Abstract
The present study aimed to evaluate the efficacy and safety of acetyl-L-carnitine (ALC) for the treatment of chemotherapy-induced peripheral neuropathy (CIPN). The study was carried out as a prospective, randomized, double-blind, placebo-controlled and paralleled clinical study. A total of 239 patients with CIPN were selected as the study subjects. Of the 239 subjects, 118 subjects received 3 g/day ALC orally for 8 weeks and 121 received a placebo. The primary endpoint was improvement of peripheral neuropathy by at least one grade. Patient status was assessed at week 4, 8 and 12 after enrollment into the study. In both the full analysis set (FAS) and the per-protocol set (PPS), peripheral sensory neuropathy was significantly ameliorated in the ALC group with 50.5 and 51.6% patients meeting the primary endpoint at week 8, compared with 24.1 and 23.1% of patients in the placebo group (P<0.001 in both sets). Secondary endpoints, such as the nerve electrophysiological examination and the Karnofsky physical score were also significantly improved in patients receiving ALC treatment, as compared with the placebo group (FAS, P=0.0463 and P=0.022; PPS, P=0.0076 and P=0.0064, respectively). Cancer-associated fatigue was significantly alleviated following ALC treatment in the PPS (P=0.0135). In the safety analysis set, the difference in adverse events incidence between the two groups was not statistically significant (P=0.3903). There were only two severe adverse events in the ALC group, which were not associated with the effect of ALC. In conclusion, the results of the present study demonstrated that in Chinese patients with cancer, oral administration of ALC is effective at ameliorating peripheral sensory neuropathy induced by chemotherapy, as well as reducing of cancer-associated fatigue and improving physical conditions.
Keywords: acetyl-L-carnitine, chemotherapy-induced peripheral neuropathy, cancer-associated fatigue, adverse events, sensory neuropathy
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common, dose-limiting adverse drug reaction in cancer treatment (1), which primarily presents as varying degrees of motor and sensory deficits, as well as autonomic dysfunction. Currently, paclitaxel, cisplatin, and vinblastine are the most commonly prescribed anti-cancer chemotherapy drugs (2). Unfortunately, these drugs all produce treatment-limiting peripheral neuropathy, for which there is no reliable clinical intervention. The primary treatment of CIPN is to reduce the chemotherapy dose and to extend the interval between treatments, or cease treatment completely (3). However, this is not an optimal choice for the long-term prognosis of the patient.
Acetyl-L-carnitine (ALC) is a nutrient supplement with the ability to stimulate the expression of nerve growth factor receptor, strengthen the tubulin of nerve cells and prevent cytoskeletal damage and cystic nerve fibrosis, as well as improve sensory nerve conduction (4,5). In addition, numerous basic and clinical studies have demonstrated that ALC alleviates CIPN without reducing the antitumor drug activity (6–8).
Sigma Tau Pharmaceuticals, Inc. developed levocarnitine acetate hydrochloride gastro-resistant tablets (Nicetile®), which is an oral drug that first appeared on the Italian market in July 1984, with peripheral nerve or nerve root mechanisms of action and inflammatory injury as the registered indication. However, the effects of Nicetile® in Chinese individuals with CIPN remains to be elucidated. The aim of the present study was to investigate the efficacy and safety of levocarnitine acetate hydrochloride gastro-resistant tablets on CIPN in a large Chinese population.
Materials and methods
Study design and approval
This study was a multicenter, randomized, double-blind, and placebo-controlled phase II clinical trial. It was approved by the Chinese State Food and Drug Administration (approval no. 2007L03540). The clinical trial registration number is NCT01526564. The clinical study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. In addition, informed consent was obtained from all participants involved in this study.
Eligible patients were aged 18–75 years without gender limitation. Eligibility criteria included: Grade ≥3 neuropathy, as determined by NCI-CTC criteria version 3.0 (9), while receiving paclitaxel, cisplatin or vinblastine treatment, and/or grade ≥2 neuropathy persisting for at least one month after the discontinuation of either drug, and neurotoxicity for <6 months; at least one abnormality on electrophysiological examination; Karnofsky physical score (KPS) of ≥60; absolute neutrophil count of ≥1.5×109/l, hemoglobin count of ≥80 g/l, platelet count of ≥75×109/l, total bilirubin counts of 1.5-fold less than normal value, glutamic-pyruvic transaminase (GPT/ALT) and glutamic-oxalacetic transaminease (GOT/AST) no more than 2.5-fold greater than the normal value; normal blood urea nitrogen, serum creatinine and electrocardiogram (ECG) findings. During the study, the use of steroids, analgesic or neuroprotectant drugs was not permitted. Patients were enrolled after providing written informed consent.
