Abstract
Purpose
The purpose of this study was to explore how adults with prediabetes perceive their risk of developing diabetes and examine their preferences for evidence-based treatment options to prevent diabetes.
Methods
A qualitative study was conducted in 2 large Midwest primary care practices, involving in-depth semistructured interviews with 35 adult patients with prediabetes.
Results
This ethnically diverse (77% nonwhite) sample of middle-aged primary care patients exhibited multiple diabetes risk factors. Knowledge gaps about prediabetes and its medical management were pervasive. Most patients overestimated the risk of developing diabetes and were not familiar with evidence-based treatment options for prediabetes. They suggested that receiving brief, yet specific information about these topics during the study interview motivated them to act. The majority of participants considered both intensive lifestyle intervention and metformin acceptable treatment options. Many preferred initial treatment with intensive lifestyle intervention but would take metformin if their efforts at lifestyle change failed and their primary care physician recommended it. Some participants expressed wanting to combine both treatments.
Conclusions
This qualitative study highlights potential opportunities to promote patient-centered dialogue about prediabetes in primary care settings. Providing patients specific information about the risk of developing diabetes and evidence-based treatment options to prevent or delay its onset may encourage action. Physicians’ prediabetes counseling efforts should be informed by the finding that most patients consider both intensive lifestyle intervention and metformin acceptable treatment options.
An estimated 38% of adults in the United States are affected by prediabetes,1 which represents an elevation of plasma glucose above the normal range but below the diagnostic threshold for diabetes. Among the 86 million American adults with prediabetes,2 the risk of developing type 2 diabetes is high, estimated at 5% to 10% per year and 70% over a lifetime.3,4 Prediabetes is associated with an increased risk of cardiovascular disease,5 neuropathy,6 retinopathy,7 and death even before the onset of diabetes.8 The burden of prediabetes frames the importance of effective strategies to identify and manage this common metabolic disorder.
The US Preventive Services Task Force’s recent recommendation for abnormal blood sugar screening will identify many patients with prediabetes who could benefit from treatment to prevent or delay diabetes.9 Large clinical trials have established that structured, intensive lifestyle interventions (ILIs) and metformin can reduce the rate of developing diabetes by as much as 58% and 31%, respectively.10–13 Follow-up studies have reported long-term reductions in diabetes incidence with ILIs and metformin, in addition to reduced cardiovascular mortality and all-cause mortality with ILIs.14–16 Despite expert recommendations to use ILIs and metformin as effective treatments for prediabetes,17,18 recent reports show that neither treatment is being offered to the vast majority of high-risk Americans.19,20
Survey studies have provided some insight into challenges hindering the widespread adoption of ILIs and metformin. For adults with prediabetes, these include limited awareness of having the condition and inaccurate perceptions regarding their risk of developing diabetes.21–25 National surveys have also documented infrequent counseling by health care providers about prediabetes and its management.26,27 In addition, prior research examining how individuals’ perceived diabetes risk affects their engagement in behaviors to prevent diabetes has yielded inconclusive results.28–31 Qualitative research exploring patients’ perceptions, motivations, and preferences can complement these quantitative findings and help inform evidence-based prediabetes management in practice.32
With over 650 million primary care visits made by US adults annually, decisions about treatment for prediabetes will most commonly occur in this setting.33 Increasing the adoption of ILIs and metformin, thereby improving the quality of prediabetes care, requires understanding patient-centered perspectives that have not yet been studied. Therefore, the objectives of the current interview study were to explore how adults with prediabetes perceive their risk of developing diabetes and examine their preferences for evidence-based treatment options to prevent diabetes.
Methods
Research Design
A qualitative approach, through the use of in-depth, semistructured interviews, was used to examine patients’ knowledge and perceptions of prediabetes. The strength of this research design is that it provides a deeper understanding of patients’ perspectives and allows the researcher to explore the complexities of patients’ experiences in ways that quantitative methods alone do not.32 The study protocol was approved by the Northwestern University Institutional Review Board and the Erie Family Health Center Research Evaluation Committee.
Study Sample
Interviews were conducted with 35 adult primary care patients who had prediabetes. Two clinic sites contributed participants to this study, the majority of whom were recruited from a large, urban, academic primary care practice. To increase the diversity of the sample with respect to race/ethnicity and socioeconomic status, patients were also recruited from a large federally qualified health center serving a predominantly Latino population. Neither study site had clinical initiatives focused on identifying patients with prediabetes or offering treatment to those who have it.
Adult patients with prediabetes were identified by an experienced statistical analyst who queried diagnosis codes and laboratory results in clinics’ electronic health record systems for any of the following prediabetic states: impaired fasting glucose, impaired glucose tolerance, or elevated A1C.34 Potential participants also needed to express awareness of having prediabetes, which was assessed during a telephone screening. Other eligibility criteria were age 20 to 59 years and body mass index (BMI) ≥27 kg/m2. Patients with diagnosed diabetes and those who had a myocardial infarction, stroke, or cancer treatment in the previous year were excluded. The rationale for these inclusion and exclusion criteria was to select patients who could safely adopt ILIs or metformin for diabetes prevention.35 Written informed consent was obtained by the interviewer prior to the interview, and participants received $50 compensation for their time upon completion of the interview.
