Abstract
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer-related mortality worldwide. In the present study, we focused on LIM and SH3 protein 1 (LASP-1), a key molecule involved in the development of multiple cancers, and attempted to elucidate its effect on the oncogenesis of lung cancer. We determined the expression level of LASP-1 in lung cancer using reverse transcription-quantitative polymerase chain reaction and western blot analysis, and also studied the potential function of LASP-1 in lung cancer cell growth, apoptosis and migration by small interfering RNA transfection. The results revealed that the levels of LASP-1 mRNA and protein were abnormally high in lung cancer cells. Following RNA interference of LASP-1, the proliferation and migration ability of the human cancer cell line A549 were significantly decreased. In addition, fluorescence-activated cell sorting analysis indicated that the apoptotic process in the A549 cell line was induced by the silencing of LASP-1. Our study is the first to investigate the potential of LASP-1 in lung cancer, and revealed its significant role in regulating the growth and metastasis of lung cancer cells. The present study suggests that LASP-1 has potential as a therapeutic target in the treatment of lung cancer in the clinic.
Keywords: apoptosis, LIM and SH3 protein 1, lung cancer, RNAi
Introduction
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer-related mortality worldwide in both males and females (1), accounting for more than 1.4 million mortalities per year. Moreover, with the high and increasing number of smokers in a number of Asian and developing countries, it is anticipated that the incidence of lung cancer will continue to increase over the next decades. The rates of lung cancer are mainly determined by smoking patterns, although other factors, including medical, occupational and environmental radiation exposure, have also been demonstrated to increase the risk of lung cancer (2).
Lung cancer is a heterogeneous disease that has historically been divided into two main types based on the disease patterns and treatment strategies: small cell-lung cancer (SCLC) and non-small cell-lung cancer (NSCLC) (3,4). The expressions of various oncogenes have been investigated in NSCLC and SCLC. The complex multiple genetic lesions in lung cancer are composed of two major forms (5): mutations activating the dominant cellular proto-oncogenes, and as those inactivating the tumor suppressor genes. The activation of RAS, MYC and HER-2/NEU genes is usually associated with an adverse prognosis of lung cancer (6–8), while the overexpression of tumor suppressor genes including VHL, RB and p53 demonstrate a positive outcome in the recovery of lung cancer patients (2).
Chemotherapy remains the cornerstone of treatment for limited- and extensive-stage lung cancer. Surgery is also considered for certain stages (9). However, these traditional treatments only provide a modest benefit with considerable toxicity for advanced lung cancer (10). There is an urgent requirement to develop alternative therapies to enable the monitoring of lung cancer treatment efficacy and assessment of the risk of metastases. Advances in our understanding of molecular genetics in lung cancer have led to the identification of key genetic aberrations in lung cancer. The potential of certain clinically relevant driver molecular lesions, including epidermal growth factor receptor (EFGR) mutations, ROS1 translocation and BRAF mutations, has already been confirmed (11).
In the present study, we focused on the LIM and SH3 protein 1 (LASP-1), another key molecule involved in development of multiple cancers, and attempted to elucidate its effect on the oncogenesis of lung cancer. The gene encodes a membrane-bound protein containing 261 amino acids with an N-terminal LIM domain (12,13). The exact function of LASP-1 in lung cancer is still not well understood; however, its expression is noted to be increased in a number of malignant tumors, including breast, colorectal, hepatocellular and bladder cancer (14–18). For the first time, we determined the expression level of LASP-1 in lung cancer using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis. We also studied the potential function of LASP-1 in lung cancer cell growth, apoptosis and migration by small interfering RNA (siRNA) transfection. The aim of our study was to evaluate the function of LASP-1 in lung cancer and clarify the prognostic value of LASP-1 in the clinic.
