Abstract
The global incidence of melanoma is increasing. Mortality from melanoma is influenced primarily by metastasis in advanced stages of the disease. Current treatments are largely ineffective; thus, novel gene delivery approaches that target tumor-specific markers may be useful for the treatment of melanoma. Systemic administration of encapsulated RNA-interference plasmids targeted against tumor cells is a potential alternative therapy for cancer. Formulations of transferrin (Tf)-conjugated polyethylene glycol (PEG) liposomes loaded with short hairpin RNA (shRNA) against WT1 (Lip + RNAi + Tf), PEG liposomes loaded with shRNA against WT1 (Lip + RNAi), Tf-conjugated PEG liposomes loaded with pEGFP-N3 (Lip + GFP + Tf) and saline solution as negative control (untreated) were administered systemically to C57BL/6 mice implanted subcutaneously with a melanoma cell line. Tumor volume, body weight, tumor weight, survival and relative expression of WT1 were evaluated. No significant differences in net body weight were identified between groups. The tumor volume decreased from 7,871 mm3 (SD±2,087) in the untreated group to 5,981 mm3 (SD±2,099) in the Lip + RNAi + Tf group. The tumor weight was reduced, from 8.8 g (SD±0.30) in the untreated group to 5.5 g (SD±0.87) in the Lip + RNAi + Tf group. An increase of 37% in survival was also observed in the group treated with Lip + RNAi + Tf in comparison to the untreated group. Tumors treated with Lip + RNAi + Tf also showed a decrease in the mean relative expression of WT1 of 0.21 (SD±0.28) folds compared with 1.8 (SD±2.49) folds in untreated group, 1.34 (SD±0.43) folds in Lip + RNAi group and of 1.89 (SD±0.69) folds in Lip + GFP + Tf group. Systemic administration of transferrin-conjugated PEG liposomes loaded with shRNA against WT1 reduced WT1 expression and tumor size and increased survival.
Keywords: transferrin-targeted liposome, WT1, melanoma, RNA interference
Introduction
Melanoma originates in pigmented melanocytes derived from neural crest cells that are normally present in the epidermis and dermis (1). Melanoma represents <5% of skin tumors but carries the greatest mortality rate of all skin neoplasms (2). The global incidence of melanoma has increased markedly (3). Melanoma is able to metastasize through the hematogenous or lymphatic system (4,5). Advanced-stage, metastatic disease confers a poor prognosis, with a median survival of less than one year (3,6).
The Wilms tumor 1 (WT1) protein is a transcription factor that regulates the expression of genes involved in cell proliferation and apoptosis (7–11). WT1 expression is essential for genitourinary development, and ~10% of nephroblastomas exhibit WT1 mutations (12). By contrast, wild-type WT1 is overexpressed in a variety of neoplasms, including lung, breast, thyroid and melanoma (13–17).
WT1 is expressed in >80% of malignant melanoma cells, but is not present in vivo in normal skin or benign melanocytic nevi (17). Expression of WT1 is associated with melanoma cell proliferation and is a possible marker of melanocytic invasion into the dermis (18,19). In vitro knockdown of WT1 induces apoptosis and increases sensitivity to chemotherapy in B16F10 melanoma cells. Furthermore, in vivo WT1 short hairpin RNA (shRNA) applied by aerosol reduces the number and size of tumors (20,21).
Gene therapy using tumor-targeted liposomes as delivery systems has opened a new era in cancer treatment (22). Liposomes are essentially phospholipid bilayer envelopes capable of systemically delivering drugs or genetic material. The advantage of using phospholipids for systemic delivery is that they are biodegradable, minimally toxic and easily removed (23,24). Recent studies show that the addition of polyethylene glycol (PEG) to phospholipids increases the half-life of liposomes in circulation; this modification also improves the ability to incorporate ligand molecules that specifically recognize superficial tumor targets (25–27).
Transferrin (Tf), a glycoprotein required for cellular absorption of iron, is often used in gene or drug delivery systems (28–37). As the Tf receptor (CD71) is overexpressed on the surface of cancer cells, it is a useful target for the delivery of therapeutic agents such as small-molecule drugs and nucleic acids (26,31,37–40).
The aim of the present study was to analyze the antitumor effects of Tf-conjugated PEG liposomes loaded with WT1 shRNA applied systemically to a subcutaneous model of melanoma in C57BL/6 mice.
Materials and methods
Materials
One-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (POPC), dimethyldioctadecyl ammonium bromide (DDAB), pegylated distearoylphosphatidylethanolamine (DSPE-PEG 2000) and DSPE-PEG 2000 conjugated to maleimide were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). DNase I was purchased from Sigma-Aldrich (St. Louis, MO, USA). Polycarbonate membranes of 100 nm were purchased from Whatman International, Ltd. (Maidstone, UK). Amicon Centriprep (molecular weight cut-off, 30 kDa) concentrator was purchased from EMD Millipore (Billerica, MA, USA). Holo-transferrin and 2-iminothiolane hydrochloride (Traut's reagent) were purchased from Sigma-Aldrich. WT1.1 DNA plasmid construction was performed as described by Zamora-Avila et al (20). B16F10 murine melanoma cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA). Rat anti-mouse CD71-(FITC) antibody clone C2 was purchased from BD Biosciences (San Jose, CA, USA). The pEGFP-N3 vector that expressed GFP protein was obtained from Clontech Laboratories, Inc. (Mountain View, CA, USA). Anti-WT1 F6 antibody (sc-7385) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; SAB2500451-100UG) antibody was purchased from Sigma-Aldrich. Anti-goat secondary antibody (#170-6515) was purchased from Bio-Rad Laboratories, Inc. (Hercules, CA, USA), and anti-mouse secondary antibody (sc-516086) was purchased from Santa Cruz Biotechnology, Inc.