Exclusion criteria included: Neuropathy caused by other antineoplastic treatment except paclitaxel, cisplatin or vinblastine; pre-existing diabetes mellitus and/or neuropathy caused by vitamin deficiency, infection, trauma, poisoning, oppression, ischemia, metabolic disorders; genetic neuropathy and/or peripheral sensory nerve dysfunction due to central nervous system lesions; use of other drug therapy for neuropathy in the last 30 days (such as nerve growth factor, amifostine reduced glutathione, vitamin E or B, glucocorticoids, ethosuximide, carbamazepine, gabapentin, sodium thiosulfate, glutamic acid, lamotrigine, α-fatty acid, lithium salt, lithium salt or magnesium salt); participation in other clinical trials in the past 30 days; out of control clinical problems (such as serious mental, nerve, cardiovascular and/or respiratory system disease); pregnant or lactating women; and poor compliance.
During the trial, patients were withdrawn if serious adverse events occurred, and/or the patient became pregnant.
Patients
A total of 240 patients met the criteria and were recruited for the present study at Shanghai Changzheng Hospital (Shanghai, China) between September 4, 2010 and November 7, 2013. All patients were treated with at least one type of taxoid, either satraplatin (80 mg/m2; GPC Biotech, Munich, Germany) or vincristine (0.05 mg/kg2; Pharmachemic Hisun, Zhejiang, China). Their peripheral sensory neuropathy grading following chemotherapy was ≥ grade 3 or 2, lasted for ≥ 1 month, and the course of neuropathy was ≤6 months. The patient either no longer required chemotherapy or did not choose to undergo chemotherapy. Each patient was administered one ALC hydrochloride enteric-coated tablet (oral administration; 500 mg/tablet, 2 tablets/time) three times a day for eight consecutive weeks. The patients of the control group received a placebo (lactose, 500 mg/tablet, 2 tablets/time) in the same manner. Patients were instructed that the interval between doses should be at ≥4 h and were monitored at week 4, 8 and 12. Since drug treatment ceased after week 8, the results obtained after week 12 represented the long-term effects of ALC intervention.
Safety analysis set (SS) was an analysis in those who received at least one dose of the investigational drug and safety valuation. Full analysis set (FAS) was analysis in which patients are included in the group to which they were randomized irrespective of compliance, administrative errors (such as error in eligibility), or other protocol deviations. Per protocol set (PPS) was an analysis in which patients are included in the group corresponding to the treatment they actually received. Patient compliance and “switchovers” were considered in the analysis.
Primary endpoint
The primary endpoint of the study was to demonstrate an improvement of ≥1 grade in neurotoxicity, as determined by NCI-CTC criteria (version 3.0) at week 8 of ALC administration (9).
Secondary endpoints
Prior to ALC administration at 4, 8 and 12 weeks, electrophysiological examinations were carried out at each time point, which included examination of the nerves (handedness, median nerve, ulnar nerve, common peroneal nerve, tibial nerve, superficial peroneal nerve and sural nerve). Three neural electricity experts compared the electrophysiology examination results at the 8th week (including nerve conductive velocity, latency period and amplitude) to the results obtained prior to ALC administration. The unified curative effect standard to centralized assess was followed and the evaluation results were recorded and signed by the experts. The results were categorized into improved, effective improved and no change, based on the efficient rate which was calculated by the following formula: Efficient rate = (Number of patients that improved + Number of effective improved patients) / (All patients - Number of patients that could not be evaluated) × 100%.
Evaluation of safety and efficacy
The case histories of the patients, previous chemotherapy information, and disease and treatment histories were recorded during screening. Prior to 4, 8, and 12 weeks of testing, all patients underwent baseline assessment, which included comprehensive physical examinations, laboratory examinations, and electrophysiological and 12-lead ECG examination. The physical examinations included the measurement of vital signs (temperature, pulse, respiration and blood pressure), the grading of neurotoxicity (9), cancer-associated fatigue classification (10) and KPS assessment (11). Laboratory examination included a routine blood panel, liver and kidney function (12) and fasting glucose measurement (13). Electrophysiology examination was performed as described and the results were categorized into improved, effective improved and no change. During the study, all disease factors, drug combinations and adverse events were recorded in the case report form (CRF).