Materials and methods
Patients and sample collection
Twenty patients were enrolled in the current study. Sections were obtained from tumor tissues and para-carcinoma tissues, and examined independently by senior pathologists. Parts of each sample were frozen immediately following surgery and stored at −135°C for RNA and protein extraction. The human lung cancer cell line A549 was purchased from ATCC (Manassas, VA, USA). The ethics committee of the Cancer Hospital of Shantou University Medical College approved the screening, inspection and data collection of the patients, and all subjects signed a written informed consent form. The study was undertaken in accordance with the provisions of the Declaration of Helsinki.
RNA isolation and RT-qPCR
LASP-1 mRNA expression in cancer tissues was analyzed by one-step RT-qPCR using an RNA Master SYBR-Green I kit (Roche Applied Science, Indianapolis, IN, USA). The whole RNA of the lung tissue of each sample was extracted with TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. cDNA was synthesized with the PrimeScript RT reagent kit (Takara Biotechnology Co. Ltd., Dalian, China). RT-qPCR was then performed using 2X SYBR-Green PCR master mix (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in an ABI PRISM 7500 system. The relative level of LASP-1 mRNA was normalized to that of β-actin and analyzed by the comparative Ct method.
Western blot analysis
The protein products of LASP-1 and the reference GAPDH were extracted from the lung tissue of the patients. The extracts were boiled using loading buffer for 5 min and then subjected to 10% sodium dodecylsulfate polyacrylamide gel electrophoresis. The protein products were then transferred onto polyvinylidene difluoride sheets. The membranes were washed with Tris-buffered saline and Tween-20 for 20 min and the procedure was repeated three times. Then the membranes were incubated with antibody overnight at room temperature. After three more washes, secondary antibody was added and the membrane was incubated for another 4 hours. The blots were developed using Beyo ECL Plus reagent (Beyotime Institute of Biotechnology, Haimen, China) after three final washes. The results of analysis were determined in the Gel Imaging system (Liuyi Factory, Beijing, China). The expression levels were calculated with Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
siRNA transfection
The target sequence for LASP-1-specific siRNA (5′-CTGGATAAGTTCTGGCATA-3′) and the negative control (NC) siRNA were obtained from Genechem Biotech (Shanghai, China). The human lung cancer cell line A549 was subjected to one of three treatments: no treatment (control group), treatment with NC-siRNA, and treatment with LASP-1-siRNA. Transfections were conducted using Lipofectamine 2000 reagent (Invitrogen Life Technologies) following the manufacturer's instructions, and subsequent selection was conducted using 400 µg/ml puromycin (Amresco, Solon, OH, USA). The protein products of LASP-1 and reference protein GAPDH were extracted from the cells, and western blot analysis was conducted as described above.
Cell proliferation
The cell proliferation ability of the different treatments was analyzed using a WST-8 Cell Counting kit-8 (Beyotime Institute of Biotechnology). Briefly, 50 µl exponentially growing cells (2×105 cells/ml) in RPMI-1640 medium (100 µl) containing 10% fetal bovine serum were seeded into a 96-well plate. For each group, cells were incubated for 0, 24, 48, 72 and 96 h, respectively, and each time point was represented by three replicates. Following incubation, CCK-8 solution (10 µl) was added to each well and the cultures were incubated at 37°C for 90 min. The optical density values in the different wells were recorded using a microplate reader (Rayto Life and Analytical Science Co., Ltd, Shenzhen, China) at 450 nm.