Data Collection
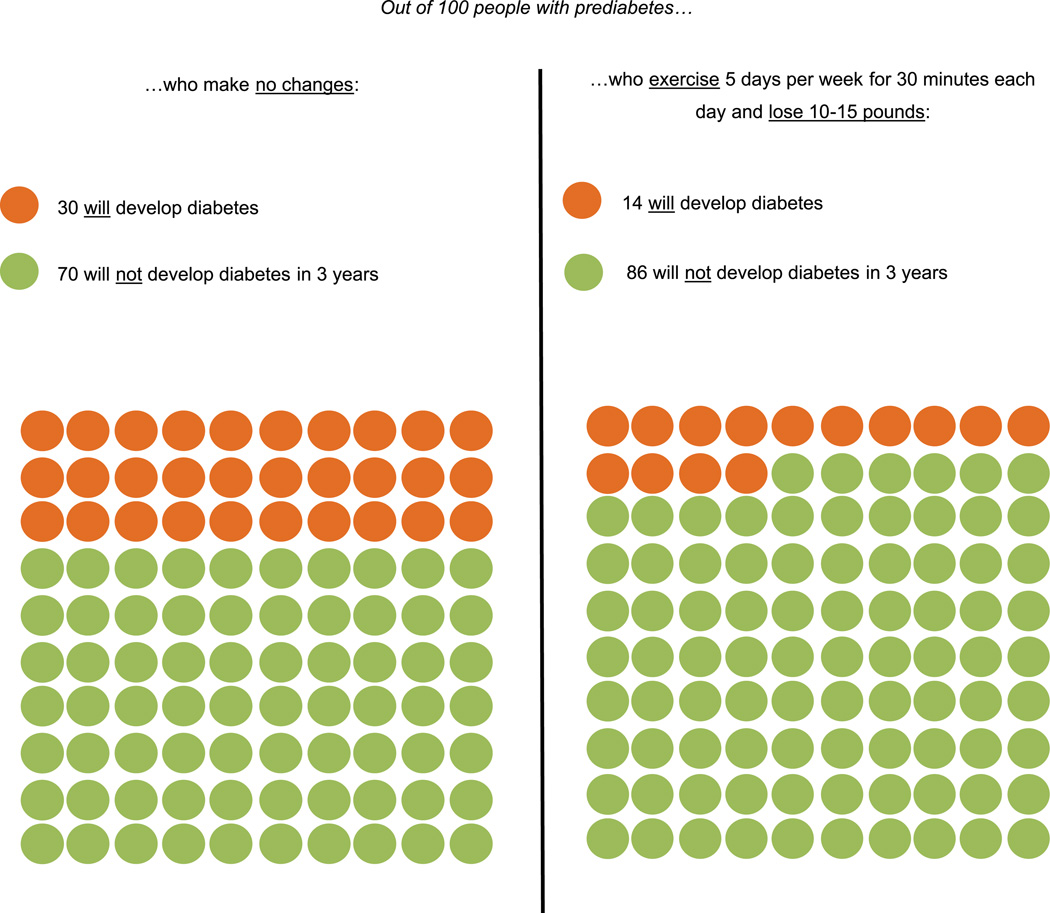
In-depth interviews were conducted to elicit the following patient perspectives: (1) knowledge about the risk of developing diabetes, (2) reactions to information about future diabetes risk with and without treatment, and (3) treatment preferences. The interview guide was informed by shared decision-making theory, which represents an effective, patient-centered framework for using evidence in routine clinical practice, including extensive application in diabetes care.36,37 Shared decision making has been defined as “an approach where clinicians and patients share the best available evidence when faced with the task of making decisions, and where patients are supported to consider options and to achieve informed preferences.”38 During the interview, patients were presented with visual depictions of diabetes risk without treatment, followed by separate images displaying the risk reduction associated with ILIs and metformin (Figures 1–3). Using 3-year data from the landmark Diabetes Prevention Program clinical trial,12 these materials were developed to convey risk information simply and facilitate conversation. Prior to participants viewing these images, the interviewer briefly described each treatment, including the principal goals of ILIs (7% weight loss and 150 min/wk of moderate-intensity physical activity). Interviews lasted approximately 45 minutes and were conducted in the participants’ preferred language (English or Spanish) by either the project manager, who had previous training and experience in qualitative data collection, or a bilingual medical student who was trained by the project manager to conduct the interviews. Data collection was continued until thematic saturation was reached.39 Each interview was digitally recorded and professionally transcribed verbatim. Spanish transcripts were professionally translated into English to enable analysis by all members of the investigative team. Two authors (M.R.M., M.C.V.) compared the transcripts with original audio recordings to ensure their accuracy.