Nerve conductive velocity (NCV)
NCV was examined to evaluate the changes prior to ALC administration and after 8 weeks of ALC administration. Treatment was defined as effective if the NCV was improved after ALC administration, whereas, it was defined as ineffective if the NCV was reduced or unchanged. Effective analysis was conducted according to the results using the following formula: Efficient rate = number of patients effectively treated/(number of patients that could not be evaluated) ×100%.
Statistical analysis
Statistical analysis was performed using SAS software version 9.2 (SAS Institute, Cary, North Carolina, USA). Independent sample t-tests or Wilcoxon tests were used to compare continuous variables between groups, and paired t-test or Wilcoxon tests were used for comparison within groups. For the categorical variable analysis, a χ2 test or exact test were used for comparisons between groups. The Cochran-Mantel-Haenszel-χ2 test was used to analyze ordinal categorical data. Data were presented as the mean±standard deviation. P<0.05 was considered to indicate a significantly significant result.
Results
Subjects
A total of 240 patients were originally eligible for this study, although the final full study group included 239 CRF subjects. The safety analysis set (SS) contained 236 patients, 118 in the experimental group and 118 in the control group. The full analysis set (FAS) contained 225 patients, 109 in the experimental group and 116 in the control group. The per-protocol set (PPS) contained 203 patients, 95 in the experimental group and 108 in the control group.
Primary endpoint-neurotoxicity
As shown in Table I, in the FAS, at the 8th week of the study, ALC treatment reduced neurotoxicity in 50.5% of the experimental patients, compared with a 24.1% reduction in the control group. The difference between the ALC and placebo groups was statistically significant [95% confidence interval (CI), 14.1–38.5%; P<0.001]. The 8-week efficacy in the PPS was 51.6% (ALC) and 23.1% (control), which was significantly different (95% CI, 15.6–41.2%; P<0.001). The statistical significance of these findings was maintained following correction for the effect of different hospital centers (P<0.001; data not shown). The reduction of neurotoxicity was time-dependent for the patients in both treatment groups. A comparison of the effect of ALC between the 4th, 8th, and 12th week revealed that the improvement in neurotoxicity was significantly different between the experimental and control group (P<0.05; Table I). As shown in Table II, there was a observable improvement on the 4th week, with a decrease in the number of patients displaying grade ≥3 neuropathy, and an increase in the number of patients displaying grade 2, although the differences between the two groups did not reach statistical significance (P>0.05). By the 8th week of treatment, the difference between the experimental and control group was statistical significant (P<0.05).
Table I.
Peripheral sensory neurotoxicity evaluation at each visit at weeks 4, 8 and 12.
| Full analysis set | Per-protocol set | |||||||
|---|---|---|---|---|---|---|---|---|
| Time | ALC (%) | Placebo (%) | χ2 | Pa | ALC (%) | Placebo (%) | χ2 | Pa |
| Week 4 | ||||||||
| Valid | 29 (26.6) | 16 (13.8) | 5.766 | 0.016 | 26 (27.4) | 14 (13.0) | 6.629 | 0.010 |
| Invalid | 80 (73.4) | 100 (86.2) | 69 (72.6) | 94 (87.0) | ||||
| Week 8 | ||||||||
| Valid | 55 (50.5) | 28 (24.1) | 16.722 | <0.001 | 49 (51.6) | 25 (23.1) | 17.636 | <0.001 |
| Invalid | 54 (49.1) | 88 (75.9) | 46 (48.4) | 83 (76.9) | ||||
| Week 12 | ||||||||
| Valid | 63 (57.8) | 46 (39.7) | 7.406 | 0.007 | 57 (60.0) | 41 (38.0) | 9.830 | 0.002 |
| Invalid | 46 (42.2) | 70 (60.3) | 38 (40.0) | 67 (62.0) | ||||
Neurotoxicities were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). Neurotoxicities were evaluated in patients before enrollment, and at weeks 4, 8 and 12. Neurotoxicity was defined as valid if the grade at weeks 4, 8 and 12 decreased compared with prior to enrollment. Otherwise, it was defined as invalid. ALC, acetyl-L-carnitine group
P-values were calculated with a two-sided χ2 test.
Table II.