Construction of WT1 shRNA plasmid
WT1 RNAi was designed using siRNA design software available online (Ambion; Thermo Fisher Scientific, Inc., Austin, TX, USA), and synthesized at 0.05 mg (Ambion; Thermo Fisher Scientific, Inc.) to create one recombinant plasmid (named WT1-1). Oligonucleotides for the WT1-1 plasmid were 5′-GATCCGGCTGTCCCACTTACAGATGGAAGCTTGCATCTGTAAGTGGGACAGCTTTTTTGGAAG-3′ and 3′-GCCGACAGGGTGAATGTCTACCTTCGAACGTAGACATTCACCCTGTCGAAAAAACCTTCGCCGG-5′. The oligonucleotides were resuspended to a final concentration of 1 mg/ml and annealed and ligated into pGSH1-GFP (Gene Therapy Systems, Inc., San Diego, CA, USA).
Western blot analysis
Tumoral tissue samples (25 mg) were lysed with TRIzol reagent according to manufacturer's instructions (Thermo Fisher Scientific, Inc., Gaithersburg, MD, USA). Protein concentration was determined using a DC protein assay kit. Proteins (50 µg whole-cell lysates) was electrophoresed on 12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Blocking was realized with 5% lactose-free milk and Tween 20 in incubation for 1 h. Three washes were performed after each hybridizing with the above antibodies. Monoclonal anti-WT1 F6 was used at a dilution of 1:2,500 in Tris-buffered saline (TBS) buffer, and secondary antibody was used at 1:5,000 in TBS buffer. To control for protein loading, anti-GAPDH was used at 1:10,000 in phosphate-buffered saline (PBS), and anti-goat secondary antibody was used at 1:5,000. All antibody incubations were 90 min at room temperature. Protein bands were visualized by enhanced chemiluminescence using Lumi-Light Western blotting substrate Roche Diagnostics (Indianapolis, IN, USA) .
Flow cytometric analysis
B16F10 melanoma cells (1×106) were resuspended in 200 µl PBS buffer and stained with anti-mouse CD71 (FITC) antibody. Cells were then incubated at 4°C for 30 min and spun at 400 × g for 10 min at 4°C. Cells were then washed twice with PBS buffer and spun at 1,600 rpm for 10 min at 4°C. Cells were suspended in 200 µl PBS and analyzed using a Accuri C6 flow cytometer (BD Biosciences) to assess cellular expression of CD71.
Liposomal preparation
Liposomal formulation was performed according to Shi and Pardridge (41). POPC (19.2 µmol), DDAB (0.2 µmol), DSPE-PEG 2000 (0.6 µmol), and DSPE-PEG 2000-maleimide (30 nmol) were dissolved in a mixture of chloroform/methanol (2:1 in a total volume of 3 ml) and subsequently evaporated using nitrogen gas. Lipids were then suspended in 0.2 ml PBS buffer containing 200 µg plasmid DNA and vortexed vigorously for 2 min. The liposome/DNA suspension was frozen in ethanol/dry ice for 4 min and thawed at 40°C for 2 min in a cycle 10 times. Adjustments to the size of the liposomes were performed using polycarbonate membranes containing a 100-nm pore size; this step was repeated five times. After extrusion, the liposomes were graduated to 2 ml and stored in the dark at 4°C. To remove unincorporated DNA inside the liposome, 5 U endonuclease I and 5 mM MgCl2 were added to the mixture and incubated for 1 h at 37°C. DNA concentration was calculated at ~50 and 60% using NanoDrop 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Anchoring of the protein to liposome was performed using 10 nmol Tf protein (Sigma-Aldrich) and thiolated using 400 µmol 2-iminothiolane (Traut's reagent) in 100 µl borate-EDTA buffer, pH 8.5 (0.15 M sodium borate and 0.1 mM EDTA). The mixture was incubated for 2 h in the dark at room temperature with shaking. Loaded Tf was washed with PBS in a concentration column (Centricon 30; Amicon) and concentrated to a final volume of 0.2 ml. The loaded Tf was immediately added to the liposomes and incubated for 24 h at 4°C to allow reaction of the maleimide group to generate PEG-immunoliposome complexes. The complexes were dialyzed using an 50-nm membrane to exclude non-incorporated materials. The mean vesicle diameters were determined using a Zetasizer Nano ZS90 particle size analyzer (Malvern Instruments, Ltd., Malvern, UK).
Subcutaneous in vivo model of melanoma
Female C57BL/6 mice (age, 7–8 weeks; weight, 20–25 g) were obtained from Harlan Laboratories S.A. De C.V. (Distrito Federal, Mexico). Seven mice in each group were used in the assay. Animals were housed under a 12-h light/dark cycle and received an autoclaved rodent diet and water ad libitum. All experiments were performed with prior approval from the local animal ethics committee. Four groups of seven mice were used for the in vivo assay. A subcutaneous model of melanoma was developed using the B16F10 cell line (CRL-6475; ATCC), which is derived from the same mouse species. A suspension of 200 µl containing 5×105 cells was subcutaneously injected into the posterior right flank of the mouse. Complete complex of Tf-conjugated PEG liposomes (50 µl) were loaded with shRNA against WT1 (Lip + RNAi + Tf), PEG liposomes (50 µl) and loaded with shRNA against WT1 (Lip + RNAi), Tf-conjugated PEG liposomes (50 µl) and loaded with pEGFP-N3 vector (Lip + GFP + Tf) and saline solution (50 µl; untreated group) were delivered intravenously through the tail vein on day 4 and every 5 days subsequently until day 29. Surviving mice were sacrificed on day 30 by cervical dislocation according the good management practices guidelines of laboratory animals, and tumors were collected for further analyses. Mouse weight, tumor weight and date of death were recorded.
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from 25 mg tumoral tissue using 1 ml TRIzol according to manufacturer's instructions. The cDNA samples were obtained using 5 µg total RNA, 200 U Superscript III and 0.5 µg oligo dT (12–17) at 42°C for 90 min, followed by heating at 70°C for 10 min. TaqMan® One-Step RT-PCR Master Mix Reagents Kit manufactured by Applied Biosystems (Thermo Fisher Scientific, Inc., Foster City, CA, USA).
