 code indicating acute organ dysfunction). In the latter group septic shock was the largest subgroup, including 1562 patients. Mean age and mortality in different subgroups are shown in Table 3, in which patients with septic shock are also listed. The risk for death from sepsis increases with the number of organs with dysfunction (Fig. 1).
Table 3.
Mean age and mortality in different subgroups of patients with sepsis
| Group | n | Mean age (years) | Mean length of stay (days) | Mortality (n [%]) |
| All | 6665 | 57.9 | 14.9 | 897 (13.5%) |
| Primary sepsis | 3517 | 57.9 | 10.4 | 508 (14.4%) |
| Secondary sepsis | 3148 | 56.9 | 20.3 | 389 (12.4%) |
| Sepsis without organ failure | 4544 | 57.1 | 14.3 | 323 (7.1%) |
| Severe sepsis | 2121 | 57.9 | 16.1 | 574 (27%) |
| Septic shock | 1562 | 54.2 | 14.3 | 457 (29.3%) |
Figure 1.
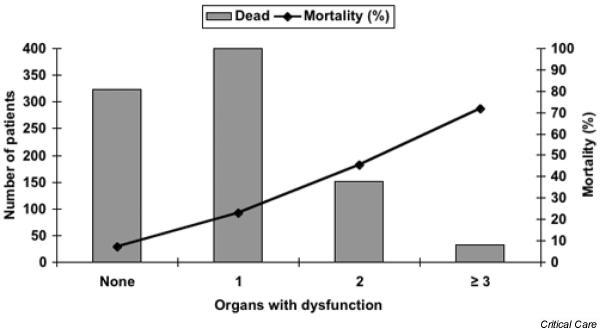
Association between organ failure and mortality.
The incidence of sepsis was low in those aged under 1 year (1.1/1000) and stayed low up to approximately 50 years of age (<1/1000). From this age the incidence of sepsis increased to 8.7/1000 in those older than 80 years. Mortality from sepsis increased with age, to a maximum of 28% in those older than 80 years (Fig. 2). Bacterial identification in patients with sepsis was coded in 2020 cases (30.3%), and the distribution is shown in Fig. 3.
Figure 2.
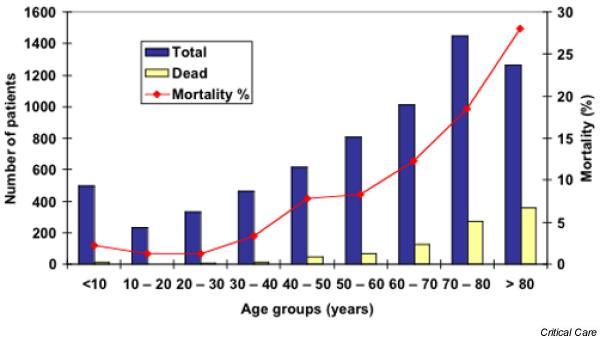
Incidence of mortality from sepsis in different age groups: Norway 1999
Figure 3.
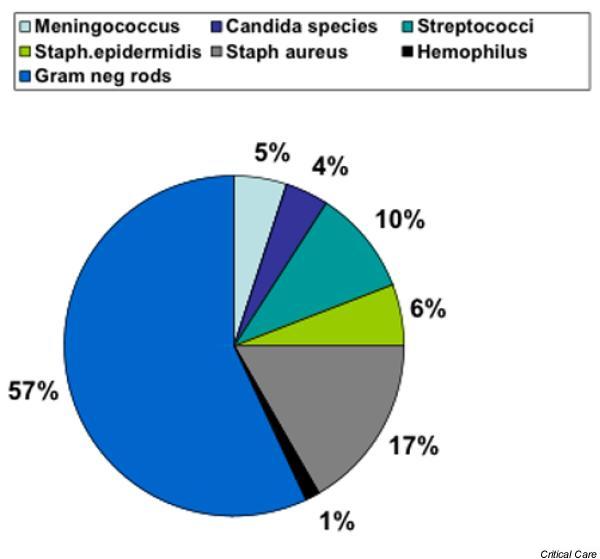
Bacteriological identity of infecting agents when coded for (n = 2020)
Resource consumption in patients with sepsis is considerable, as illustrated by the mean hospital stay of 14.9 days, and the total number of days spent in hospital was 97,178. The sepsis rate calculated from the number of hospital admissions was 9.5/1000 admissions, and severe sepsis occurred in 3.0/1000 admissions.