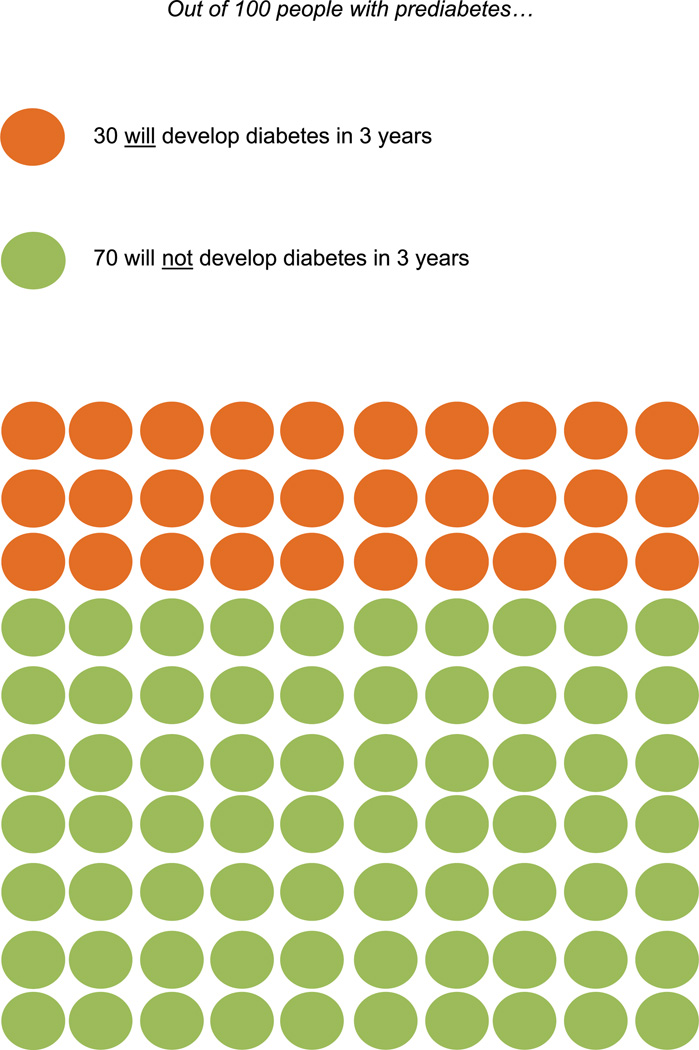
Figure 1.
Risk of developing diabetes without treatment.
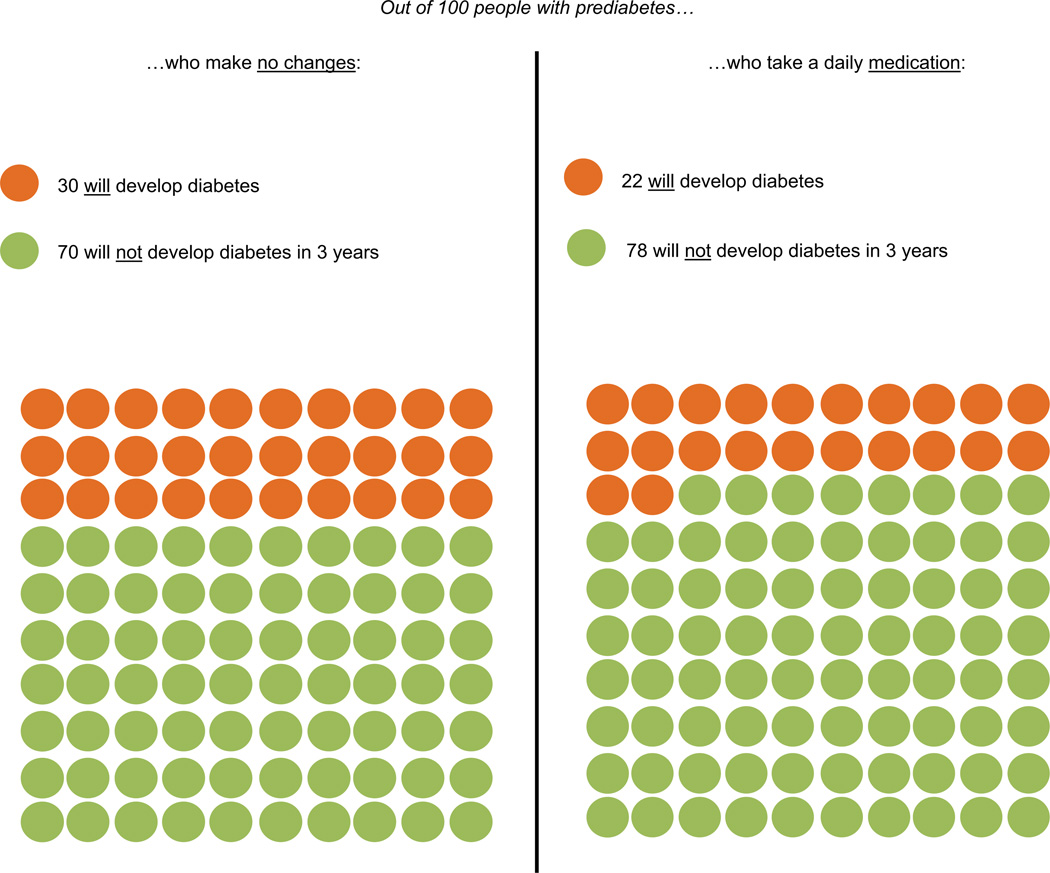
Figure 3.
Risk of developing diabetes on daily metformin.