Each qPCR reaction was performed with 2 µl cDNA and WT1 forward primer, TCTGCGGAGCCCAATACAG, reverse primer, CACATCCTGAATGCCTCTGAAGA, and probe FAM-CACCGTGCGTGTGTATT-NFQ. As an endogenous control, a mouse β-actin primer set was used, manufactured by Applied Biosystems. For each reaction, we used Universal PCR Master Mix manufactured by Roche Molecular Systems, Inc. (Branchburg, NJ, USA). The protocol was performed for 40 cycles at 94°C for 30 sec and 64°C for 30 sec using a Real-Time Thermal cycler CROMO4 (Bio-Rad Laboratories, Inc.). Relative quantification was performed using the Livak method (42). All samples were run in duplicate, destilled water was including as negative control of reacction.
Statistical analysis
Significance of different treatments was determined by analysis of variance by Dunnett's test. To survival rate were performed Kaplan-Meier curves, using SPSS software, version 13 (SPSS, Inc., Chicago, IL, USA) All data are expressed as the mean ± the standard errors of the mean. P<0.05 was considered to indicate a statistically significant difference.
Results
Size distribution according to intensity of liposomes
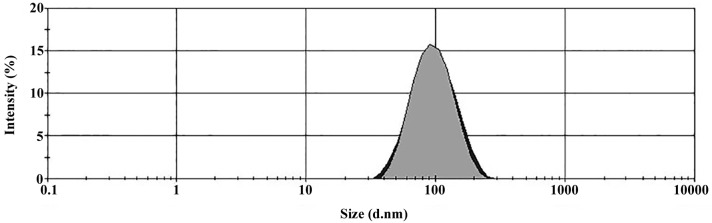
Sizes of the Tf-conjugated PEG liposomes loaded with WT1 shRNA are shown in Fig. 1. The mean diameter of the PEGylated Tf-conjugated PEG liposomes loaded with shRNA against WT1 was 86 nm. The poly-dispersity index (PDI) was 0.154, indicating that the vesicles have a high level of homogeneity. Liposome formulation by vortexing and repeated cycles of freezing-thawing allowed the formation of unilamellar liposomes. Liposomes contained a high concentration of the neutral phospholipid POPC and a minor quantity of cationic lipid DDAB (0.2 mol), which improved the incorporation of nucleic acid into the aqueous liposome center. A smaller quantity of DSPE-PEG 2000 (0.6 mol) and DSPE-PEG 2000-maleimide (30 nmol) provided steric properties, and the maleimide group allowed the addition of thiolated transferrin.
Figure 1.
Size distribution according to intensity of liposomes. Approximately 50 µl complex was suspended in 1 ml phosphate-buffered saline and analyzed using a Zetasizer Nano ZS90. Mean diameters of Tf-conjugated PEG liposomes loaded with shRNA against WT1 were of 86 nm and 0.154 of poly-dispersity index.
Transferrin receptor and WT1 expression in the Bl6F10 cell line
B16F10 cells showed 87% positivity for the Tf receptor according to flow cytometry results (Fig. 2A). In addition, western blotting confirmed expression of WT1 in B16F10 cells (Fig. 2B). Thus, B16F10 cells were selected as a suitable model to test Tf-conjugated PEG liposomes loaded with WT1 shRNA.
Figure 2.
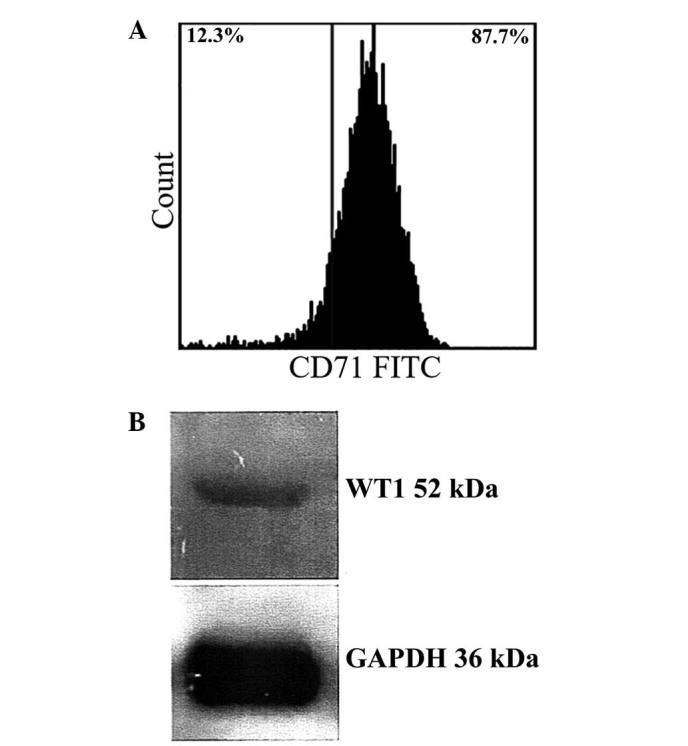
Presence of transferrin receptor and WT1 protein in B16F10 melanoma cells. (A) 1×106 B16F10 cells were incubated with anti-mouse CD71 antibody, then analyzed using flow cytometry. The figure shows the percentage of positivity to FITC dye. (B) 50 µg total protein extraction of B16F10 were analyzed using western blot analysis. The figure shows proteins of 52–54 kDa WT1, and the endogenous control GAPDH.
In vivo assay
Administration of different treatments Lip + RNAi + Tf, Lip + RNAi, Lip + GFP + Tf and untreated was initiated four days after cells were injected subcutaneously into mice. Treatments were administered six times through intravenous tail vein injection during a period of five days.
The mean percentage of weights of mice previously inoculated with B16F10 cells and treated with Lip + RNAi + Tf, Lip + RNAi, Lip + GFP + Tf and untreated is shown in Fig. 3A. Untreated and Lip + RNAi + Tf groups showed increases of 34% (SD±24.92) and 41% (SD±12.78) respectively, without significant differences between groups. In Lip + RNAi and Lip + GFP + Tf groups showed a low increase of 1.5% (SD±12.97) and 10% (SD±6.87) respectively, against final reading on day 19.
Figure 3.