Cell apoptosis
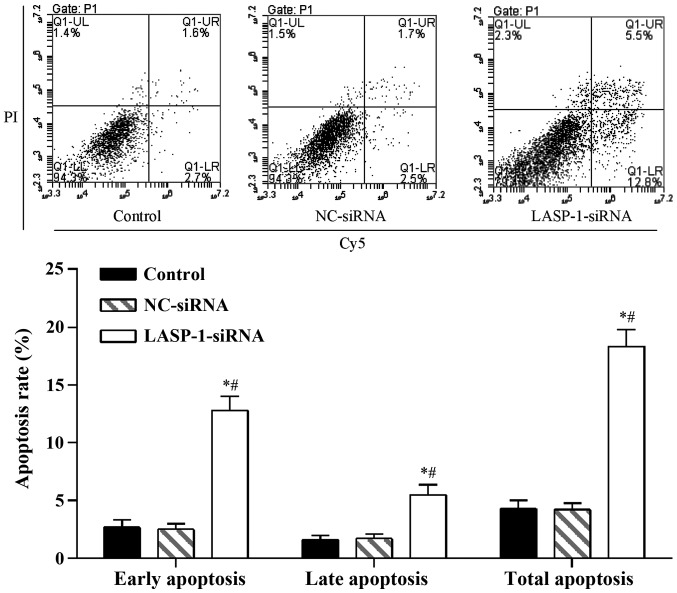
Cells with different treatments were seeded at 2×105 per 35-mm culture dish for 48 h. Apoptosis was assessed by Annexin V-Cy5 and propidium iodide (PI) staining (K103-25; BioVision, Milpitas, CA, USA) followed by fluorescence-activated cell sorting (FACS) analysis. The cells were pelleted and resuspended in Annexin V binding buffer (10 mM HEPES, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2; pH 7.4) containing Annexin V-Cy5 and 1 mg/ml PI. Following incubation at room temperature for 5 min, the cells were analyzed with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). The total apoptotic cell percentage of all cells was determined as UR + LR, which is the sum of the later apoptosis rate (UR, upper-right quadrant, showing advanced stage apoptosis cell percentage) and the early apoptosis rate (LR, lower-right quadrant, showing the prophase apoptosis cell percentage).
Cell migration
Cells with different treatments were incubated for 48 h and starved overnight, then 100 µl incubation medium (with 1 mM MgCl2) containing 1×105 cells was seeded in the upper chamber of bovine serum albumin-coated 8-µM pore size Transwell chambers (Corning, Cambridge, MA, USA). Cells were allowed to migrate through the porous membrane for 4 h at 37°C. Cells remaining on the upper surface were removed with a cotton carrier. The lower surfaces of the membranes were stained in a solution of 1% (w/v) crystal violet in 2% ethanol for 30 sec and then rinsed in distilled water. Cell-associated crystal violet was extracted by incubation in 10% acetic acid for 20 min. Non-migrated cells were removed with cotton swabs. Cell images were obtained under a phase-contrast microscope (Olympus, Tokyo, Japan).
Statistical analysis
All the data were expressed as the means ± standard deviation. Multiple comparisons were conducted using the least significant difference method; P<0.05 was considered to indicate a statistically significant difference. All statistical analyses were conducted using SPSS version 19.0 (IBM SPSS, Armonk, NY, USA).
Results
Expression of LASP-1 is significantly upregulated in tumor tissues
Based on the results of RT-qPCR, the relative expression of the LASP-1 gene in tumor tissues was significantly higher than that in para-carcinoma tissues (Fig. 1A). A similar pattern was observed in western blot analysis. In Fig. 1B, the expression of LASP-1 in four patients is illustrated, and the results reveal significantly enhanced synthesis of LASP-1 in the tumor tissues.
Figure 1.
