Abstract
The present study aimed to investigate the blocking of tumor cell adhesion to the extracellular matrix, the prevention of tumor metastasis by the β peptide trimer β3, as well as the influence of β3 on the recurrence and survival time of hepatocellular carcinoma (HCC) nude mice model LCI-D20 after early resection. To this end, the DNA fragment of the β3 peptide (DLYYLMDLSYSMKGGDLYYLMDLSYSMKGGDLYYLMDLSYSMK) was cloned into the expression vector pET-His and the fusion protein His-β3 was expressed in E. coli BL21 (DE3) plysS. The anti-adhesion effect of β3 on the highly metastatic HCC cell line HCCLM6 to fibronectin (FN) was measured by MTT assay. The inhibition of HCCLM6 cell invasion by β3 was analyzed using a Transwell (modified Boyden chamber) system and Matrigel. The influence of β3 on the recurrence of HCC and mouse survival time after early resection was investigated using the HCC metastasis nude mice model LCI-D20. HCCLM6 cells incubated with 10, 20, 50 or 100 µmol/l β3 for 3 h demonstrated a marked reduction in adhesion to FN. The adhesion inhibition rates were 11.8, 21.7, 37.5 and 66.4%, respectively. In addition, cell invasion was reduced by 51.3% in HCCLM6 cells cultured with 100 µmol/l β3. Treatment with β3 also inhibited tumor recurrence at the incisal edge and prolonged the survival time of LCI-D20 mice following early resection. The present study provided evidence that β3 peptide specifically blocked the adhesion and invasion of HCCLM6 cells, inhibited HCC recurrence in vivo and prolonged the survival time of HCC nude mice LCI-D20 following hepatectomy. Therefore, β3 may be further investigated as a novel anti-tumor drug.
Keywords: trimer β peptide, hepatocellular carcinoma, anti-adhesion, invasion, recurrence
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer mortality worldwide. In 2010, liver cancer resulted in 754,00 mortalities, representing an increase of 62.4% since 1990. Despite significant advances in the treatment and the prevention of postoperative metastasis, the 5-year postoperative recurrence rate of HCC remains high and the recurrence and metastasis of HCC also remains a problem in clinical practice (1,2). Efforts have been made to develop more efficient drugs to inhibit and prevent tumor metastasis. Cancer metastasis involves tumor cells dissociating from the primary locus, invading the surrounding tissue, entering and extravasating from the circulation, and growing in distant organs (3,4). During this complex process, cell adhesion is one of the most important events (5). A number of previous studies have focused on synthesized anti-adhesion peptides (6–8). However, the application of these short peptides is limited due to their short half-life and high dosage required. To prolong the half-life of synthesized peptide, multimers and derivatives were designed (9–11). It has been demonstrated that the anti-metastasis effect of multimers of synthesized peptides was stronger than that of monomer peptides (12,13).
Integrins are a family of adhesion molecules located on cells and in the extracellular matrix. The expression levels of integrins are closely associated with the migration ability of cells (14). The anti-adhesion peptide β (DLYYLMDLSYSMK, β1) was designed by Liu et al based on the conserved sequence of the integrin a and β units (15). In our previous study, this peptide was shown to block the interaction between HCC cells and the extracellular matrix, in addition to inhibiting intrahepatic and pulmonary metastases following carcinosectomy in a nude mouse model with human HCC that has high metastatic potential (LCI-D20) (16,17). On the basis of these studies, the trimeric peptide β (β3) was designed. The present study aimed to determine the effects of β3 on the adhesive properties of the human liver cancer cell line HCCLM6 to fibronectin (FN), the invasion of HCCLM6 cells through reconstituted basement membrane. In addition the rate of liver cancer recurrence and LCI-D20 mouse survival time following early carcinosectomy were investigated.