Peripheral sensory neurotoxicity grading.
| CTC grade | ALC (%) | Placebo (%) | χ2 | Pa |
|---|---|---|---|---|
| FAS | ||||
| Baseline | ||||
| I | 0 (0.0) | 0 (0.0) | 1.175 | 0.278 |
| II | 42 (38.5) | 53 (45.7) | ||
| III | 67 (61.5) | 63 (54.3) | ||
| 4th week | ||||
| I | 6 (5.6) | 8 (7.3) | 0.873 | 0.646 |
| II | 61 (57.0) | 56 (50.9) | ||
| III | 40 (37.4) | 46 (41.8) | ||
| 8th week | ||||
| I | 27 (25.2) | 20 (18.2) | 6.242 | 0.0441 |
| II | 54 (50.5) | 46 (41.8) | ||
| III | 26 (24.3) | 44 (40.0) | ||
| 12th week | ||||
| I | 37 (34.6) | 28 (25.5) | 3.7594 | 0.153 |
| II | 48 (44.9) | 48 (43.6) | ||
| III | 22 (20.6) | 34 (30.9) | ||
| PPS | ||||
| Baseline | ||||
| I | 0 (0.0) | 0 (0.0) | 1.910b | 0.167 |
| II | 34 (35.8) | 49 (45.4) | ||
| III | 61 (64.2) | 59 (54.6) | ||
| 4th week | ||||
| I | 5 (5.3) | 8 (7.8) | 1.007b | 0.605 |
| II | 52 (55.3) | 50 (49.0) | ||
| III | 37 (39.4) | 44 (43.1) | ||
| 8th week | ||||
| I | 22 (23.4) | 18 (17.6) | 5.364b | 0.068 |
| II | 48 (51.1) | 42 (41.2) | ||
| III | 24 (25.5) | 42 (41.2) | ||
| 12th week | ||||
| I | 32 (34.0) | 25 (24.5) | 4.299b | 0.117 |
| II | 43 (45.7) | 44 (43.1) | ||
| III | 19 (20.2) | 33 (32.4) | ||
P-values were calculated with the two-sided or CMH χ2 tests.
CMH χ2 tests. CTC, common toxicity criteria; CMH, Cochran-Mantel-Haenszel; ALC, acetyl-L-carnitine; FAS, full analysis set; PPS, per-protocol set.
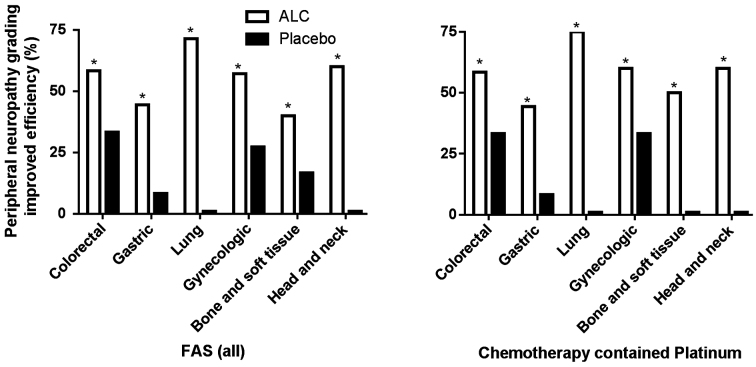
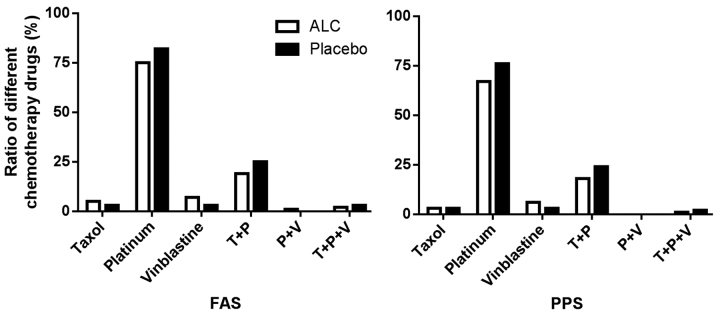
To further examine whether the efficacy of ALC was affected by chemotherapy treatment and cancer type, the cancer type in the treatment and placebo groups were classified prior to comparison of ALC efficacy. In the FAS, ALC was able to significantly improve the peripheral neuropathy grading in all cancer types, results which were not observed in the placebo group (P<0.05; Fig. 1). ALC's effect was not affected by treatment with platinum-containing chemotherapy (Fig. 1). In addition, chemotherapy had no difference on the effect of ALC, compared with the (Fig. 2).
Figure 1.
Peripheral neuropathy grading improved efficiency in patients with various types of cancer. Cancer types of the patients enrolled in the study include colorectal, gastric, lung, gynecological, bone and soft tissue, and head and neck cancers. Peripheral neuropathy grading improved efficiency was measured in the patients receiving either ALC or placebo. *P<0.05 vs. placebo. ALC, acetyl-L-carnitine; FAS, full analysis set.
Figure 2.