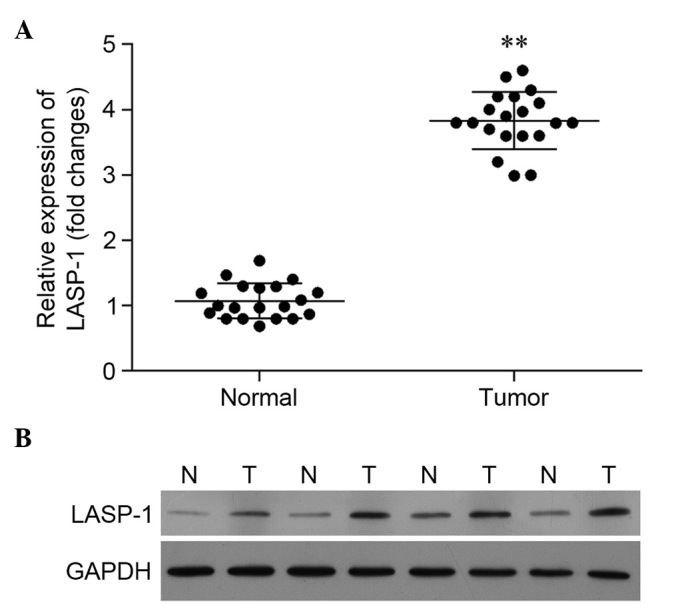
Reverse transcription-quantitative polymerase chain reaction and western blot analysis of the expression of LIM and SH3 protein 1 (LASP-1) gene in lung cancer tumor tissues (T) and normal para-carcinoma tissues (N).
siRNA transfection of LASP-1 gene blocks the synthesis of LASP-1
The human lung cancer cell line A549 was subjected to one of three different treatments and then the synthesis of LASP-1 was detected using western blot analysis. It was demonstrated that the stable transfection of LASP-1-siRNA significantly blocked the synthesis of LASP-1 compared with the other two treatments (Fig. 2).
Figure 2.
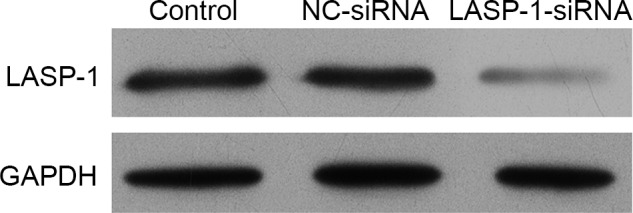
Western blot analysis of LIM and SH3 protein 1 (LASP-1) expression following siRNA tranfection. Transfection using LASP-1-specific siRNA significantly inhibited the synthesis of LASP-1. NC, negative control.
Downregulation of LASP-1 influences the proliferation, apoptosis and migration of A549 cell line
Proliferation of A549 cells was influenced by RNAi of LASP-1, and the effect was time-dependent; with the increase in treatment time, the negative regulatory effect of LASP-1-siRNA became evident (Fig. 3). A similar pattern was also observed in the FACS results, where RNAi with LASP-1-specific siRNA significantly increased the apoptosis percentage (18.3%) of the A549 cell line compared with the other two groups (4.3% for the control group and 4.2% for the NC-siRNA group) (Fig. 4). The results of CKK-8 assay and FACS were indicative of the induction of the autophagy process in lung cancer cells by downregulation of LASP-1.
Figure 3.
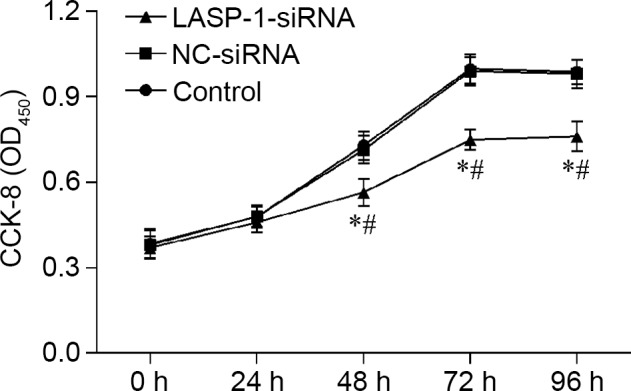
LIM and SH3 protein 1 (LASP-1)-specific siRNA treatment impairs the proliferative ability of A549 cell line. *Significantly different from control group, P<0.05; #significantly different from negative control (NC)-siRNA group, P<0.05.
Figure 4.
LIM and SH3 protein 1 (LASP-1)-specific siRNA treatment induces apoptosis in A549 cells. *Significantly different from control group, P<0.05; #significantly different from negative control (NC)-siRNA group, P<0.05.