Materials and methods
Design and production of β3
The anti-adhesion trimeric β peptide (DLYYLMDLSYSMKGGDLYYLMDLSYSMKGGDLYYLMDLSYSMK, β3) was expressed in E. coli as described previously (18). Briefly, the DNA fragment of β3 (Sangon Biotech Co., Ltd., Shanghai, China) was cloned into the pET-His expression vector (Gene Power Lab Ltd., Shenzhen, China) and the fusion protein His-β3 was expressed in E. coli BL21 (DE3) plysS (Gene Power Lab Ltd.). Following 1.5 h of induction with isopropyl-β-D-thiogalactoside (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA), 20 mg of β3 peptide was obtained from 1 L of culture medium following purification with metal-chelating sepharose 6B FF (Vector Gene Technology Company Ltd., Beijing, China). The purity of β3 was 92.2% according to Gel-Pro Analyzer 3.1 software (Media Cybernetics, Inc., Rockville, MD, USA).
Cell culture
The highly metastatic HCC cell line HCCLM6, was initially established and preserved by the Liver Cancer Institute, Fudan University (Shanghai, China) and was cultured in Gibco Dulbecco's modified eagle's medium (DMEM, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.), 100 U/ml penicillin at 37°C under an atmosphere of 5% CO2. The medium was replenished every 3 days to maintain cell growth.
Coating the 96-well high binding microplate with FN
FN (Sigma-Aldrich, St. Louis, MO, USA) solution (containing 10 µg/ml FN, 20 mmol/l Tris-Cl, pH 7.4, 150 mmol/l NaCl, 1 mmol/l MgCl2, 1 mmol/l CaCl2, 1 mmol/l MnCl2) was added to a 96-well high binding microplate (100 ml per well), and incubated at 4°C overnight. The plate was then incubated with blocking buffer (10 mmol/l Hepes, pH 7.4, 140 mmol/l NaCl, 5.4 mmol/l KCl, 5.56 mmol/l glucose, 3% BSA, 1 mmol/l MgCl2, 2 mmol/l CaCl2, 1 mmol/l MnCl2) at 37°C for 2 h, and air dried for future use.
Cell adhesion assay
A total of 100 µl of HCCLM6 cell suspension (2×105 cells/ml) was plated in each well of an FN-coated 96-well high binding microplate. A total of 100 ml of DMEM medium containing β3 at concentrations of 20, 40, 100 or 200 µmol/l was added at the same time. The final concentrations of β3 were 10, 20, 50 or 100 µmol/l, respectively. The same volume of cell culture medium without β3 was added to the control group and 200 ml of cell culture medium only was added in the plate for the blank group. The assay was conducted in quintuplicate for each sample. Following incubation for 3 h at 37°C in 5% CO2, the unattached cells were gently washed away with HANKS buffer (Sangon Biotech Co., Ltd.). The attached cell number in each well was measured using the MTT assay (see below). The inhibition rate of β3 on cell adhesion to FN was calculated with the following equation: Cell adhesion inhibition rate = (average OD of control well - average OD of β3-treated well) / (average OD of control well - average OD of blank well) × 100%.
MTT assay
The number of attached cells in each well was analyzed by the MTT assay, and quantified by a micro-titer plate reader (Amersham, USA). Briefly, 100 µl DMEM and 20 µl MTT (5 mg/ml; Sigma-Aldrich) were added to each well. After incubation at 37°C for 4 h, the medium and MTT was discarded. A total of 200 µl of 0.04 mol/l hydrogen chloride-2-propanol solution was added to each well. The amount of MTT formazan product, which reflects the number of cells adhering to FN, was determined by measuring absorbance with a microplate reader at a wavelength of 570 nm and a reference wavelength of 630 nm.
Invasion assay
The invasion assay was performed as described previously (19). Briefly, the upper portions of Transwell chambers (Corning, New York, NY, USA) were coated with 75 µl of Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) diluted 1:10 in serum-free DMEM and incubated at 37°C for 2 h. The supernatants of HCCLM6 cells cultured with DMEM containing 10% FCS were harvested once the cells had grown to confluence, and after the addition of FN at a final concentration of 5 µg/ml, resulting in conditioned medium. The cells were harvested by trypsinization and diluted to a 2×106/ml cell suspension with serum-free DMEM. A total of 100 µl of the cell suspension and 100 µl of 200 µmol/l β3 peptide in serum-free DMEM (or serum-free DMEM only as a control) were added in the upper chambers. Then, 600 µl of conditioned medium was added to the bottom chamber of the Transwell plate. After incubation at 37°C for 48 h under a 5% CO2 atmosphere, the non-invading cells and the gel were gently removed from the upper chamber with cotton-tipped swabs. After rinsing with PBS, cells on the filters were fixed with formaldehyde and stained in Giemsa staining solution for 30 min. The number of invaded cells on the filters were counted in five randomly selected high-powered (×200) fields per filter under a microscope (Leica, Heerbrugg, Switzerland). The invasion inhibition rate was calculated using the following equation: Invasion inhibition rate = [1 - (invaded cell number in β3 chamber / invaded cell number in control chamber)] × 100%.