Ratio of various chemotherapy drugs. Chemotherapy drugs used in the patients receiving either ALC or placebo, including taxol, platinum and vinblastine. FAS, full analysis set; PPS, per-protocol set; ALC, acetyl-L-carnitine.
Secondary endpoints
Electrophysiological examination
Although the results from week 8 in the NCV test for the median nerve, ulnar nerve, common peroneal nerve, tibial nerve, superficial peroneal nerve and sural nerve demonstrate various degrees of improvement compared with the baseline at week 0, only the NCV of the sural nerve was significantly different between the experimental and control groups (P<0.05; data not shown).
Evaluation of electrophysiology
In the FAS, ALC treatment significantly improved NCV in the experimental group (60.7%), as compared with the control group (56.9%; P<0.05). Similar results could be observed in the PPS group, with a 76.8% improvement in the experimental group and a 59.3% in the control group (P<0.05; Table III).
Table III.
Nerve electrophysiological function (Week 8).
| Full analysis set | Per-protocol set | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect | ALC (%) | Placebo (%) | χ2 | Pa | ALC (%) | Placebo (%) | χ2 | Pa |
| Marked effect | 22 (20.2) | 17 (14.7) | 4.133 | 0.126 | 21 (22.1) | 16 (14.8) | 7.330 | 0.026 |
| Improved | 54 (49.5) | 49 (42.2) | 52 (54.7) | 48 (44.4) | ||||
| Unchanged | 33 (30.3) | 50 (43.1) | 22 (23.2) | 44 (40.7) | ||||
| Valid | 76 (60.7) | 66 (56.90) | 3.972 | 0.046 | 73 (76.8) | 64 (59.3) | 7.121 | 0.008 |
| Invalid | 22 (23.2) | 44 (40.7) | ||||||
Valid, marked effect + improved; invalid, unchanged. A marked effect was defined if the decreased rate was ≥50%. Improved was defined if the decreased rate was ≥16% and <50%. Unchanged was defined if the decreased rate was <16%.
P-values were calculated with a two-sided χ2 test. ALC, acetyl-L-carnitine.
Cancer-associated fatigue
In the FAS, ALC treatment reduced cancer-associated fatigue, however, the difference between the control (19.8%) and treatment (31.2%) groups was not statistically significant after 8 weeks treatment (P=0.0501). Conversely, in the PPS, the effect of ALC treatment was significantly different between the two groups at both week 8 (P<0.05) and 12 (P<0.05; Table IV).
Table IV.
Cancer-associated fatigue.
| CTC grade | ALC (%) | Placebo (%) | χ2 | P |
|---|---|---|---|---|
| FAS | ||||
| 4th week | ||||
| Valid | 17 (15.6) | 16 (13.8) | 0.146 | 0.702 |
| Invalid | 92 (84.4) | 100 (86.2) | ||
| 8th week | ||||
| Valid | 34 (31.2) | 23 (19.8) | 3.837 | 0.050 |
| Invalid | 75 (68.8) | 93 (80.2) | ||
| 12th week | ||||
| Valid | 41 (37.6) | 31 (26.7) | 3.063 | 0.080 |
| Invalid | 68 (62.4) | 85 (73.3) | ||
| PPS | ||||
| 4th week | ||||
| Valid | 17 (17.9) | 14 (13.0) | 0.950 | 0.330 |
| Invalid | 78 (82.1) | 94 (87.0) | ||
| 8th week | ||||
| Valid | 32 (33.7) | 20 (18.5) | 6.100 | 0.014 |
| Invalid | 63 (66.3) | 88 (81.5) | ||
| 12th week | ||||
| Valid | 39 (41.1) | 27 (25.0) | 5.936 | 0.015 |
| Invalid | 56 (58.9) | 81 (75.0) | ||
FAS, full analysis set; PPS, per-protocol set; ALC, acetyl-L-carnitine. P-values were calculated with a two sided χ2 test.
KPS
In the FAS, ALC treatment induced a statistically significant improvement (P<0.05) in KPS (29.3%) compared with the control group (13.0%). In the PPS, the improvement rate was 31.6% in the experimental group, and 12.0% in the control group, a difference that was statistically significant (P<0.05). In both PPS and FAS, the improvement of KPS after 12 weeks of treatment was also statistically significant compared with the baseline (P<0.01).