Data Analysis
NVivo software (v. 9; NVivo, Victoria, Australia) was used to assist the investigative team with organizing and analyzing the qualitative data. Transcripts were analyzed using deductive and inductive content analysis simultaneously.40 Deductive content analysis began by grouping participant quotes into codes that followed predefined topics from the interview guide. Three study team members with previous qualitative research experience (M.J.O., M.R.M., and N.R.K.) developed a codebook after independently reviewing the same 6 transcripts. The resulting codebook included codes reflecting topics from the interview guide, in addition to previously unexpected topics identified inductively. These investigators used the codebook to review an additional 6 transcripts and then revised the codebook by consensus. When the codebook was finalized, at least 2 of these investigators reviewed the remaining 23 transcripts and organized participants’ responses by the corresponding codes. Common themes were developed inductively during face-to-face meetings, synthesizing participants’ responses across codes to reflect their values, needs, and preferences about prediabetes and its treatment. All members of the investigative team agreed on the final themes and the most representative quotes supporting them.
Results
All 35 study participants had prediabetes, and many had other diabetes risk factors (Table 1). Over three-fourths of the middle-aged participants were African American or Latino, and 71% had not completed college. Three major themes were identified that reflect participants’ perceptions about their diabetes risk and treatment preferences to prevent or delay diabetes: (1) multiple knowledge gaps about prediabetes and its treatment are pervasive, (2) evidence about diabetes risk and treatment options for prediabetes is motivating to patients, and (3) both ILIs and metformin are considered acceptable treatments.
Table 1.
Participant Characteristics (n = 35)
| Sociodemographic Characteristic | n (%) |
|---|---|
| Age, mean (range), y | 48 (38–58) |
| Female | 19 (54) |
| Race/ethnicity | |
| Hispanic | 14 (40) |
| Non-Hispanic black | 13 (37) |
| Non-Hispanic white | 8 (23) |
| Educational attainment | |
| ≤High school | 13 (37) |
| Some college | 12 (34) |
| ≥College | 10 (29) |
| Clinical characteristic | |
| Obesitya | 25 (71) |
| Physical inactivityb | 14 (40) |
| History of diabetes in first-degree relative | 25 (71) |
| History of gestational diabetesc | 1 (5) |
| Dyslipidemiad | 18 (51) |
| Hypertensiond | 22 (63) |
| Taking daily prescription medicationd | 27 (77) |
Obesity status is defined by a body mass index of ≥30 kg/m2 based on self-reported height and weight.
Defined by participants’ response to the following question: “Do you do physical activity or exercise at least 3 times/week?”
Percentage is calculated among women only.
History of dyslipidemia, hypertension, and prescription medication use is based on self-report.
Knowledge Gaps Are Pervasive
This diverse sample of primary care patients with pre-diabetes had little knowledge about the condition or its medical management. While all participants were aware of having prediabetes, some demonstrated remarkably limited understanding of its meaning: “I never heard about the higher chance of developing diabetes from prediabetes … I always heard about diabetes and thought it was something you either had or not, something like black and white.” Prior to the study interview, only 1 participant reported receiving specific information about the risk of developing diabetes. Almost all participants expressed surprise that approximately 30% of people with prediabetes will develop diabetes within 3 years, most having assumed this proportion would be higher: “I would have expected that the number of those [with prediabetes] who would actually develop diabetes would be much higher.” Some, however, believed diabetes was inevitable: “As soon as I hear prediabetes … I automatically think you are going to get [diabetes]. I think people usually go that way.” Many participants knew that lifestyle changes could lower their risk of diabetes, which some reported hearing from their primary care provider: “The doctor suggested to me to reduce the meals and to exercise. But just that.” Others had not received such counseling but conveyed an intuitive understanding of this fact: “I mean just logically I knew that if you lose some weight and exercise, obviously your chances would go down. But I did not know any of the statistics.” However, none of the participants had previous knowledge of evidence-based goals for weight loss and physical activity that are the cornerstone of ILIs. In addition, none knew about metformin as a treatment option to prevent or delay diabetes, but a few had heard of it as a treatment for diabetes: “I’ve heard that people who have type 2 diabetes can control it with medication. But I never heard that if you take a medication, it can prevent diabetes. I never heard that.”
Evidence About Prediabetes and Diabetes Prevention Is Motivating
While the participants knew very little about their risk of developing diabetes, they appreciated learning this information during the study interview. For some participants, it gave them hope that diabetes could be prevented:
With that information there, I know I can come back. I have to either get off the borderline or go overboard, so that means I’ve got to work hard.
There’s still some time and the time is now…. It encourages me more now seeing the numbers how I still have time. I feel a little better seeing the numbers and seeing that there is a possibility to just really prevent diabetes.
For others, learning about their risk of developing diabetes served as a wakeup call or motivator for adopting treatment:
I did not know the numbers, of course. But it’s sort of like a threat, I think, like a warning sign for me.
I think if being explained clearly the seriousness and how close you are to being a diabetic would be key in how serious I would be about [treatment].
Participants’ reactions to the evidence about treatments to prevent diabetes were also positive. Learning the risk reduction associated with ILIs and metformin seemed to encourage participants to take action. Some expressed that this information would enhance their confidence to tackle prediabetes: “This [information] is letting me know that I can beat this … that I can try to develop an exercise and eating plan that will get me back to being almost normal, almost a normal human being.” Others suggested that hearing the evidence about diabetes prevention treatments persuaded them to act, which they may not have previously considered: “It changes in terms of how serious I take the medicine or the lifestyle change…. Before, I was kind of leaning toward, ‘If [diabetes] is going to come, let it be here.’ And now I am saying I guess I better figure this out.” The fact that modest weight loss can substantially reduce diabetes risk was particularly motivating to some participants, who felt they should learn this from their primary care provider: “These are things that should be shared with patients because it doesn’t seem like it would be difficult to lose 10 or 15 pounds. Sometimes when you are told you need to lose weight, you immediately go to the big number… and it feels like it is impossible.” The desire for more information about how to prevent diabetes was common: “Information never hurts and it is nice to know that there are other options besides diet and exercise. Once again, you have to look at the complete picture when you are making decisions on what to do.” Several participants asked if there would be additional risk reduction from adopting both treatments simultaneously.