Animal model and treatment
A total of 24 6-week-old male BALB/cA nude mice were obtained from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences (Shanghai, China). A tumor block from tumor-bearing mice was implanted into the left lobe of the nude mouse liver as described previously (20). Briefly, a left upper abdominal transverse incision was made under anesthesia. The left lobe of the liver was exposed and a part of the liver surface was mechanically injured with scissors. Next, a tumor block of 0.2×0.2×0.2 cm was fixed within the liver tissue. After the surgery, mice were kept in laminar-flow cabinets under pathogen-free conditions and given free access to food and water. Liver cancer early resection was performed at 0.2 cm from the edge of the tumor at day 10 following implantation, prior to metastasis.
Measurement of tumor recurrence and mouse survival time
Animals were randomly assigned to 2 groups (each group, n=12). At day 1 following resection, the animals were subcutaneously administrated 100 µl of 1 mg/ml of β3 or normal saline (NS) as a control every other day for 10 doses. Half of the mice (n=6) in each group were sacrificed at day 55 post-implantation with an overdose of 6% chloral hydrate (0.5 ml/100 g of body weight; Sigma-Aldrich). At autopsy, the viscera of the animals were examined macroscopically. If recurrence of tumors at the incisal margin was observed, the lesions were resected and weighed.
The other 6 mice of each group were housed in pathogen-free cages. Each animal was examined daily until death. The number of days that each animal survived following resection was recorded. Survival curves were constructed using the Kaplan-Meier method.
All animal experiments were conducted in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. This study was approved by the Experimental Animal Ethics Committee of Shanghai Medical College, Fudan University (approval no. 20021101).
Statistical analysis
All data were entered into Excel spreadsheets (Excel, Microsoft, Seattle, USA). Statistical analysis was performed using Student's t-test, or the Mann Whitney U test when the data were not normally distributed. Values of P<0.05 in a two-tailed fashion were considered to indicate a statistically significant difference. All analyses were performed using SAS (SAS Institute Inc., Cary, NC, USA).
Results
The inhibitory effect of β3 on the adhesion of HCCLM6 cells to FN
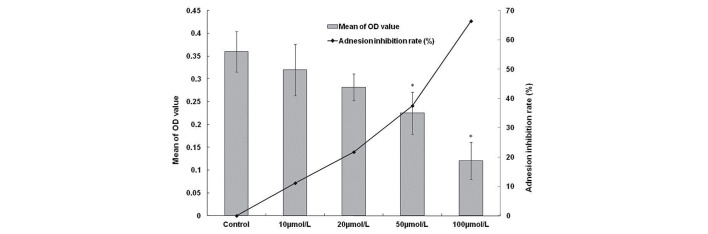
HCCLM6 cells incubated with 10, 20, 50 and 100 µmol/l β3 for 3 h exhibited a marked reduction in cell adhesion. The adhesion inhibition rates were 11.8, 21.7, 37.5 and 66.4%, respectively (Fig. 1). These findings indicate that β3 is able to inhibit the adhesion of HCCLM6 cells to FN, and β3 may obstruct the invasion of HCC cells to paratumor liver parenchyma.
Figure 1.
The inhibitory effect of β3 on the adhesion of HCCLM6 cells to fibronectin (n=5). HCCLM6 cells incubated with 10, 20, 50 and 100 µmol/l β3 for 3 h exhibited a marked reduction in cell adhesion. The adhesion inhibition rates were 11.8, 21.7, 37.5 and 66.4%, respectively. * P<0.05, compared with control group.
The inhibitory effect of β3 on the invasion ability of HCCLM6 cells
Following incubation with 100 µmol/l β3, the number of invaded HCCLM6 cells was reduced significantly, with an inhibition rate of 51.3% (Table I). Therefore, β3 may block HCC cells from invading the surrounding tissue and entering and extravasating from the circulation in vivo.