Safety evaluation: Analysis of adverse reactions and events
Of the 236 patients, 41 reported a total of 62 incidents of adverse reactions. There was no significant difference in the number of adverse events between the experimental (19.5%) and control (15.3%) group (P>0.05; Table V). The adverse reaction rate was 6.8% (8/118) for the trial group and 5.1% (6/118) for the control group, a difference that was not statistically significant (P>0.05; Table V). The primary adverse reactions were gastrointestinal reactions such as vomiting, abdominal distension and diarrhea. No statistically significant differences were observed between the treatment and control group (P>0.05). The most common adverse event was diarrhea [three cases in the ALC group (2.5%) and two cases in the control group (1.7%)]. Secondary events were decreased white blood cell count, liver dysfunction and insomnia. Notably, the three cases of decreased white blood cell count were all in the ALC group. There were three cases of liver dysfunction [one in the ALC group (0.8%) and two in the control group (1.7%)], and three cases of insomnia [two in the ALC group (1.7%) and one in the control group (0.8%)]. Four subjects in the ALC group withdrew from the study due to adverse events, but the adverse events in only one of these patients were associated with the drug. In total, seven severe adverse events (SAEs) occurred, two in the experimental group (1.7%) and five in the control group (4.2%). The incidence of adverse events was not statistically different between the treatment and control group (P>0.05), and none of the SAEs were associated with the drugs.
Table V.
Comparison of adverse reaction and adverse event incidence.
| Incident | ALC (%) | Placebo (%) | χ2 | Pa |
|---|---|---|---|---|
| Adverse events | ||||
| Yes | 23 (19.5) | 18 (15.3) | 0.738 | 0.3903 |
| No | 95 (80.5) | 100 (84.7) | ||
| Adverse reaction | ||||
| Yes | 8 (6.8) | 6 (5.1) | 0.3037 | 0.5816 |
| No | 110 (93.2) | 112 (94.9) | ||
ALC, acetyl-L-carnitine
P-values were calculated with a two sided χ2 test.
Discussion
Chemotherapy has a crucial role in the comprehensive treatment of cancer (14). However, CIPN is one of the most common dose-limiting adverse drug reactions (15–17). CIPN usually affects the dorsal root ganglia of primary sensory neurons. However, other sites, such as nerve terminals (distal terminations of the branches of an axon), may also be affected (14). Clinical features of CIPN vary depending on the type of agent involved and the site of action, and may include pure sensory or sensory-motor peripheral nerve damage of large myelinated or small unmyelinated fibers (14). The present study evaluated the efficacy and safety of ALC in Chinese patients with CIPN.
In this clinical trial, the therapeutic effect of ALC on neurotoxicity became evident after 8 weeks of treatment, and neuropathy was significantly reduced in patients treated with ALC. However, improvement of CIPN was a slow process, and statistically significant differences were not observed until week 8 after treatment. At week 12, there remained a significant difference between the ALC and the placebo group, demonstrating that the improvement in neurotoxicity persists without further clinical intervention (including discontinuation of chemotherapeutics).
Electrophysiological tests of neuronal function revealed statistical differences between the experimental and control group, with improvement of electrophysiological function after 8 weeks of treatment. These findings indicate that ALC is able to improve neuronal function in patients following chemotherapy, consistent with previous studies which demonstrated that prophylactic administration of oral ALC prevents the development of paclitaxel-induced painful peripheral neuropathy (18,19). Nerve conduction velocity, one of the objective indicators of peripheral neuropathy was also improved, but this improvement was less marked than the improvement in the subjective symptoms of the patients. Comparison of the neuronal conduction velocity between the baseline and week 8 of the trial indicated that only the sural sensory nerve exhibited a significant improvement in function. The following factors may be responsible: i) Motor neuron toxicity appears in the early stages of chemotherapy, thus, following timely treatment, neuronal function was improved and the difference in conduction speed of the two groups of motor neurons was not statistically significant; ii) the median, ulnar, and superficial peroneal nerves are composed of finer fibers and are located peripherally compared with the nervus suralis neural fiber of the leg, and the coarser fibers are thus more resistant to CIPN; iii) the sensory nerve fibers recover more rapidly, and therefore only the sural sensory nerve demonstrated significant treatment differences.
At the 4th week of this study, the number of patients with grade 3 decreased, but there was no statistical difference between the two groups. By the 8th week, the difference between the two groups gained statistical significance, suggesting that the onset and process of CIPN improvement is slow. Therefore, the treatment of CIPN requires long-term medication. After 4 weeks of ALC withdrawal (12th week of trial), the two groups returned to being statistically indistinguishable from one another. The trial group improved more than the control group with 37 grade 1 patients (34.6%), compared with 28 (25.5%) in the control group, and 22 cases (20.6%) of grade 3 patients in the trial group, compared with 34 cases (30.9%) in the control group. It was hypothesized that if this treatment period was extended to 12 weeks, the improvement would be further increased.