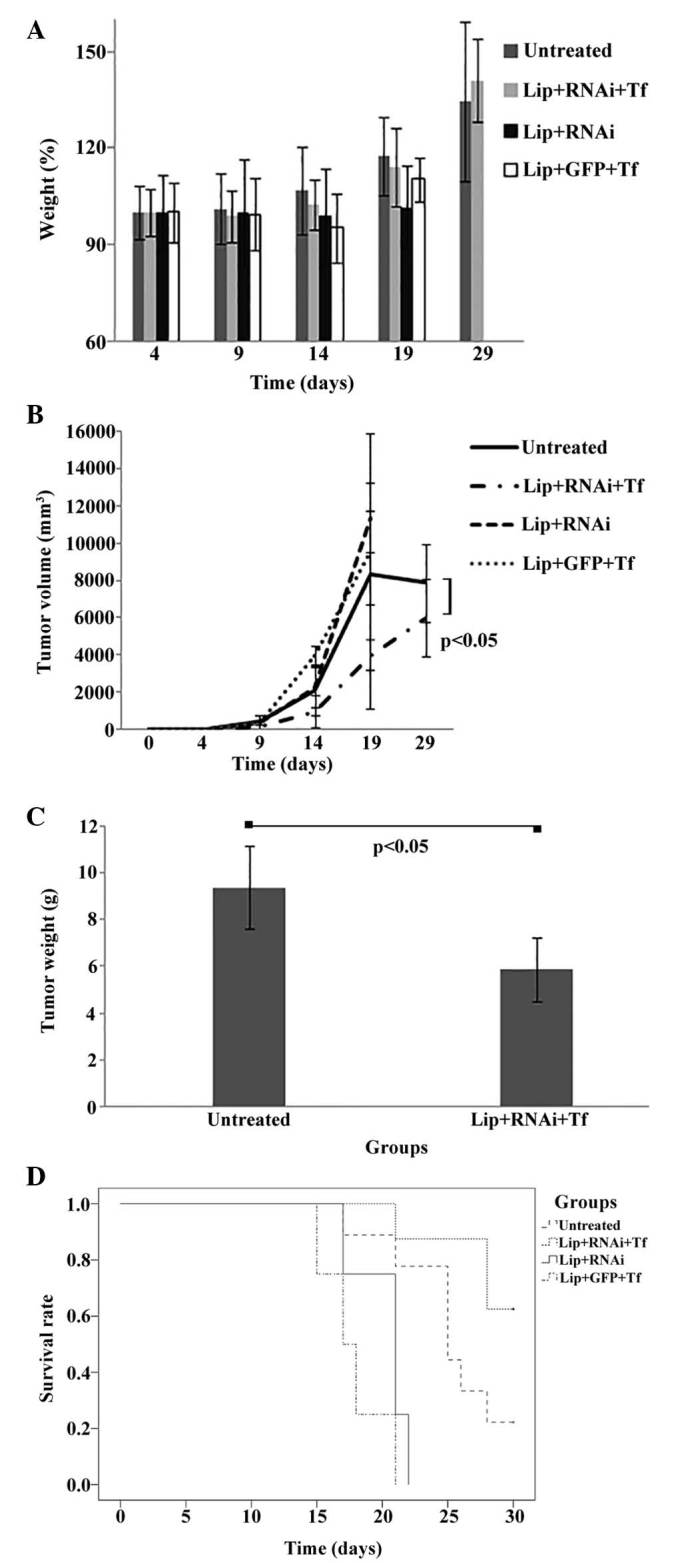
Antitumor activity of treatments in model in vivo. (A) Analysis of body weight of ‘in vivo’ assay. Mean percentages of the total weight of mice groups (untreated, Lip + RNAi + Tf, Lip + RNAi and Lip + GFP + Tf) during the time (days) of assay. Not significant difference was observed between groups. (B) Analysis of tumor volume in vivo assay. B16f10 cells were implanted subcutaneously into C57BL/6 mice. Intravenous injection of Lip + RNAi + Tf, Lip + RNAi, Lip + GFP + Tf and untreated treatments were performed. Data are expressed as the mean ± standard deviation (SD). Tumor volume was calculated using the following formula: Length × width2 x 0.5 and presented as mm3. (C) Analysis of tumor weight in vivo assay. The figure shows the weight mean of collected tumors in grams from groups untreated and Lip + RNAi + Tf. The standard deviation and statistical significance (P<0.05) were included. Data are expressed as mean including SD. (D) Analysis of rate survival in vivo assay. Kaplan-Meier curves of survival rate of mice groups. The assay was censored at 30 days after initial seeded of B16F10 cells (+).
Fig. 3B we found that Lip + RNAi and Lip + GFP + Tf groups grew, and there was a decrease in survival. For this reason, we were only able to record measurements to day 19 post-implant. The Lip + RNAi + Tf treatment showed a significant decrease (P<0.05) in tumor volume compared to the untreated group. The final readings presented a mean of 7,871 mm3 (SD±2,087) for the untreated group and 5,981 mm3 (SD±2,099) for Lip + RNAi + Tf group. This change represents a reduction in tumor volume of 24%.
Fig. 3C shows the mean final tumors weight of the groups of mice sacrificed at the end of the trial. Only the untreated and Lip + RNAi + Tf groups were analyzed because the mice of the other groups died after day 30. The results showed a reduction in tumor mass in the Lip + RNAi + Tf group and a mean tumor weight of 5.5 g (SD±0.87) compared with the untreated group that showed to mean tumor weight of 8.8 g (SD±0.30). The decrease of 34% in weight of the tumors collected shows that tumor size was significantly reduced (P<0.05) in Lip + RNAi + Tf group.
Fig. 3D the survival rate of C57BL/6 mice with implanted B16F10 melanoma cells subcutaneously into the right thigh of the hind limb was 25.22 days (SD±1.31). The untreated group presented a survival rate of 22.2% and Lip + RNAi + Tf group showed a survival rate of 62.5%, equivalent to an increase of 37%. This difference suggests that treatment with Lip + RNAi + Tf significantly increases survival in melanoma model employed (P<0.060). The Lip + RNAi and Lip + GFP + Tf groups showed a reduced survival compared to the untreated group, with a mean survival estimated at 20.25 and 17.75 days, respectively. The surviving mice were sacrificed on day 30 for humane reasons (+ Censored groups).