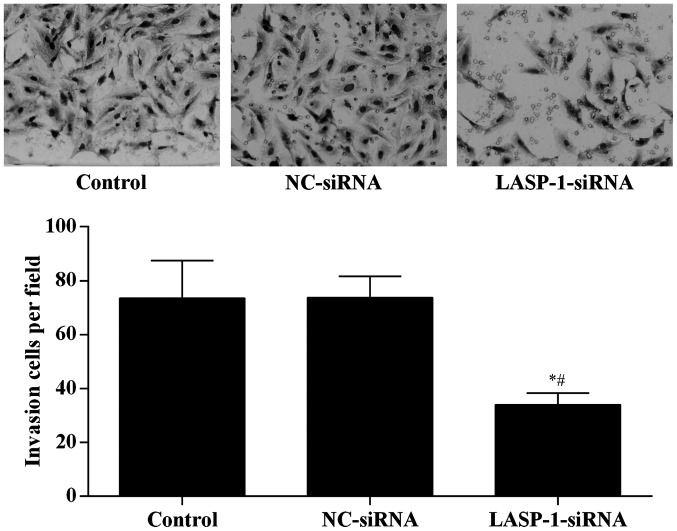
To directly examine the function of LASP-1 on the migration of A549 cells, Transwell assay was performed in a modified Boyden chamber with A549 cells transfected with different treatments. After 4 h, the number of A549 cells that had migrated through the porous membrane was recorded. Silencing of LASP-1 in A549 cells significantly reduced cell migration (Fig. 5), suggesting that LASP-1 was necessary for the metastasis of lung cancer cells.
Figure 5.
LIM and SH3 protein 1 (LASP-1)-specific siRNA treatment reduces the migration ability of A549 cells. *Significantly different from control group, P<0.05; #significantly different from negative control (NC)-siRNA group, P<0.05.
Discussion
Advances in our understanding of molecular genetics in lung cancer have led to the identification of key genetic aberrations, which have potential as therapeutic targets in the clinic. Currently, molecular subsets identified in lung cancer include mutation EGFR, KRAS, HER2, PIK3CA, BRAF and MET genes, and gene rearrangement in ALK, ROS1 and RET (11). Based on the identification of these driver oncogenes, lung cancer has been taken as a heterogeneous disease composed of various molecular subsets. In the present study, we investigated the expression of another tumor-associated factor, LASP-1, in lung cancer tissues for the first time. We observed that LASP-1 mRNA and protein levels were significantly increased in lung cancer tissues compared with para-carcinoma tissues. By silencing the expression of the LASP-1 gene in the human lung cancer A549 cell line, we established that the proliferation and migration ability of the cancer cells were notably weakened while the apoptotic process was induced, suggesting a key role of LASP-1 in the growth and metastasis of lung cancer. Our results were consistent with those previous studies, which revealed the significance of LASP-1 in the progression and metastasis of a variety of tumor types, including breast, colorectal, ovarian and hepatocellular cancer (14–18).
To test whether the decreased proliferation of the A549 cell line could be due to apoptosis, we conducted FACS analysis. Our data revealed significant cell death in response to LASP-1-specific siRNA treatment. However, our result was contrary to the conclusion of Grunewald et al (14), which demonstrated no notable influence of silencing of LASP-1 on the apoptotic process in BT-20 and MCF-7 cell lines. Although the two studies focused on different tumor types, the results were indicative of the complex mechanism of LASP-1 in regulating cancer cell apoptosis.
Cell migration requires temporal and spatial organization of signal transduction processes that regulate actin polymerization and focal adhesion turnover. To date, more than 50 different adhesion proteins regulating the rate and organization of actin polymerization and focal adhesion turnover have been identified. As a well-known focal adhesion adaptor protein, LASP-1 is closely associated with the cytoskeleton (12,19–21) and involved in cell migration and invasion. The molecule is capable of interacting and co-localizing with a series of focal adhesion proteins, including F-actin, zyxin and LPP. Since cells depleted of LASP-1 still attach to the extracellular matrix and form focal adhesions, it is hypothesized that LASP-1 plays a supportive role in focal adhesion dynamics rather than actual formation of the related structures (22). Thus, silencing of LASP-1 may cause a cellular localization change of its binding partners at the focal adhesion site and influence the migration ability of cells.