In the current study, the efficacy of ALC on CIPN improvement was not associated with the subject cancer types or the chemotherapy. Although chemotherapy may induce CIPN via various pathways, the main characteristics are inflammation and neuronal necrosis. Given the potential influence of chemotherapy, all patients in the study had not previously received chemotherapy (20–23). Another important point is all the subjects in the study has stopped and would not receive the chemotherapy. Therefore, the influence of chemotherapy may be eliminated.
In the PPS, the ALC group had significantly diminished cancer-associated fatigue compared with the control group. In both the FAS and PPS, the ALC group exhibited significant improvements in KPS compared with the control group. These results demonstrate that ALC is able to ease cancer-associated fatigue and improve the physical condition of patients following chemotherapy. Fatigue, caused by tumors and their associated treatment is a common problem for patients with cancer, and chronic fatigue seriously diminishes patient quality of life. Currently, there is a lack of pharmacological therapies for the treatment of cancer-associated fatigue. The results of this clinical study demonstrate that ALC is able to reduce cancer-associated fatigue, thus improving patient quality of life.
The overall incidence of adverse reactions in this clinical study was 5.9%, with 6.8% for the ALC group. In total, 3.4% of the patients withdrew from the study due to adverse reactions. These results demonstrate that ALC is well tolerated in patients, which is consistent with previous studies (24–27). Seven cases of SAE [two in the trial group (1.7%) and five in the control group (4.2%)] were recorded, although none of the SAEs were determined to be associated with drug administration. These findings provide evidence of the high safety profile of this type of treatment intervention.
In conclusion, to the best of our knowledge, the present study provides the first demonstration of the efficacy and safety of ALC for reducing chemotherapy-induced peripheral neuropathy toxicity and its associated symptoms. Based on these results, ALC may have an important role in the treatment of chemotherapy-induced peripheral neuropathy in China.
Acknowledgements
The authors of the present study would like to thank Professor Zhao Naiqing and Professor Luo Jiangfeng for their advice and help with the statistical analysis involved in the present study, and are grateful to Dr. Yao Chaoya and Dr. Qian Xuemei for their participation with the electrophysiology examination. This study was funded by Lee's Pharmaceutical (Hong Kong) Ltd. and Sigma-Tau Pharmaceutical, Inc., (Pomezia, Italy).
References
- 1.Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: Pathogenesis and emerging therapies. Support Care Cancer. 2004;12:619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 2.Urba SG, Orringer MB, Ianettonni M, Hayman JA, Satoru H. Concurrent cisplatin, paclitaxel and radiotherapy as preoperative treatment for patients with locoregional esophageal carcinoma. Cancer. 2003;98:2177–2183. doi: 10.1002/cncr.11759. [DOI] [PubMed] [Google Scholar]
- 3.Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol. 2016;140:176–183. doi: 10.1016/j.ygyno.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taglialatela G, Angelucci L, Ramacci MT, Werrbach-Perez K, Jackson GR, Perez-Polo JR. Stimulation of nerve growth factor receptors in PC12 by acetyl-L-carnitine. Biochem Pharmacol. 1992;44:577–585. doi: 10.1016/0006-2952(92)90452-O. [DOI] [PubMed] [Google Scholar]
- 5.Westlund KN, Lu Y, Werrbach-Perez K, Hulsebosch CE, Morgan B, Pizzo DP, Eisenberg HM, Perez-Polo JR. Effects of nerve growth factor and acetyl-L-carnitine arginyl amide on the human neuronal line HCN-1A. Int J Dev Neurosci. 1992;10:361–373. doi: 10.1016/0736-5748(92)90026-V. [DOI] [PubMed] [Google Scholar]
- 6.Pisano C, Pratesi G, Laccabue D, Zunino F, Lo Giudice P, Bellucci A, Pacifici L, Camerini B, Vesci L, Castorina M, et al. Paclitaxel and Cisplatin-induced neurotoxicity: A protective role of acetyl-L-carnitine. Clin Cancer Res. 2003;9:5756–5767. [PubMed] [Google Scholar]