Discussion
In this analysis of the incidence of sepsis in Norwegian hospitals in 1999, a frequency of 1.49 sepsis cases/1000 inhabitants was found, with an overall mortality of 13.5%. Severe sepsis was found in 31.8% of the patients, with a hospital mortality of 27%.
These figures clearly show that sepsis in Norway is common and carries a poor prognosis, with 897 deaths in 1999. However, this is still far from the number of deaths that follow acute myocardial infarction (5599 persons in 1999), which is contrary to the situation in USA [1].
It must be underlined that data taken from such a registry are associated with a certain degree of uncertainty. Data are not prospectively collected with the use of strict definitions of sepsis and severe sepsis. Sepsis is a syndrome (systemic inflammatory response) caused by an infection [7], and it is not considered as a single diagnosis or diagnostic group in the ICD-10 coding system. The definition has been challenged recently, and other methods to define and describe sepsis have been suggested [8]. The extent to which Norwegian physicians use the same definition of sepsis is therefore very difficult to estimate. Because data are registered anonymously in the NPR, it is not possible to perform a quality check on the data in retrospect. Such shortcomings are present in most similar registries.
In Norway there has been a tendency to equate sepsis to a positive blood culture (i.e. growth of bacteria or fungi in blood). This is clearly not the case, as was once again demonstrated in the most recent, large clinical trial in patients with sepsis, in which the use of recombinant human activated protein C in severe sepsis was evaluated [9]. In that study only one-third of all included patients had a positive blood culture. Moreover, growth of bacteria in the blood (bacteraemia) does not mean that the patient will necessarily develop sepsis.
Severe sepsis is not found in the ICD-10 coding system. In order to code for severe sepsis, a code for sepsis (or severe infection) with one or more codes for organ dysfunction must be applied. Coding organ dysfunction is a problem in itself because organ dysfunction is poorly defined, and no internationally accepted definitions exist, even for the most common organ dysfunctions. Short episodes (lasting less than 24 hours) of organ dysfunction, such as respiratory failure with hypoxaemia or circulatory failure with hypotension, can be overlooked. Only when the organ dysfunction is severe enough to initiate specific treatment (such as the use of vasoactive drugs, ventilator treatment, and renal replacement therapy) is there a greater chance that it will be included in the diagnostic records at discharge.
Another uncertainty is the inclusion of severe infections in our search as substitutes for diagnosis of sepsis. Some of these patients might have had only localized infections, with little or no systemic inflammation. However, most probably fulfil at least three of the four sepsis criteria, and hence were included in the search.
In Haukeland University Hospital the number of patients with growth in blood culture increased from 4.3/1000 admissions in the period 1974–1979 to 8.7/1000 admissions in 1988–1989 [10]. In the present study, conducted 10 years later, we found the incidence of sepsis to be 9.5/1000 admissions.
In general, few studies have been published on the national incidence of sepsis in different countries. In a literature search, only two such studies, both from the USA, were found [1,2]. Several studies have been published regarding the occurrence of sepsis in patients confined to the ICU [11-13] or in different hospitals [10,14], or the occurrence of specific forms of sepsis, such as meningococcal sepsis [15,16]. Such studies, however, are not easy to compare with the present one. In a recent large prospective study conducted in Australia and New Zealand [13] the calculated annual incidence of severe sepsis was found to be 0.77/1000 population per year, which is similar to the 0.8/1000 population per year found in the study reported by Martin and coworkers [2] using retrospective data from the National Centre for Health Statistics (similar to the NPR). In the latter study the occurrence of all sepsis was found to be 2.5 cases/1000 inhabitants per year. These figures are higher but similar to our finding of 1.5 and 0.5 cases of sepsis and severe sepsis/1000 inhabitants per year.