ILIs and Metformin Are Acceptable Treatment Options
Most participants preferred ILIs as their first choice to prevent or delay diabetes. They cited some of the following perceptions about ILIs to support this preference: (1) it has additional benefits beyond lowering diabetes risk, (2) it constitutes “personal responsibility,” and (3) it is a “natural way” for managing prediabetes:
(1) “I think that you feel much better when you work out and eat better, as opposed to taking a pill where you are really not doing anything proactively to change the situation.”
(2) “I’m taking the position of more aggressive personal responsibility [with lifestyle changes] because I can, and I want to explore that potential first. But if I do all those things and my physical structure and DNA just says, ‘Man, you’re predisposed and it’s linked in that DNA chain,’ then I’d be all over [metformin] and be on board with it.”
(3) “I would prefer to exercise more and change my diet. I would probably want to try the natural way for a month or two because that is kind of where I am already anyway. So it would support what I am starting now anyway.”
Some participants chose ILIs as their preferred initial treatment because it is associated with a greater risk reduction than metformin, and they wished to avoid medication unless it was necessary: “You have a better chance with working out and losing weight than you would with the medication … I prefer not to take any medication, because I don’t really take medication now for anything. But if my doctor said, ‘I think you need to,’ then I would take it. If she said ‘I need you to take metformin every day,’ then I would say ‘okay.’”
While many patients preferred to begin treatment with ILIs, most believed that they would take metformin if their lifestyle efforts failed and their primary care provider recommended it:
If I can’t lose the weight and I’m at risk for developing diabetes, then I would take the medication because the alternative is worse…. At a certain point I have to be realistic. If I’m not making enough progress to affect my blood sugar [with lifestyle changes], then yes, I would take the medication.
I would fight it for a period of time until my doctor said, “You’re really not making enough progress, I think you should try this alternative.” And then I would try [metformin].
Several participants mentioned that the possibility of losing a small amount of weight was a motivation to take metformin: “Possibly, what is impressive about metformin is weight loss … I am so used to medications causing weight gain, to hear you say possible weight loss is like, ‘Wow, that is different.’” Questions about the recommended duration of metformin therapy, in addition to its safety, side effects, and cost, were common. Some participants noted that they would use metformin as an adjunct to intensive lifestyle therapy, because they presumed the combination of treatments may reduce diabetes risk more than either treatment alone:
We need to eat well not only due to diabetes. We need to have a complete healthy body, no? Since there are no serious side effects and this can help you escape the disease, I think [metformin] would work well together with [lifestyle changes] to have a complete healthy body.
If you’re going to take the medication and you stop exercising or not having a good nutritive diet, I would say “What’s the point?” If I take the medication, I’m telling you, I will make the combination of exercise and nutrition so it makes the effect better.
Of these, some participants wanted to take both treatments initially, while others would add metformin to lifestyle change if they did not achieve desired weight loss or glycemic outcomes. Only 3 participants reported an unwillingness to take metformin for diabetes prevention under any circumstances. Two of these were generally opposed to taking medicine, and the other was not convinced by the risk reduction associated with metformin.
Discussion
These interviews present some of the first evidence describing patients’ perspectives about prediabetes and its treatment. The findings suggest that these individuals have inaccurate perceptions of diabetes risk and incomplete knowledge of treatment options. Patients with pre-diabetes wanted specific information on these topics, which may encourage them to take action to prevent or delay diabetes. This study is the first to report that most primary care patients are willing to adopt ILIs and met-formin to lower their diabetes risk, which should encourage shared decision-making approaches by patients and health care providers.
Patient-centered research about prediabetes is limited by low levels of awareness among those who have the condition.21 While participants who were aware of having prediabetes were included in this study, they had little previous knowledge about the magnitude of their diabetes risk or the corresponding time horizon for developing diabetes. Most participants overestimated the risk of developing diabetes. Other studies of adults with prediabetes have also reported poor knowledge about the likelihood of developing diabetes,22–25 which may affect their motivation to adopt treatment and the effectiveness of treatment.41,42 We did not observe that participants who overestimated their diabetes risk were less motivated to prevent diabetes. A large body of research suggests that individuals’ perceived risk of disease shapes their preventive health behavior. However, this literature has identified several dimensions of perceived risk that influence behavior, including patients’ perceived susceptibility to the disease and its severity, in addition to the likelihood of developing it.43
This study found that patients with prediabetes have little knowledge about how to manage the condition. Consistent with previous research, many participants reported a general awareness that lifestyle changes can help prevent or delay diabetes.44,45 However, none was aware of evidence-based goals for lifestyle change or the reduction in diabetes risk expected from achieving those goals. Given that setting specific goals is a central strategy for changing health behavior,46 lacking this information may hinder individuals’ diabetes prevention efforts. Participants’ knowledge about metformin as a treatment option for prediabetes was similarly poor. The only other study examining primary care patients’ awareness of medications for diabetes prevention also found that it was low.30 These prominent knowledge gaps threaten shared decision making in prediabetes, which requires that patients have accurate information about their condition, its associated health risks, and evidence-based treatment options.38
Existing research suggests that providers rarely discuss prediabetes during primary care encounters.26,27,47 Studies on weight loss counseling in primary care have found that lack of time is the most important barrier.48 In this context, primary care providers cannot deliver ILIs for diabetes prevention during regular office visits, and there is currently no evidence supporting this practice. Therefore, primary care providers’ role is limited to providing brief counseling about prediabetes, eliciting patients’ treatment preferences, and prescribing or referring them for treatment. This is analogous to how other cardiovascular risk factors are managed in primary care.49–51 The information about prediabetes and diabetes prevention contained in the interview guide was brief, taking <3 minutes to administer, and was understood by patients from diverse backgrounds. Providing brief informational interventions about prediabetes is likely feasible and replicable during primary care visits, compared with in-depth lifestyle counseling, which is estimated to take at least 12 minutes in this setting.52 Even brief information offered during study interviews seemed to motivate participants to take action. Previous research suggests that simply delivering a diagnosis of obesity promotes patients’ weight loss attempts.53 The impact of provider-delivered information or brief counseling about prediabetes has not been investigated.