Analysis of WT1 expression in mouse tumor tissues
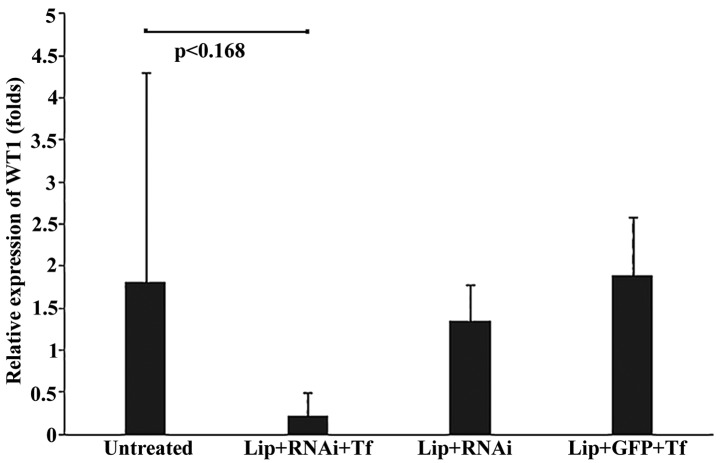
The WT1 expression levels in all groups of mice were analyzed using RT-qPCR analysis. The samples analyzed of Lip + RNAi and Lip + GFP + Tf groups were extracted soon after death of the mouse. In Fig. 4 show the relative expression of WT1 standardized according to the Livak method. To accomplish this, the average obtained from samples of untreated group was established as a calibrator and β-actin was used as endogenous control. Fig. 4 shows that the Lip + RNAi + Tf group showed a mean relative expression level of 0.21 (SD±0.28)-fold, representing a decrease in expression of WT1, compared with the other groups of mice which presented mean relative expression levels of 1.8 (SD±2.49)-fold for the untreated group of mice, 1.34 (SD±0.43)-fold for the Lip + RNAi group, and 1.89 (SD±0.69)-fold for the Lip + GFP + Tf group. The reduction in the expression levels of WT1 in the Lip + RNAi + Tf group suggests that tumor samples decrease in the expression of WT, which in turn suggests internalization directed by Tf.
Figure 4.
Analysis of WT1 expression in mouse tumor tissues. Figure presents the mean relative expression folds of WT1 in samples analyzed of the different groups of mice used in the in vivo model. Quantitative polymerase chain reactions were run in duplicate and β-actin was used as an endogenous gene. Data are expressed as the mean including standard deviation, and the statistical value were included in the graph (P<0.168).
Discussion
During the last three decades, global incidence of melanoma has rapidly increased, particularly among Caucasian populations (3). The increased mortality rate from melanoma is associated with a relatively late-stage diagnosis and resistance to chemotherapeutic agents (3).
The search for new therapeutic strategies has helped identify new tumor-specific molecules suitable for gene therapy (9). Restoration of tumor suppressor genes, such as p53, is a major strategy used for tumor reduction (43). Antisense and RNA interference are additional strategies for targeting genes involved in cancer. Well-defined tumor markers in melanoma include mutations in the oncogenes NRAS, BRAF, c-KIT, GNAQ and GNA11 or reduced function of the tumor suppressor genes PTEN and p53 (44–48). Between 40 and 60% of melanomas exhibit mutations in BRAF, and 90% have mutations in p53, although only 10% of these mutations actually disable the tumor suppressor activity of p53 (49). Recent studies show high expression of wild-type WT1 in a large proportion of solid and non-solid tumors, including melanoma (13–16,50). WT1 is a multifaceted protein involved in cell proliferation, cell death and angiogenesis. WT1 expression is found in 39% of melanomas, and >80% of WT1-positive melanomas is diagnosed in advanced stages of the disease. Thus, WT1 is a potential therapeutic target in melanoma. Antisense oligonucleotide and RNA interference strategies that decrease the expression of WT1 decreased the proliferation of cancer cells (51–54).
One of the limitations of gene therapy is tumor-specific delivery. The use of viral vectors is a good method for delivering genetic material in vitro (46–48); this strategy is limited by the host immune response in vivo (55–57).
The main advantages of liposomes include considerable concentration of large quantities of antitumor agent and intracellular delivery, increased half-life of tumor agents due to reduced degradation, gradual delivery of antineoplastic molecules, easy removal and reduced toxicity (24).
Phospholipid derivatives are currently used to improve circulating of liposomes and reduce their elimination by the liver and macrophages (58). The incorporation of thiol groups allows crosslinking with lysines in proteins, which can yield liposomes targeted to the ligand-receptor. The Tf receptor is widely used as delivery vehicle targeting tumor cells (41). The Tf receptor, CD71, is involved in the intracellular uptake of iron. The high expression of CD71 in many tumors, including melanoma, and the association with high iron requirements by dividing cells, makes this a good target (59). Tf is a serum protein found in high concentrations in the blood and is the natural ligand for the Tf receptor. Due to the versatility of the Tf receptor, it is useful for the delivery of transferrin antitumor agents, such as toxins, proteins and genetic materials (28,29,32,33,40).
In a previous study, we demonstrated the ability of shRNA to silence the WT1 gene and induce apoptosis in B16F10 murine melanoma cells (41). In a model of lung metastasis, application of this shRNA via aerosol was successful in reducing the size and number of tumors (60). The delivery system was effectively applied using PEI-DNA complexes, which reached high concentrations in the lung; however, this form application works exclusively in the lung (60). Systemic application of targeted liposomes opens the possibility of targeting WT1 in tumors in other parts of body (60). Liposome therapy would not be exclusive for melanoma, as other types of tumors express WT1 and the Tf receptor. The present results showed a 24% decrease in tumor size without changes in body weight. The final weight of the tumor for the experimental group differed by 34% from that of the control mice (20). In addition, an improvement in survival of 37% was observed in mice treated with Tf-conjugated PEG liposomes loaded with WT1 shRNA. These are important results, considering that B16F10 cells form very aggressive tumors in C57BL/6 mice (14).
A reduction in WT1 expression was noted in the Lip + RNAi + Tf experimental treatment group. Although transfection efficiency was not analyzed, RT-qPCR suggests that decreased expression of WT1 was associated with a reduction in tumor mass. Tumors were collected within 24 h of the last treatment. In order to identify transfected cells with decreased expression of WT1, we would have to assess expression of beta-galactosidase about 2–6 h after in vivo inoculation (41).