- 7.Ghirardi O, Lo Giudice P, Pisano C, Vertechy M, Bellucci A, Vesci L, Cundari S, Miloso M, Rigamonti LM, Nicolini G, et al. Acetyl-L-Carnitine prevents and reverts experimental chronic neurotoxicity induced by oxaliplatin, without altering its antitumor properties. Anticancer Res. 2005;25:2681–2687. [PubMed] [Google Scholar]
- 8.Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain. 2008;135:262–270. doi: 10.1016/j.pain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argyriou AA, Velasco R, Briani C, Cavaletti G, Bruna J, Alberti P, Cacciavillani M, Lonardi S, Santos C, Cortinovis D, et al. Peripheral neurotoxicity of oxaliplatin in combination with 5-fluorouracil (FOLFOX) or capecitabine (XELOX): A prospective evaluation of 150 colorectal cancer patients. Ann Oncol. 2012;23:3116–3122. doi: 10.1093/annonc/mds208. [DOI] [PubMed] [Google Scholar]
- 10.Portenoy RK, Itri LM. Cancer-related fatigue: Guidelines for evaluation and management. Oncologist. 1999;4:1–10. [PubMed] [Google Scholar]
- 11.de Kock I, Mirhosseini M, Lau F, Thai V, Downing M, Quan H, Lesperance M, Yang J. Conversion of Karnofsky Performance Status (KPS) and Eastern Cooperative Oncology Group Performance Status (ECOG) to Palliative Performance Scale (PPS), and the interchangeability of PPS and KPS in prognostic tools. J Palliat Care. 2013;29:163–169. [PubMed] [Google Scholar]
- 12.Iwasaki N, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Yano N, Iwamoto Y. Liver and kidney function in Japanese patients with maturity-onset diabetes of the young. Diabetes Care. 1998;21:2144–2148. doi: 10.2337/diacare.21.12.2144. [DOI] [PubMed] [Google Scholar]
- 13.Fisman EZ, Motro M, Tenenbaum A, Boyko V, Mandelzweig L, Behar S. Impaired fasting glucose concentrations in nondiabetic patients with ischemic heart disease: A marker for a worse prognosis. Am Heart J. 2001;141:485–490. doi: 10.1067/mhj.2001.113219. [DOI] [PubMed] [Google Scholar]
- 14.Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: A comprehensive update of the literature. Cancer Manag Res. 2014;6:135–147. doi: 10.2147/CMAR.S44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brzezinski K. Chemotherapy-induced polyneuropathy. Part I. Pathophysiology. Contemp Oncol (Pozn) 2012;16:72–78. doi: 10.5114/wo.2012.27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci Lett. 2015;596:90–107. doi: 10.1016/j.neulet.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: A systematic review. Support Care Cancer. 2014;22:2261–2269. doi: 10.1007/s00520-014-2255-7. [DOI] [PubMed] [Google Scholar]
- 18.Flatters SJ, Xiao WH, Bennett GJ. Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful peripheral neuropathy. Neurosci Lett. 2006;397:219–223. doi: 10.1016/j.neulet.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin HW, Flatters SJ, Xiao WH, Mulhern HL, Bennett GJ. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: Effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exp Neurol. 2008;210:229–237. doi: 10.1016/j.expneurol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 21.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 22.Uceyler N, Kafke W, Riediger N, He L, Necula G, Toyka KV, Sommer C. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology. 2010;74:1806–1813. doi: 10.1212/WNL.0b013e3181e0f7b3. [DOI] [PubMed] [Google Scholar]
- 23.Areti A, Yerra VG, Naidu V, Kumar A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malaguarnera M, Gargante MP, Cristaldi E, Colonna V, Messano M, Koverech A, Neri S, Vacante M, Cammalleri L, Motta M. Acetyl L-carnitine (ALC) treatment in elderly patients with fatigue. Arch Gerontol Geriatr. 2008;46:181–190. doi: 10.1016/j.archger.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Maestri A, De Pasquale Ceratti A, Cundari S, Zanna C, Cortesi E, Crinò L. A pilot study on the effect of acetyl-L-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori. 2005;91:135–138. doi: 10.1177/030089160509100206. [DOI] [PubMed] [Google Scholar]
- 26.Evans JD, Jacobs TF, Evans EW. Role of acetyl-L-carnitine in the treatment of diabetic peripheral neuropathy. Ann Pharmacother. 2008;42:1686–1691. doi: 10.1345/aph.1L201. [DOI] [PubMed] [Google Scholar]
- 27.Bianchi G, Vitali G, Caraceni A, Ravaglia S, Capri G, Cundari S, Zanna C, Gianni L. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-L-carnitine. Eur J Cancer. 2005;41:1746–1750. doi: 10.1016/j.ejca.2005.04.028. [DOI] [PubMed] [Google Scholar]