While this study found that patients with prediabetes consider both ILIs and metformin acceptable treatments to prevent or delay diabetes, their use is infrequent in practice.19,20 Some evidence supports the finding that patients with prediabetes find these treatments acceptable and may use them for this purpose. For example, a recent pragmatic trial demonstrated that the majority of participants with prediabetes who were offered ILIs participated at least minimally and achieved clinically significant weight loss.54 While previous studies have not examined patients’ perceptions of taking metformin to prevent diabetes, the widespread use of aspirin for the primary prevention of myocardial infarction suggests that many patients are willing to take daily medication for a preventive indication.55 Aspirin and metformin are both inexpensive, have similar side effects, and are associated with a comparable risk reduction for their respective outcome.56,57
This study makes a unique contribution to the diabetes prevention literature but has the following limitations. The qualitative interviews provide rich descriptions of participants’ perceptions and motivations related to prediabetes but do not allow us to predict their future behavior or generalize to broad populations. Only patients who were aware of having prediabetes were included to avoid the possibility of nonclinical study staff being the first to inform them about this diagnosis. Reactions to risk information and treatment preferences may differ among patients who are unaware of having the condition. Self-selection bias may have affected the findings about participants’ motivation to address prediabetes.58 Finally, this study was not designed to empirically develop or test strategies for delivering information about prediabetes and engaging in dialogue about treatment. Future research in this area is needed.
Conclusions/Future Directions
Given new diabetes screening guidelines that will identify greater numbers of individuals with prediabetes, this study highlights potential opportunities for counseling and discussing this condition with patients. First, giving patients specific information about their risk of developing diabetes and the expected risk reduction associated with preventive treatments is an untapped opportunity that may encourage action. Participants expressed interest in adopting prediabetes treatments after discussing their personal values, needs, and preferences with the interviewer. While primary care providers may face barriers to engaging patients in the same way, these findings suggest a benefit to patient-centered dialogue about these topics that incorporates the best available evidence. This personalized approach to helping patients make treatment decisions is already recommended for managing dyslipidemia and hypertension, as suggested by new guidelines and evidence.59,60
Implications
This study also has implications for research to promote the adoption of diabetes prevention treatments in practice. Because ILI programs are most often delivered outside of health care settings,61 future research is needed to promote patient engagement with community-based ILI programs and to develop effective linkages between primary care providers and delivery sites. Increasing the use of metformin for diabetes prevention will require understanding barriers to its use among both patients and providers. Interestingly, some participants sought information about the recommended duration of metformin and the incremental benefit of combined treatment with ILIs. Neither of these questions can be answered definitively using existing evidence and suggest future directions for diabetes prevention research. Ongoing policy developments, including a recent decision by Medicare to reimburse ILI delivery,62 may present opportunities for natural experiments while augmenting individual efforts to prevent or delay diabetes.
Figure 2.
Risk of developing diabetes with intensive lifestyle intervention.
Acknowledgments
We acknowledge Robert Whitaker, MD, MPH, and Marshall Chin, MD, MPH, for their thoughtful comments on an earlier draft of the manuscript and Teresa Gomez, MD, MPH, for her role in data collection. We also thank the study participants for the time the invested in this study and for the valuable information they contributed.
Funding: This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases through the following grants: the Chicago Center for Diabetes Translation Research Pilot and Feasibility grant program P30-DK092949 (Dr Kandula, principal investigator) and K23-DK095981 (Dr O’Brien, principal investigator).
Footnotes
Conflict of Interest: Dr Kandula received salary support from the American Medical Association that was not related to her participation in this study. The other authors have no potential conflicts of interest.
Disclosures: An abstract based on this article was presented at the Society for General Internal Medicine Annual Meeting on May 12, 2016.