Complete tumor eradication was not possible; thus, further studies should examine increased concentrations of liposome complexes or additional anticancer molecules. Previous studies have shown that WT1 silencing sensitizes cells to chemotherapeutic agents, such as cisplatin, doxorubicin and radiation. Increased sensitivity to apoptotic stimuli in response to silencing of WT1 is likely due to the fact that WT1 regulates Bcl-2, multi-drug resistance 1 (MDR1) and P-glycoprotein expression, which are involved in chemoresistance (21,61,62).
Acknowledgements
This study was supported by the Microbiology and immunology Department, Biological Sciences Faculty, Autonomous University of Nuevo León (UANL).
References
- 1.Cichorek M, Wachulska M, Stasiewicz A, Tymińska A. Skin melanocytes: Biology and development. Postepy Dermatol Alergol. 2013;30:30–41. doi: 10.5114/pdia.2013.33376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Situm M, Buljan M, Bulić SO, Simić D. The mechanisms of UV radiation in the development of malignant melanoma. Coll Antropol 31 (Suppl 1) 2007:13–16. [PubMed] [Google Scholar]
- 3.Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol. 2014;170:11–19. doi: 10.1111/bjd.12492. [DOI] [PubMed] [Google Scholar]
- 4.Leiter U, Buettner PG, Eigentler TK, Garbe C. Prognostic factors of thin cutaneous melanoma: An analysis of the central malignant melanoma registry of the german dermatological society. J Clin Oncol. 2004;22:3660–3667. doi: 10.1200/JCO.2004.03.074. [DOI] [PubMed] [Google Scholar]
- 5.Leiter U, Meier F, Schittek B, Garbe C. The natural course of cutaneous melanoma. J Surg Oncol. 2004;86:172–178. doi: 10.1002/jso.20079. [DOI] [PubMed] [Google Scholar]
- 6.Lee ML, Tomsu K, Von Eschen KB. Duration of survival for disseminated malignant melanoma: Results of a meta-analysis. Melanoma Res. 2000;10:81–92. [PubMed] [Google Scholar]
- 7.Hewitt SM, Hamada S, McDonnell TJ, Rauscher FJ, III, Saunders GF. Regulation of the proto-oncogenes bcl-2 and c-myc by the Wilms' tumor suppressor gene WT1. Cancer Res. 1995;55:5386–5389. [PubMed] [Google Scholar]
- 8.Englert C, Hou X, Maheswaran S, Bennett P, Ngwu C, Re GG, Garvin AJ, Rosner MR, Haber DA. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 1995;14:4662–4675. doi: 10.1002/j.1460-2075.1995.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maheswaran S, Englert C, Bennett P, Heinrich G, Haber DA. The WT1 gene product stabilizes p53 and inhibits p53-mediated apoptosis. Genes Dev. 1995;9:2143–2156. doi: 10.1101/gad.9.17.2143. [DOI] [PubMed] [Google Scholar]
- 10.Haber DA, Englert C, Maheswaran S. Functional properties of WT1. Med Pediatr Oncol. 1996;27:453–455. doi: 10.1002/(SICI)1096-911X(199611)27:5<453::AID-MPO11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Englert C, Maheswaran S, Garvin AJ, Kreidberg J, Haber DA. Induction of p21 by the Wilms' tumor suppressor gene WT1. Cancer Res. 1997;57:1429–1434. [PubMed] [Google Scholar]
- 12.Scholz H, Kirschner KM. A role for the Wilms tumor Protein WT1 in organ development. Physiology (Bethesda) 2005;20:54–59. doi: 10.1152/physiol.00048.2004. [DOI] [PubMed] [Google Scholar]
- 13.Oji Y, Miyoshi S, Maeda H, Hayashi S, Tamaki H, Nakatsuka S, Yao M, Takahashi E, Nakano Y, Hirabayashi H, et al. Overexpression of the Wilms' tumor gene WT1 in de novo lung cancers. Int J Cancer. 2002;100:297–303. doi: 10.1002/ijc.10476. [DOI] [PubMed] [Google Scholar]
- 14.Loeb DM, Evron E, Patel CB, Sharma PM, Niranjan B, Buluwela L, Weitzman SA, Korz D, Sukumar S. Wilms' tumor suppressor gene (WT1) is expressed inprimary breast tumors despite tumor-specific promoter methylation. Cancer Res. 2001;61:921–925. [PubMed] [Google Scholar]
- 15.Miyoshi Y, Ando A, Egawa C, Taguchi T, Tamaki Y, Tamaki H, Sugiyama H, Noguchi S. High expression of Wilms' tumor suppressor gene predicts poor prognosis in breast cancer patients. Clin Cancer Res. 2002;8:1167–1171. [PubMed] [Google Scholar]
- 16.Oji Y, Miyoshi Y, Koga S, Nakano Y, Ando A, Nakatsuka S, Ikeba A, Takahashi E, Sakaguchi N, Yokota A, et al. Overexpression of the Wilms' tumor gene WT1 in primary thyroid cancer. Cancer Sci. 2003;94:606–611. doi: 10.1111/j.1349-7006.2003.tb01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner N, Panelos J, Massi D, Wagner KD. The Wilms' tumor suppressor WT1 is associated with melanoma proliferation. Pflugers Arch. 2008;455:839–847. doi: 10.1007/s00424-007-0340-1. [DOI] [PubMed] [Google Scholar]
- 18.Garrido-Ruiz MC, Rodriguez-Pinilla SM, Pérez-Gómez B, Rodriguez-Peralto JL. WT 1 expression in nevi and melanomas: A marker of melanocytic invasion into the dermis. J Cutan Pathol. 2010;37:542–548. doi: 10.1111/j.1600-0560.2009.01379.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilsher M, Cheerala B. WT1 as a complementary marker of malignant melanoma: An immunohistochemical study of whole sections. Histopathology. 2007;51:605–610. doi: 10.1111/j.1365-2559.2007.02843.x. [DOI] [PubMed] [Google Scholar]
- 20.Zamora-Avila DE, Franco-Molina MA, Trejo-Avila LM, Rodríguez-Padilla C, Reséndez-Pérez D, Zapata-Benavides P. RNAi silencing of the WT1 gene inhibits cell proliferation and induces apoptosis in the B16F10 murine melanoma cell line. Melanoma Res. 2007;17:341–348. doi: 10.1097/CMR.0b013e3282efd3ae. [DOI] [PubMed] [Google Scholar]