LSAP-1 has also been identified as a p53 target in a global screen of p53 binding sites (23). The mutation of p53 causes a loss of tumor suppression function, promoting cellular proliferation. These mutations in p53 tumor suppressor genes are reported in over 60% of lung cancer cases (24). A previous study clearly demonstrated that LASP-1 might be the link for p53 to mediate its function on the cytoskeleton and in turn alter cell behavior (25). As a result, the p53-LASP-1-focal adhesion plaque pathway may represent a potential mechanism by which LASP-1 modulates cell motility and growth. However, the expression of p53 was not investigated in the current study and so we are unable to explain the underlying interaction of p53 and LSAP-1 in lung cancer.
In summary, we quantified the expression of mRNA and protein of LASP-1 in lung cancer tissues for the first time and observed an abnormally high level of LASP-1 in lung cancer cells. After silencing the expression of LASP-1 in the human lung cancer cell line A549, we noted that the proliferation and migration ability of the cells were significantly decreased. Moreover, data from FACS analysis revealed the induction of apoptosis by silencing LASP-1 in the A549 cell line. Our results indicated the significant role of LASP-1 in regulating the growth and metastasis of lung cancer cells. Although we do not have a detailed explanation of the influence of LASP-1 on lung cancer, the present study inferred that the molecule had the potential to form a therapy for lung cancer in the clinic. To elaborate the role of LASP-1 in lung cancer, more comprehensive studies are due to be conducted in the future.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Singh CR, Kathiresan K. Molecular understanding of lung cancers - a review. Asian Pac J Trop Biomed. 2014;4:S35–S41. doi: 10.12980/APJTB.4.2014C597. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novaes FT, Cataneo DC, Junior RL Ruiz, Defaveri J, Michelin OC, Cataneo AJM. Lung cancer: histology, staging, treatment and survival. J Bras Pneumol. 2008;34:595–600. doi: 10.1590/S1806-37132008000800009. (In English and Portuguese) [DOI] [PubMed] [Google Scholar]
- 4.Youlden DR, Cramb SM, Baade PD. The international epidemiology of lung cancer: geographical distribution and secular trends. J Thorac Oncol. 2008;3:819–831. doi: 10.1097/JTO.0b013e31818020eb. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JL, Pillai S, Chellappan SP. Genetic and biochemical alterations in non-small cell lung cancer. Biochem Res Int. 2014;2012:940405. doi: 10.1155/2012/940405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodenhuis S, Slebos RJ, Boot AJ, Evers SG, Mooi WJ, Wagenaar SS, van Bodegom PC, Bos JL. Incidence and possible clinical significance of K-ras oncogene activation in adenocarcinoma of the human lung. Cancer Res. 1988;48:5738–5741. [PubMed] [Google Scholar]
- 7.Liu H, Radisky DC, Yang D, Xu R, Radisky ES, Bissell MJ, Bishop JM. MYC suppresses cancer metastasis by direct transcriptional silencing of αv and β3 integrin subunits. Nat Cell Biol. 2012;14:567–574. doi: 10.1038/ncb2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prins J, De Vries EG, Mulder NH. The myc family of oncogenes and their presence and importance in small-cell lung carcinoma and other tumour types. Anticancer Res. 1993;13:1373–1385. [PubMed] [Google Scholar]
- 9.Kurup A, Hanna NH. Treatment of small cell lung cancer. Crit Rev Oncol Hematol. 2004;52:117–126. doi: 10.1016/S1040-8428(04)00151-9. [DOI] [PubMed] [Google Scholar]