Table I.
The inhibitory effects of β3 on the invasion of HCCLM6 cells (n=5).
| Group | Invaded cells (mean ± SD) | Invasion inhibitory rate (%) |
|---|---|---|
| Control group | 22.6±4.77 | – |
| β3 group | 11±2.74a | 51.3 |
P<0.05, compared with control group.
The influence of β3 on the intrahepatic recurrence of the LCI-D20 model following early resection
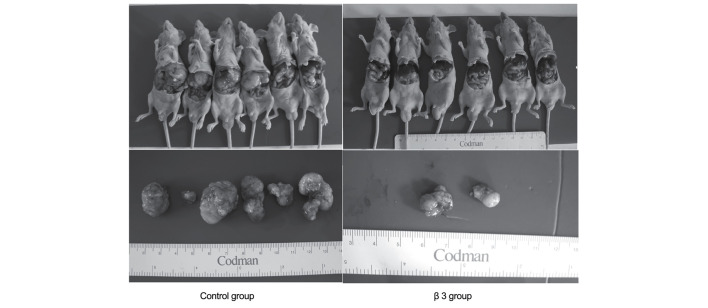
On the 10th day post-tumor-implantation, LCI-D20 tumors were resected, and β3 or the same volume of NS was subcutaneously injected. On day 55, mice were sacrificed to check for intrahepatic recurrence. The recurrent tumors were located around the incisal margins. There were 2 (2/6) mice with intrahepatic recurrent tumors in the β3 group, while there were 6 (6/6) mice with intrahepatic recurrent tumors in the control group (Fig. 2 and Table II). Compared with the control group, the weight of recurrent tumors of the β3 group were markedly reduced. These results indicate that β3 may have inhibitory effects on tumor recurrence at the incisal margin.
Figure 2.
Liver cancer recurrence at the incisal margins after early resection. On the 10th day post-tumor-implantation, LCI-D20 tumors were resected, and β3 or the same volume of saline was subcutaneously injected. On day 55, mice were sacrificed to check for intrahepatic recurrence. A total of 2/6 mice developed intrahepatic recurrent tumors in the β3 group, while 6/6 mice developed intrahepatic recurrent tumors in the control group. Compared with the control group, the weights of recurrent tumors of the β3 group were markedly reduced.
Table II.
Liver cancer recurrence after early resection.
| Group | Number of mice examined | Weight of recurrent lesions (mean ± SD) | Number of mice with recurrent lesions |
|---|---|---|---|
| Control group | 6 | 2.31±1.57a | 6 |
| β3 group | 6 | 0.17±0.26ab | 2 |
weight in grams
P<0.05, compared with control group.
Survival analysis of LCI-D20 mice after early resection
Twelve animals were randomly assigned to 2 groups (each group, n=6). At day 1 following resection, the animals were subcutaneously administrated 100 µl of 1 mg/ml of β3 or NS as a control every other day for 10 doses. The mice were housed in pathogen-free cages, and given free access to autoclaved food and water. The survival curves were constructed using the Kaplan-Meier method (Fig. 3). The median survival times after early resection were 136.5 days (control group) and 166.5 days (β3 group), respectively. This result indicates that β3 treatment prolonged the survival time of mice following early resection.
Figure 3.
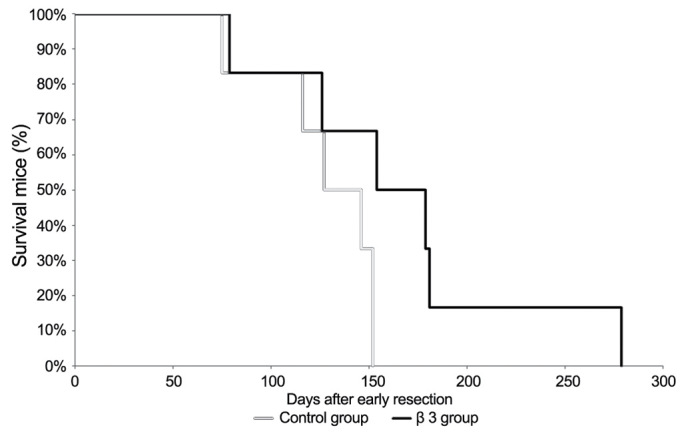
Kaplan-Meier analysis of mouse survival time following early resection. At day 1 after resection, the animals were subcutaneously administrated 100 µl of 1 mg/ml of β3 or saline alone as a control every other day for 10 doses. The median survival times after early resection were 136.5 d (control group) and 166.5 d (β3 group), respectively.