- 21.Zapata-Benavides P, Manilla-Muñoz E, Zamora-Avila DE, Saavedra-Alonso S, Franco-Molina MA, Trejo-Avila LM, Davalos-Aranda G, Rodríguez-Padilla C. WT1 silencing by RNAi synergizes with chemotherapeutic agents and induces chemosensitization to doxorubicin and cisplatin in B16F10 murine melanoma cells. Oncol Lett. 2012;3:751–755. doi: 10.3892/ol.2012.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu KF, Zhang WQ, Luo LM, Song P, Li D, Du R, Ren W, Huang D, Lu WL, Zhang X, Zhang Q. The antitumor activity of a doxorubicin loaded, iRGD-modified sterically-stabilized liposome on B16-F10 melanoma cells: In vitro and in vivo evaluation. Int J Nanomedicine. 2013;8:2473–2485. doi: 10.2147/IJN.S46962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maruyama K, Ishida O, Kasaoka S, Takizawa T, Utoguchi N, Shinohara A, Chiba M, Kobayashi H, Eriguchi M, Yanagie H. Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT) J Control Release. 2004;98:195–207. doi: 10.1016/j.jconrel.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63:131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Boerman OC, Oyen WJ, van Bloois L, Koenders EB, van der Meer JW, Corstens FH, Storm G. Optimization of technetium-99m-labeled PEG liposomes to image focal infection: Effects of particle size and circulation time. J Nucl Med. 1997;38:489–493. [PubMed] [Google Scholar]
- 26.Derycke AS, De Witte PA. Transferrin-mediated targeting of hypericin embedded in sterically stabilized PEG-liposomes. Int J Oncol. 2002;20:181–187. [PubMed] [Google Scholar]
- 27.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, et al. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stavridis JC, Deliconstantinos G, Psallidopoulos MC, Armenakas NA, Hadjiminas DJ, Hadjiminas J. Construction of transferrin-coated liposomes for in vivo transport of exogenous DNA to bone marrow erythroblasts in rabbits. Exp Cell Res. 1986;164:568–572. doi: 10.1016/0014-4827(86)90056-X. [DOI] [PubMed] [Google Scholar]
- 29.Vidal M, Sainte-Marie J, Philippot JR, Bienvenue A. The influence of coupling transferrin to liposomes or minibeads on its uptake and fate in leukemic L2C cells. FEBS Lett. 1987;216:159–163. doi: 10.1016/0014-5793(87)80776-7. [DOI] [PubMed] [Google Scholar]
- 30.Di Giulio A, D'Andrea G, Saletti MA, Impagnatiello A, D'Alessandro AM, Oratore A. The binding of human serum transferrin to its specific receptor reconstituted into liposomes. Cell Signal. 1994;6:83–90. doi: 10.1016/0898-6568(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 31.Egea MA, García ML, Alsina MA, Reig F. Coating of liposomes with transferrin: Physicochemical study of the transferrin-lipid system. J Pharm Sci. 1994;83:169–173. doi: 10.1002/jps.2600830211. [DOI] [PubMed] [Google Scholar]
- 32.Corley P, Loughrey HC. Targeting of doxorubicin loaded liposomes to T-cells via the transferrin receptor. Biochem Soc Trans. 1998;26:S37. doi: 10.1042/bst026s037. [DOI] [PubMed] [Google Scholar]
- 33.Simões S, Slepushkin V, Gaspar R, de Lima MC, Düzgüneş N. Gene delivery by negatively charged ternary complexes of DNA, cationic liposomes and transferrin or fusigenic peptides. Gene Ther. 1998;5:955–964. doi: 10.1038/sj.gt.3300674. [DOI] [PubMed] [Google Scholar]
- 34.Singh M. Transferrin as a targeting ligand for liposomes and anticancer drugs. Curr Pharm Des. 1999;5:443–451. [PubMed] [Google Scholar]
- 35.Eavarone DA, Yu X, Bellamkonda RV. Targeted drug delivery to C6 glioma by transferrin-coupled liposomes. J Biomed Mater Res. 2000;51:10–14. doi: 10.1002/(SICI)1097-4636(200007)51:1<10::AID-JBM2>3.3.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Ishida O, Maruyama K, Tanahashi H, Iwatsuru M, Sasaki K, Eriguchi M, Yanagie H. Liposomes bearing polyethyleneglycol-coupled transferrin with intracellular targeting property to the solid tumors in vivo. Pharm Res. 2001;18:1042–1048. doi: 10.1023/A:1010960900254. [DOI] [PubMed] [Google Scholar]
- 37.Voinea M, Dragomir E, Manduteanu I, Simionescu M. Binding and uptake of transferrin-bound liposomes targeted to transferrin receptors of endothelial cells. Vascul Pharmacol. 2002;39:13–20. doi: 10.1016/S1537-1891(02)00165-9. [DOI] [PubMed] [Google Scholar]
- 38.Anabousi S, Laue M, Lehr CM, Bakowsky U, Ehrhardt C. Assessing transferrin modification of liposomes by atomic force microscopy and transmission electron microscopy. Eur J Pharm Biopharm. 2005;60:295–303. doi: 10.1016/j.ejpb.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Sharma G, Modgil A, Layek B, Arora K, Sun C, Law B, Singh J. Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: Biodistribution and transfection. J Control Release. 2013;167:1–10. doi: 10.1016/j.jconrel.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi T, Ishida T, Okada Y, Ise S, Harashima H, Kiwada H. Effect of transferrin receptor-targeted liposomal doxorubicin in P-glycoprotein-mediated drug resistant tumor cells. Int J Pharm. 2007;329:94–102. doi: 10.1016/j.ijpharm.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 41.Shi N, Pardridge WM. Noninvasive gene targeting to the brain. Proc Natl Acad Sci USA. 2000;97:7567–7572. doi: 10.1073/pnas.130187497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) Method. Methods. 