- 10.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Eastern Cooperative Oncology Group: Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 11.Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC) Respirology. 2015;20:370–378. doi: 10.1111/resp.12490. [DOI] [PubMed] [Google Scholar]
- 12.Chew CS, Chen X, Parente JA, Tarrer S, Okamoto C, Qin HY. Lasp-1 binds to non-muscle F-actin in vitro and is localized within multiple sites of dynamic actin assembly in vivo. J Cell Sci. 2002;115:4787–4799. doi: 10.1242/jcs.00174. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa H, Terasaki AG, Suzuki H, Ohashi K, Miyamoto S. Short-term retention of actin filament binding proteins on lamellipodial actin bundles. FEBS Lett. 2006;580:3223–3228. doi: 10.1016/j.febslet.2006.04.082. [DOI] [PubMed] [Google Scholar]
- 14.Grunewald TG, Kammerer U, Schulze E, Schindler D, Honig A, Zimmer M, Butt E. Silencing of LASP-1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp Cell Res. 2006;312:974–982. doi: 10.1016/j.yexcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L, Wang H, Liu C, Liu Y, Wang X, Wang S, Sun X, Li J, Deng Y, Jiang Y, Ding Y. Promotion of colorectal cancer growth and metastasis by the LIM and SH3 domain protein 1. Gut. 2010;59:1226–1235. doi: 10.1136/gut.2009.202739. [DOI] [PubMed] [Google Scholar]
- 16.Salvi A, Bongarzone I, Miccichè F, Arici B, Barlati S, De Petro G. Proteomic identification of LASP-1 down-regulation after RNAi urokinase silencing in human hepatocellular carcinoma cells. Neoplasia. 2009;11:207–219. doi: 10.1593/neo.81076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Li W, Jin X, Cui S, Zhao L. LIM and SH3 protein 1, a promoter of cell proliferation and migration, is a novel independent prognostic indicator in hepatocellular carcinoma. Eur J Cancer. 2013;49:974–983. doi: 10.1016/j.ejca.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Chiyomaru T, Enokida H, Kawakami K, Tatarano S, Uchida Y, Kawahara K, Nishiyama K, Seki N, Nakagawa M. Functional role of LASP1 in cell viability and its regulation by microRNAs in bladder cancer. Urol Oncol. 2012;30:434–443. doi: 10.1016/j.urolonc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Butt E, Gambaryan S, Göttfert N, Galler A, Marcus K, Meyer HE. Actin binding of human LIM and SH3 protein is regulated by cGMP-and cAMP-dependent protein kinase phosphorylation on serine 146. J Biol Chem. 2003;278:15601–15607. doi: 10.1074/jbc.M209009200. [DOI] [PubMed] [Google Scholar]
- 20.Li B, Zhuang L, Trueb B. Zyxin interacts with the SH3 domains of the cytoskeletal proteins LIM-nebulette and Lasp-1. J Biol Chem. 2004;279:20401–20410. doi: 10.1074/jbc.M310304200. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber V, Moog-Lutz C, Régnier CH, Chenard MP, Boeuf H, Vonesch JL, Tomasetto C, Rio MC. Lasp-1, a novel type of actin-binding protein accumulating in cell membrane extensions. Mol Med. 1998;4:675–687. [PMC free article] [PubMed] [Google Scholar]
- 22.Chew CS, Parente JA, Jr, Zhou CJ, Baranco E, Chen X. Lasp-1 is a regulated phosphoprotein within the cAMP signaling pathway in the gastric parietal cell. Am J Physiol. 1998;275:C56–C67. doi: 10.1152/ajpcell.1998.275.1.C56. [DOI] [PubMed] [Google Scholar]
- 23.Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 24.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Feng P, Xiao Z, Ren EC. LIM and SH3 protein 1 (Lasp1) is a novel p53 transcriptional target involved in hepatocellular carcinoma. J Hepatol. 2009;50:528–537. doi: 10.1016/j.jhep.2008.10.025. [DOI] [PubMed] [Google Scholar]