Discussion
The adhesion molecules on the surface of both tumor cells and endothelial cells are associated with tumor metastasis and recurrence. Blocking the interaction between tumor cell adhesion molecules and their ligands is a major strategy to prevent cancer metastasis (21,22). A number of previous studies have focused on the synthesized anti-adhesion peptides (13,23). One such peptide is RGD, derived from the common conserved sequence of the main matrix proteins such as fibronectin, collagen and fibrinogen (24,25). A second peptide is YIGSR, which originates from the basement membrane protein laminin (26). The third peptide is EILDV, which stems from the core sequence of fibronectin (27). The application of these short peptides is limited due to their short half-life, ease of degradation and the requirement for a high dosage. To prolong the peptides' half-lives, multimers and derivatives of these peptides were designed (28). The anti-metastasis effect of multimers of synthesized peptides was stronger compared to peptide monomers. The more times the sequence is repeated, the stronger the anti-metastasis effect is.
FN is an important cell adhesion molecule in the extracellular matrix. It mediates cell adhesion and migration, and serves a significant role in tumor invasion and metastasis (29). Examination of tumor cell adhesion to FN is a common method for studying tumor cell metastasis. In the present study, the extracellular matrix was simulated by coating cell culture plates with FN, after which the inhibitory effects of β3 peptide on liver cancer cell adhesion to FN were investigated. The results demonstrated that after treatment of HCCLM6 cells with β3 peptide for 3 h, an inhibitory effect on cell adhesion to FN was observed. There are two possible mechanisms by which β3 peptide blocked tumor cell adhesion to FN. First, the β3 peptide may occupy the integrin binding site through binding to the RGD sequence of the matrix protein. Second, β3 may also interact with integrin because β3 was designed based on the conserved sequence of the integrin α and β units.
During metastasis, tumor cells must penetrate the basement membrane through three steps: Dislodging from the original site, entering blood circulation, and migrating from blood flow into remote sites (30). Matrigel, used as an artificial basement membrane matrix, is produced from mouse Engelbreth-Holm-Swarm sarcoma rich in extracellular matrix protein (31). The artificial basement membrane is plated on a Millipore filter in Transwell culture chambers, and forms a membrane structure similar to natural basement membrane. Invasive tumor cells can penetrate the membrane under the induction of chemotactics, simulating the invasion of tumor cells through the basement membrane in vivo. The results indicated that β3 exerted significant inhibitory effects on the invasion of HCCLM6 cells.
In addition, the anti-tumor effect of β3 was observed in vivo. In LCI-D20 mice, β3 treatment was shown to reduce the weights of recurrent tumors at the incisal margins, in addition to the number of mice with intrahepatic recurrent tumors after early resection. Notably, β3 prolonged the survival time of LCI-D20 mice after early hepatectomy.
Metastasis and recurrence of liver cancer are major determinants for the prognosis and long-term survival of liver cancer patients. Polypeptide therapy is a newly developed treatment for tumors. Taken together, these cell and animal studies demonstrated that the β3 peptide had anti-adhesion and anti-recurrence effects, in addition to the capability to prolong survival time after early resection. Therefore, the β3 peptide is worthy of further investigation as a potential drug for blocking tumor metastasis and recurrence.