2001;25:402–418. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Xu L, Pirollo KF, Chang EH. Tumor-targeted p53-gene therapy enhances the efficacy of conventional chemo/radiotherapy. J Control Release. 2001;74:115–128. doi: 10.1016/S0168-3659(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 44.Carlino MS, Haydu LE, Kakavand H, Menzies AM, Hamilton AL, Yu B, Ng CC, Cooper WA, Thompson JF, Kefford RF, et al. Correlation of BRAF and NRAS mutation status with outcome, site of distant metastasis and response to chemotherapy in metastatic melanoma. Br J Cancer. 2014;111:292–299. doi: 10.1038/bjc.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pilloni L, Bianco P, Difelice E, Cabras S, Castellanos ME, Atzori L, Ferreli C, Mulas P, Nemolato S, Faa G. The usefulness of c-Kit in the immunohistochemical assessment of melanocytic lesions. Eur J Histochem. 2011;55:105–111. doi: 10.4081/ejh.2011.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khalili JS, Yu X, Wang J, Hayes BC, Davies MA, Lizee G, Esmaeli B, Woodman SE. Combination small molecule MEK and PI3K inhibition enhances uveal melanoma cell death in a mutant GNAQ and GNA11 dependent manner. Clin Cancer Res. 2012;18:4345–4355. doi: 10.1158/1078-0432.CCR-11-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X-P, Gimm O, Hampel H, Niemann T, Walker MJ, Eng C. Epigenetic PTEN silencing in Malignant Melanomas without PTEN Mutation. Am J Pathol. 2000;157:1123–1129. doi: 10.1016/S0002-9440(10)64627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terzian T, Torchia EC, Dai D, Robinson SE, Murao K, Stiegmann RA, Gonzalez V, Boyle GM, Powell MB, Pollock PM, et al. p53 prevents progression of nevi to melanoma predominantly through cellcycle regulation. Pigment Cell Melanoma Res. 2010;23:781–794. doi: 10.1111/j.1755-148X.2010.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Box NF, Vukmer TO, Terzian T. Targeting p53 in melanoma. Pigment Cell Melanoma Res. 2014;27:8–10. doi: 10.1111/pcmr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyagi T, Ahuja H, Kubota T, Kubonishi I, Koeffler HP, Miyoshi I. Expression of the candidate Wilm's tumor gene, WT1, in human leukemia cells. Leukemia. 1993;7:970–977. [PubMed] [Google Scholar]
- 51.Zapata-Benavides P, Tuna M, Lopez-Berestein G, Tari AM. Downregulation of Wilms' tumor 1 protein inhibits breast cancer proliferation. Biochem Biophys Res Commun. 2002;295:784–790. doi: 10.1016/S0006-291X(02)00751-9. [DOI] [PubMed] [Google Scholar]
- 52.Chen MY, Clark AJ, Chan DC, Ware JL, Holt SE, Chidambaram A, Fillmore HL, Broaddus WC. Wilms' tumor 1 silencing decreases the viability and chemoresistance of glioblastoma cells in vitro: A potential role for IGF-1R de-repression. J Neurooncol. 2011;103:87–102. doi: 10.1007/s11060-010-0374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huo X, Ren L, Shang L, Wang X, Wang J. Effect of WT1 antisense mRNA on the induction of apoptosis in ovarian carcinoma SKOV3 cells. Eur J Gynaecol Oncol. 2011;32:651–656. [PubMed] [Google Scholar]
- 54.Chen Y, Bathula SR, Yang Q, Huang L. Targeted nanoparticles deliver siRNA to melanoma. J Invest Dermatol. 2010;130:2790–2798. doi: 10.1038/jid.2010.222. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Jin Y, Zhang B, Chen H. Preparation of recombinant adenovirus Ad5/F35 containing human WT1 and identification after recombinant adenovirus infected dendritic cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014;30:135–138. (In Chinese) [PubMed] [Google Scholar]
- 56.Sundarasetty BS, Singh VK, Salguero G, Geffers R, Rickmann M, Macke L, Borchers S, Figueiredo C, Schambach A, Gullberg U, et al. Lentivirus-induced dendritic cells for immunization against high-risk WT1(+) acute myeloid leukemia. Hum Gene Ther. 2013;24:220–237. doi: 10.1089/hum.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shan XY, Liu ZL, Wang B, Guo GX, Wang MS, Zhuang FL, Cai CS, Zhang MF, Zhang YD. Construction of recombinant lentiviral vector of Tie2-RNAi and its influence on malignant melanoma cells in vitro. Zhonghua Zheng Xing Wai Ke Za Zhi. 2011;27:277–283. (In Chinese) [PubMed] [Google Scholar]
- 58.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51:691–743. [PubMed] [Google Scholar]
- 59.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigen: A national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zamora-Avila DE, Zapata-Benavides P, Franco-Molina MA, Saavedra-Alonso S, Trejo-Avila LM, Reséndez-Pérez D, Méndez-Vázquez JL, Isaias-Badillo J, Rodríguez-Padilla C. WT1 gene silencing by aerosol delivery of PEI:RNAi complexes inhibits B16-F10 lung metastases growth. Cancer Gene Ther. 2009;16:892–899. doi: 10.1038/cgt.2009.35. [DOI] [PubMed] [Google Scholar]
- 61.Clark AJ, Chan DC, Chen MY, Fillmore H, Dos Santos WG, Van Meter TE, Graf MR, Broaddus WC. Down-regulation of Wilms' tumor 1 expression in glioblastoma cells increases radiosensitivity independently of p53. J Neurooncol. 2007;83:163–172. doi: 10.1007/s11060-006-9317-8. [DOI] [PubMed] [Google Scholar]
- 62.Shen H, Xu W, Wu Z, Tang H, Xie Y, Zhong X. Down-regulation of WT1/+17AA gene expression using RNAi and modulating leukemia cell chemotherapy resistance. Haematologica. 2007;92:1270–1272. doi: 10.3324/haematol.11010. [DOI] [PubMed] [Google Scholar]