Both sepsis and severe sepsis carry high hospital mortality. In the study by Angus and coworkers [1] the mortality of severe sepsis was 28.6%, which is very similar to the 27% that we found in patients with severe sepsis. In the study by Martin and coworkers [2], increased mortality with increasing numbers of organs in failure was demonstrated. They found the mortality for sepsis without organ failure to be 15%, increasing to 70% with three or more organ failures [2]; this is nearly identical to our findings.
Conclusion
Sepsis and severe sepsis are not uncommon in Norwegian hospitals and carry a severe prognosis. In severe sepsis the mortality was 27% and was found to increase with the number of organs in failure. Sepsis must be given greater priority in general health discussions and education of health personnel in our country. An international campaign to reduce the mortality from sepsis by 25% in the coming 5 years is presently ongoing [17]; it will be of interest to see whether we can achieve this goal in Norway.
Key messages
The incidences of sepsis and severe sepsis have increased over the past few decades. Sepsis carries a poor prognosis, with mortality increasing with the number of organs in failure.
The overall sepsis rate in Norway 1999 was 1.49/1000 inhabitants.
The rate of severe sepsis was 0.5/1000 inhabitants.
Competing interests
The costs for the NPR search was provided by Lilly Norway.
Abbreviations
ICU = intensive care unit; ICD = International Classification of Diseases; NPR = Norwegian Patient Registry.
See related commentary: http://ccforum.com/content/8/4/222
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- World Health Organization The International Statistical Classification of Diseases and Related Health Problems, tenth revision. http://www.who.int/whosis/icd10/ [PubMed]
- NOMESCO NOMESCO Classification of Surgical Procedures (NCSP) http://www.pubcare.uu.se/nordwho/ [in Norwegian]
- Statistics Norway Mean population, sex and age distribution [in Norwegian] http://www.ssb.no/folkemengde/arkiv/tab-2000-04-11-44.html
- Sintef Patient Data. 1999. http://www.npr.no/Somatikk/index.htm [in Norwegian]
- Bone R, Balk R, Cerra J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Vincent J, Opal S, Torres A, Bonten M, Cohen J, Wunderink R. The PIRO concept: I is for infection. Critical Care. 2003;7:252–255. doi: 10.1186/cc2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ, Jr, Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group Efficacy and Safety of Recombinant Human Activated Protein C for Severe Sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Haug JB, Harthug S, Kalager T, Digranes A, Solberg CO. Bloodstream infections at a Norwegian university hospital, 1974–1979 and 1988–1989: changing etiology, clinical features, and outcome. Clin Infect Dis. 1994;19:246–256. doi: 10.1093/clinids/19.2.246. [DOI] [PubMed] [Google Scholar]
- Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulme R, Lepage E, Le Gall R. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–121. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, Regnier B. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–974. doi: 10.1001/jama.274.12.968. [DOI] [PubMed] [Google Scholar]
- Finfer SBR, Lipman J, French C, Dobb G, Myburgh J. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. ICM. 2004;30:589–596. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- Hallan S, Naustdal T. Bacteremia at a medium-sized Norwegian hospital. Tidsskr Nor Laegeforen. 1994;114:2102–2106. [PubMed] [Google Scholar]
- Deeks S, Kertesz D, Ryan A, Johnson W, Ashton F. Surveillance of invasive meningococcal disease in Canada, 1995–1996. Can Commun Dis Rep. 1997;23:121–125. [PubMed] [Google Scholar]
- Halstensen A, Pedersen SH, Haneberg B, Bjorvatn B, Solberg CO. Case fatality of meningococcal disease in western Norway. Scand J Infect Dis. 1987;19:35–42. doi: 10.3109/00365548709032375. [DOI] [PubMed] [Google Scholar]
- Slade E, Tamber PS, Vincent JL. The Surviving Sepsis Campaign: raising awareness to reduce mortality. Crit Care. 2003;7:1–2. doi: 10.1186/cc1876. [DOI] [PMC free article] [PubMed] [Google Scholar]