References
- 1.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates and Its Burden in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 3.Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007;78(3):305–312. doi: 10.1016/j.diabres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance implications for care. Diabetes Care. 2007;30(3):753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55(13):1310–1317. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 6.Bongaerts BW, Rathmann W, Kowall B, et al. Postchallenge hyperglycemia is positively associated with diabetic polyneuropathy: the KORA F4 study. Diabetes Care. 2012;35(9):1891–1893. doi: 10.2337/dc11-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen TT, Wang JJ, Wong TY. Retinal vascular changes in prediabetes and prehypertension: new findings and their research and clinical implications. Diabetes Care. 2007;30(10):2708–2715. doi: 10.2337/dc07-0732. [DOI] [PubMed] [Google Scholar]
- 8.Brutsaert EF, Shitole S, Biggs ML, et al. Relations of postload and fasting glucose with incident cardiovascular disease and mortality late in life: the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2016;71(3):370–377. doi: 10.1093/gerona/glv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siu AL. Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(11):861–868. doi: 10.7326/M15-2345. [DOI] [PubMed] [Google Scholar]
- 10.Pan XR, Li G, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 11.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 12.Knowler W, Barrett-Connor E, Fowler S, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran A, Snehalatha C, Mary S, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49(2):289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 14.Knowler W, Fowler S, Hamman R, et al. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2(6):474–480. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 16.Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Prevention or delay of type 2 diabetes. Diabetes Care. 2015;38(suppl):S31–S32. doi: 10.2337/dc15-S008. [DOI] [PubMed] [Google Scholar]
- 18.LeFevre ML. US Preventive Services Task Force. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(8):587–593. doi: 10.7326/M14-1796. [DOI] [PubMed] [Google Scholar]
- 19.Moin T, Li J, Duru OK, et al. Metformin prescription for insured adults with prediabetes from 2010 to 2012: a retrospective cohort study. Ann Intern Med. 2015;162(8):542–548. doi: 10.7326/M14-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.YMCA’s Diabetes Prevention Program. Diabetes Prevention Program FACT SHEET: November 2015. Chicago, IL: YMCA of the USA; 2015. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Awareness of predia-betes: United States, 2005–2010. MMWR Morb Mortal Wkly Rep. 2013;62(11):209–212. [PMC free article] [PubMed] [Google Scholar]
- 22.Jones EJ, Roche CC, Appel SJ. A review of the health beliefs and lifestyle behaviors of women with previous gestational diabetes. J Obstet Gynecol Neonatal Nurs. 2009;38(5):516–526. doi: 10.1111/j.1552-6909.2009.01051.x. [DOI] [PubMed] [Google Scholar]
- 23.Maty SC, Tippens KM. Perceived and actual diabetes risk in the Chinese and Hispanic/Latino communities in Portland, OR, USA. Diabetes Med. 2011;28(6):658–667. doi: 10.1111/j.1464-5491.2010.03193.x. [DOI] [PubMed] [Google Scholar]
- 24.Gallivan J, Brown C, Greenberg R, Clark CM. Predictors of perceived risk of the development of diabetes. Diabetes Spectr. 2009;22(3):163–169. [Google Scholar]
- 25.Harwell TS, Dettori N, Flook BN, et al. Preventing type 2 diabetes: perceptions about risk and prevention in a population-based sample of adults > or =45 years of age. Diabetes Care. 2001;24(11):2007–2008. doi: 10.2337/diacare.24.11.2007. [DOI] [PubMed] [Google Scholar]
- 26.Geiss LS, James C, Gregg EW, et al. Diabetes risk reduction behaviors among U.S. adults with prediabetes. Am J Prev Med. 2010;38(4):403–409. doi: 10.1016/j.amepre.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Dorsey R, Songer T. Lifestyle behaviors and physician advice for change among overweight and obese adults with prediabetes and diabetes in the United States, 2006. Prev Chronic Dis. 2011;8(6):A132. [PMC free article] [PubMed] [Google Scholar]
- 28.Gopalan A, Lorincz IS, Wirtalla C, Marcus SC, Long JA. Awareness of prediabetes and engagement in diabetes risk-reducing behaviors. Am J Prev Med. 2015;49(4):512–519. doi: 10.1016/j.amepre.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Grant RW, O’Brien KE, Waxler JL, et al. Personalized genetic risk counseling to motivate diabetes prevention: a randomized trial. Diabetes Care. 2013;36(1):13–19. doi: 10.2337/dc12-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hivert MF, Warner AS, Shrader P, Grant RW, Meigs JB. Diabetes risk perception and intention to adopt healthy lifestyles among primary care patients. Diabetes Care. 2009;32(10):1820–1822. doi: 10.2337/dc09-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang M, Valdez R, Ned RM, et al. Influence of familial risk on diabetes risk-reducing behaviors among U.S. adults without diabetes. Diabetes Care. 2011;34(11):2393–2399. doi: 10.2337/dc11-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritholz MD, Beverly EA, Weinger K. Digging deeper: the role of qualitative research in behavioral diabetes. Curr Diabetes Rep. 2011;11(6):494–502. doi: 10.1007/s11892-011-0226-7. [DOI] [PubMed] [Google Scholar]
- 33.Hing E, Uddin S. Visits to primary care delivery sites: United States, 2008. NCHS Data Brief. 2010;(47):1–8. [PubMed] [Google Scholar]