References
- 1.Fang WQ, Li SP, Zhang CQ, Xu L, Shi M, Chen MS, Li JQ. Prophylaxis and clinical treatment for surgical margin recurrence of small primary hepatocellular carcinoma. Ai Zheng. 2005;24:834–836. [PubMed] [Google Scholar]
- 2.Zhi X, Lin L, Yang S, Bhuvaneshwar K, Wang H, Gusev Y, Lee MH, Kallakury B, Shivapurkar N, Cahn K, et al. βII-spectrin (SPTBN1) suppresses progression of hepatocellular carcinoma and Wnt signaling by regulation of Wnt inhibitor kallistatin. Hepatology. 2015;61:598–612. doi: 10.1002/hep.27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyke JA. Overview-burgeoning promise in metastasis research. Eur J Cancer. 2000;36:1589–1594. doi: 10.1016/S0959-8049(00)00182-9. [DOI] [PubMed] [Google Scholar]
- 4.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-C. [DOI] [PubMed] [Google Scholar]
- 5.Chu XY, Chen LB. Cellular adhesive molecular and the invasion and metastasis of neoplasm. Yixue Yanjiusheng Xuebao. 2000;13:42–45. [Google Scholar]
- 6.Li FH. The inhibitory effect of bioactive peptides on neoplasm metastasis. Kouqiang Hemian Waike Zazhi. 1999;9:231–234. [Google Scholar]
- 7.Liu LY, Chen ZY, Zhao TH. Investigations of a peptide with RGD and YIGSR fragments: Synthesis and its anti-tumor invasion activities. Zhongguo Xinyao Zazhi. 2005;14:729–731. [Google Scholar]
- 8.Saiki I, Yoneda J, Kobayashi H, Igarashi Y, Komazawa H, Ishizaki Y, Kato I, Azuma I. Antimetastatic effect by anti-adhesion therapy with cell-adhesive peptide of fibronectin in combination with anticancer drugs. Jpn J Cancer Res. 1993;84:326–335. doi: 10.1111/j.1349-7006.1993.tb02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu LY, Chen ZY, Zhao TH. Synthesis of RGD identical-fork-peptide derivative with inhibitive effecton adhesiveness of advanced metastatic tumor cells. Zhongguo Xinyao Zazhi. 2006;15:1661–1663. [Google Scholar]
- 10.Zhang HQ, Shinohara H, Gu N, Sasaki H, Sisido M. Cell adhesion inhibition by RGD peptides linked with a photoisomerizable nonnatural amino acid. J Southeast Univ. 2001;17:22–26. [Google Scholar]
- 11.Zhao M, Wang C, Jiang X, Pen S. Synthesis of RGD containing peptides and their bioactivities. Prep Biochem Biotechnol. 2002;32:363–380. doi: 10.1081/PB-120015464. [DOI] [PubMed] [Google Scholar]
- 12.Cao K, Zhao TH, Chen ZY, Gao W, Yang HS, Shi B. The invasive capacity of human lung great cellular xancerous PG cells on reformed basement membrane and inhibition of synthetic peptides. Zhongliu Fangzhi Yanjiu. 2002;29:20–22. [Google Scholar]
- 13.Okrój M, Dobrzańska-Paprocka Z, Rolka K, Bigda J. In vitro and in vivo analyses of the biological activity of RGD peptides towards Ab Bomirski melanoma. Cell Mol Biol Lett. 2003;8:873–884. [PubMed] [Google Scholar]
- 14.Liu J, Guo SX, Tang JG. Research progress of RGD-peptide for cancer therapy. Guowai Yixue (Zhongliuxue Fence) 2003;30:193–197. [Google Scholar]
- 15.Liu YK, Nemoto A, Feng Y, Uemura T. The binding ability to matrix proteins and the inhibitory effects on cell adhesion of synthetic peptides derived from a conserved sequence of integrins. J Biochem. 1997;121:961–968. doi: 10.1093/oxfordjournals.jbchem.a021680. [DOI] [PubMed] [Google Scholar]
- 16.Uemura T, Nemoto A, Liu YK. Synthetic peptide derived from a conserved sequence of integrin β subunit. Res Adv in Biosci & Bioeng. 2000;23:65–83. [Google Scholar]
- 17.Sun JJ, Zhou XD, Liu YK, Tang ZY, Sun RX, Zhao Y, Uemura T. Inhibitory effects of synthetic beta peptide on invasion and metastasis of liver cancer. J Cancer Res Clin Oncol. 2000;126:595–600. doi: 10.1007/PL00008470. [DOI] [PubMed] [Google Scholar]