- 34.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2016;39(suppl 1):S13–S22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 35.Diabetes Prevention Program Research Group. The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stiggelbout AM, Van der Weijden T, De Wit M, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256. doi: 10.1136/bmj.e256. [DOI] [PubMed] [Google Scholar]
- 37.Tamhane S, Rodriguez-Gutierrez R, Hargraves I, Montori V. Shared decision-making in diabetes care. Curr Diabetes Rep. 2015;15(12):1–10. doi: 10.1007/s11892-015-0688-0. [DOI] [PubMed] [Google Scholar]
- 38.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandelowski M. Sample size in qualitative research. Res Nurs Health. 1995;18(2):179–183. doi: 10.1002/nur.4770180211. [DOI] [PubMed] [Google Scholar]
- 40.Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis (Introducing Qualitative Methods Series) Thousand Oaks, CA: Sage; 2006. [Google Scholar]
- 41.Pinelli NR, Herman WH, Brown MB, Jaber LA. Perceived risk and the willingness to enroll in a diabetes prevention lifestyle intervention in Arab-Americans. Diabetes Res Clin Pract. 2010;90(2):e27–e29. doi: 10.1016/j.diabres.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grover SA, Lowensteyn I, Joseph L, et al. Patient knowledge of coronary risk profile improves the effectiveness of dyslipidemia therapy: the CHECK-UP study: a randomized controlled trial. Arch Intern Med. 2007;167(21):2296–2303. doi: 10.1001/archinte.167.21.2296. [DOI] [PubMed] [Google Scholar]
- 43.Glanz K, Rimer BK, Viswanath K. Theory, research, and practice in health behavior and health education. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. San Francisco, CA: Jossey-Bass; 2008. [Google Scholar]
- 44.Rosal MC, Borg A, Bodenlos JS, Tellez T, Ockene IS. Awareness of diabetes risk factors and prevention strategies among a sample of low-income Latinos with no known diagnosis of diabetes. Diabetes Educ. 2011;37(1):47–55. doi: 10.1177/0145721710392247. [DOI] [PubMed] [Google Scholar]
- 45.Cullen KW, Buzek BB. Knowledge about type 2 diabetes risk and prevention of African-American and Hispanic adults and adolescents with family history of type 2 diabetes. Diabetes Educ. 2009;35(5):836–842. doi: 10.1177/0145721709341851. [DOI] [PubMed] [Google Scholar]
- 46.Strecher VJ, Seijts GH, Kok GJ, et al. Goal setting as a strategy for health behavior change. Health Educ Q. 1995;22(2):190–200. doi: 10.1177/109019819502200207. [DOI] [PubMed] [Google Scholar]
- 47.Yang K, Lee YS, Chasens ER. Outcomes of health care providers’ recommendations for healthy lifestyle among U.S. adults with prediabetes. Metab Syndr Relat Disord. 2011;9(3):231–237. doi: 10.1089/met.2010.0112. [DOI] [PubMed] [Google Scholar]
- 48.Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995;24(6):546–552. doi: 10.1006/pmed.1995.1087. [DOI] [PubMed] [Google Scholar]
- 49.Hyman DJ, Pavlik VN. Self-reported hypertension treatment practices among primary care physicians: blood pressure thresholds, drug choices, and the role of guidelines and evidence-based medicine. Arch Intern Med. 2000;160(15):2281–2286. doi: 10.1001/archinte.160.15.2281. [DOI] [PubMed] [Google Scholar]
- 50.Bertoni AG, Bonds DE, Steffes S, et al. Quality of cholesterol screening and management with respect to the National Cholesterol Education’s Third Adult Treatment Panel (ATPIII) guideline in primary care practices in North Carolina. Am Heart J. 2006;152(4):785–792. doi: 10.1016/j.ahj.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 51.Hill JO, Wyatt H. Outpatient management of obesity: a primary care perspective. Obes Res. 2002;10(suppl 2):124S–130S. doi: 10.1038/oby.2002.205. [DOI] [PubMed] [Google Scholar]
- 52.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Post RE, Mainous AG, III, Gregorie SH, et al. The influence of physician acknowledgment of patients’ weight status on patient perceptions of overweight and obesity in the United States. Arch Intern Med. 2011;171(4):316–321. doi: 10.1001/archinternmed.2010.549. [DOI] [PubMed] [Google Scholar]
- 54.Ackermann RT, Liss DT, Finch EA, et al. A randomized comparative effectiveness trial for preventing type 2 diabetes. Am J Public Health. 2015;105(11):2328–2334. doi: 10.2105/AJPH.2015.302641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101(10):1206–1218. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 56.Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149–157. e142. doi: 10.1016/j.amjmed.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 57.Juul-Moller S, Edvardsson N, Jahnmatz B, et al. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Lancet. 1992;340(8833):1421–1425. doi: 10.1016/0140-6736(92)92619-q. [DOI] [PubMed] [Google Scholar]
- 58.Seawright J, Gerring J. Case selection techniques in case study research: a menu of qualitative and quantitative options. Polit Res Q. 2008;61(2):294–308. [Google Scholar]
- 59.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;129(25 suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 60.SPRINT Research Group. Wright JT, Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ackermann RT. Working with the YMCA to implement the Diabetes Prevention Program. Am J Prev Med. 2013;44(4 suppl 4):S352–S356. doi: 10.1016/j.amepre.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Pear R. Medicare proposal takes aim at diabetes. [Accessed May 16, 2016];New York Times. 2016 http://www.nytimes.com/2016/03/23/us/politics/medicare-proposal-takes-aim-at-diabetes.html?_r=0.