- 18.Wang SM, Zhu J, Li Y, Pan LF, Zha XL, Liu YK. Molecular cloning and expression of anti-tumor adhesion peptide (beta3) Sheng Wu Gong Cheng Xue Bao. 2005;21:558–562. (In Chinese) [PubMed] [Google Scholar]
- 19.Knutson JR, Lida J, Fields GB, McCarthy JB. CD44/chondroitin sulfate proteoglycan and alpha 2beta 1 integrin mediate human melanoma cell migration on type IV collagen and invasion of basement membranes. Mol Biol Cell. 1996;7:383–396. doi: 10.1091/mbc.7.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun FX, Tang ZY, Lui KD, Ye SL, Xue Q, Gao DM, Ma ZC. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int J Cancer. 1996;66:239–243. doi: 10.1002/(SICI)1097-0215(19960410)66:2<239::AID-IJC17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Syrigos KN, Karayiannakis AJ. Adhesion molecules as targets for the treatment of neoplastic diseases. Curr Pharm Des. 2006;12:2849–2861. doi: 10.2174/138161206777947759. [DOI] [PubMed] [Google Scholar]
- 22.Jiang CG, Xu HM. Research and application of anti-adhesion therapy in cancer metastasis. Guowai Yixue (Zhongliuxue Fence) 2005;32:31–34. [Google Scholar]
- 23.Wang YH, Liu YK, Li WC, Ye SL, Tang ZY. Inhibitory effect of anti-adhesion peptides on invasion/metastasis ability of hepatocellular carcinoma cells. Zhonghua Shiyan Waike Zazhi. 2004;21:1168–1169. [Google Scholar]
- 24.Liu YK, Wu WZ, Wu X, Jiang Y, Zhou XD. Liver cancer metastasis and signal transduction. In: Tang ZY. Metastasis and recurrence of hepatocellular carcinoma-basic and clinical studies. Shanghai Shanghai Scientific and technological education public house. 2003:93–104. [Google Scholar]
- 25.Maeda M, Izuno Y, Kawasaki K, Kaneda Y, Mu Y, Tsutsumi Y, Nakagawa S, Mayumi T. Amino acids and peptides. XXXI. Preparation of analogs of the laminin-related peptide YIGSR and their inhibitory effect on experimental metastasis. Chem Pharm Bull (Tokyo) 1998;46:347–350. doi: 10.1248/cpb.46.347. [DOI] [PubMed] [Google Scholar]
- 26.Kaneda Y, Yamamoto Y, Okada N, Tsutsuml Y, Nakagawa S, Kakiuch M, Maeda M, Kawasaki K, Mayumi T. Antimetastatic effect of synthetic Glu-Ile-Leu-Asp-Val peptide derivatives containing D-amino acids. Anticancer Drugs. 1997;8:702–707. doi: 10.1097/00001813-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Feng ZH, Huang B, Zhang GM, Li D, Wang HT. Inducement of antitumor-immunity by DC activated by Hsp70-H22 tumor antigen peptide. Chinese Journal of Cancer Research. 2003;15:79–85. doi: 10.1007/BF02974906. [DOI] [Google Scholar]
- 28.Wang SM, Zhu J, Li Y, Pan LF, Zha XL, Liu YK. Inhibitory effects of β peptide and polymeric β peptide on adhesion of hepatocellular carcinoma cells to fibronectin. Shiyong Zhongliu Zazhi. 2005;21:223–226. [Google Scholar]
- 29.Li NF, Gemenetzidis E, Marshall FJ, Davies D, Yu Y, Frese K, Froeling FE, Woolf AK, Feakins RM, Naito Y, et al. RhoC interacts with integrin α5β1 and enhances its trafficking in migrating pancreatic carcinoma cells. PLoS One. 2013;8:e81575. doi: 10.1371/journal.pone.0081575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sengupta N, MacDonald TT. The role of matrix metalloproteinases in stromal/epithelial interactions in the gut. Physiology (Bethesda) 2007;22:401–409. doi: 10.1152/physiol.00027.2007. [DOI] [PubMed] [Google Scholar]
- 31.Benton G, Arnaoutova I, George J, Kleinman HK, Koblinski J. Matrigel: From discovery and ECM mimicry to assays and models for cancer research. Adv Drug Deliv Rev 79–80. 2014:3–18. doi: 10.1016/j.addr.2014.06.005. [DOI] [PubMed] [Google Scholar]