Abstract
When assessing outcome in hepatocellular carcinoma (HCC), it is important to consider prognostic factors in background non-tumorous liver tissue as well as in the tumor, since multiple occurrence is associated with background liver status such as hepatitis. The current study aimed to elucidate molecular prognostic predictors that have an association with HCC background non-tumorous tissue. Microarray expression profiling identified aldo-keto reductase family 1, member B10 (AKR1B10) as a putative non-tumorous prognostic factor, and AKR1B10 gene expression was investigated in 158 curatively resected HCC cases by reverse transcription-quantitative polymerase chain reaction. AKR1B10 expression (AKR1B10 value/GAPDH value × 1,000) was significantly higher in tumor tissue (median, 9.2200; range, 0.0003–611.0200; n=158) than in the corresponding non-tumorous tissue (median, 0.5461; range, 0.0018–69.0300; n=158) (P<0.001). When the samples were grouped according to AKR1B10 expression in tumor tissue relative to non-tumorous tissue, tumor<non-tumorous expression (n=26) significantly correlated with poor recurrence-free survival (P=0.0074) and overall survival (OS) (P<0.0001), and was an independent prognostic factor for OS (P=0.0011) in a multivariate analysis. The ratio of AKR1B10 messenger RNA levels in HCC and corresponding non-tumorous tissues may predict prognosis after curative hepatectomy, with low expression in HCC tissue relative to non-tumorous tissue indicative of poor prognosis.
Keywords: AKR1B10, hepatocellular carcinoma, hepatectomy, microarray analysis, prognosis
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third most common cause of cancer-related mortality worldwide (1). Although hepatectomy is one of the most effective options for HCC without distant metastases (2–4), 80% of HCC patients experience intrahepatic recurrence even after curative resection, and 50% die within 5 years (5). The types of intrahepatic recurrence are mainly divided into two types: Intrahepatic metastasis (IM), which involves the development of HCC foci from primary tumor cells and their spread to the remnant liver via the portal vein before or during hepatectomy; and multicentric occurrence (MO), which involves the development of new HCC foci due to chronic active hepatitis or cirrhosis provoked by viruses, alcohol, toxins or other HCC risk factors (6–9). In other words, when the clinicopathological factors for HCC recurrence are divided into two categories such as tumor factors and background liver factors, IM tends to be related to tumor factors and MO is rather related to background liver factors, since IM reflects the characteristics of the primary tumor and MO exhibits different genetic features from the primary lesions.
The clinical progression and outcomes of IM and MO differ significantly, as determined by several studies (8–10). Therefore, distinguishing between these conditions is important for designing therapeutic strategies and predicting prognosis. Usually, the pattern of HCC intrahepatic recurrence is determined histologically, but it is sometimes difficult to distinguish them (11). The present authors previously examined mutations in the mitochondrial genome (12) and hypermethylation in tumor suppressor gene promoters (13) in HCC, and found distinctions between MO and IM. Our findings suggested that MO was more common than IM. However, the study of another group demonstrated that the proportion of two recurrence patterns was almost the same (14). In any case, MO recurrence pattern makes HCC totally different from other solid carcinomas in view of recurrence.
Considering this unique MO pattern in HCC, simply focusing on tumor tissue is insufficient; comparison of tumor tissue and background non-tumorous liver tissue is also important. When genetic and epigenetic changes related to HCC carcinogenesis and recurrence are tried to be elucidated, many investigators have attempted to evaluate only tumor tissue. However, we hypothesized that molecular changes in the latter may directly cause MO or indirectly affect the malignancy of the primary HCC. The present study was designed to identify a unique molecular marker of HCC, perhaps in background non-tumorous liver tissue, and to assess the predictive value of the marker.
Materials and methods
Sample collection
For microarray analysis, non-tumorous liver tissue, referred to as for corresponding normal (CN), was obtained from a typical HCC patient during hepatectomy. The patient was a 58-year-old man; his HCC resulted from chronic hepatitis, and recurred 3 years after resection at Nagoya University Hospital (Nagoya, Japan). Pathology confirmed the absence of cancerous regions from the CN sample. As controls, non-cancerous liver tissue (not affected by hepatitis), referred to as super normal (SN), was obtained from 11 patients with liver metastases who underwent hepatectomy at Nagoya University Hospital. Their primary diseases were colorectal cancer (n=5), gastrointestinal stromal tumor (n=2), or gastric cancer, esophageal cancer, cervical cancer or tongue cancer (n=1 each). The samples were collected between January 1998 and December 2011
For reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assays, HCC and CN tissue was collected from 158 consecutive patients who underwent curative primary hepatectomy at Nagoya University Hospital between January 1998 and December 2011. Median patient age was 65 years (range, 37–84 years), and the male:female ratio was 84:16. The median follow-up duration was 48.5 months (range, 0.3–193.8 months). Patient characteristics are summarized in Table I. All tumor tissue samples were histologically confirmed as HCC.
Table I.
Characteristic of patients with hepatocellular carcinoma (n=158).
| Characteristic | Value |
|---|---|
| Age (years), median (range) | 65 (37–84) |
| Sex (male:female), n (%) | 132 (84):26 (16) |
| Viral infection (HBV:HCV:non-HBV/HCV), n (%) | 41 (26):92 (58):28 (18) |
| Child-Pugh classification (A:B), n (%) | 148 (94):9 (6) |
| Liver damage classification (A:B:C), n (%) | 126 (83):25 (16):1 (1) |
| Albumin (mg/dl), median (range) | 3.9 (2.3–4.9) |
| Total bilirubin, (mg/dl), median (range) | 0.7 (0.2–7.3) |
| PT (%), median (range) | 89.7 (46.9–138.0) |
| AFP (ng/ml), median (range) | 17 (0.8–119,923.0) |
| Tumor size (cm), median (range) | 3.50 (0.15–15.00) |
| Tumor number (single:multiple), n (%) | 124 (78):34 (22) |
| ICG-R15 (%), median (range) | 11.5 (1.6–35.2) |
| Japanese stage (I:II:III:IV), n (%) | 17 (11):82 (52):40 (26):17 (11) |
HBV, hepatitis B virus; HCV, hepatitis C virus; PT, prothrombin time; AFP, alpha-fetoprotein; ICG-R15, retention rate of indocyanine green 15 min after administration.
All surgically obtained tissue samples were immediately frozen in liquid nitrogen and stored at −80°C until analysis. This study was approved by our institutional review board of at Nagoya University (Nagoya, Japan), and all patients provided written informed consent.
Microarray procedure
Total RNA was extracted from the CN and SN samples using a miRNeasy Mini-kit (Qiagen, Inc., Valencia, CA, USA). The 11 SN samples were mixed to eliminate individual differences. RNA integrity was assessed using an Agilent 2100 bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA); an RNA integrity number ≥8 was indicative of good quality RNA. RNA was labeled with cyanine-3 dye using a Quick Amp Labeling kit (Agilent Technologies, Inc.) and hybridized to Agilent whole human genome (4×44 K) microarrays (Agilent Technologies, Inc.) for 17 h in a rotating SciGene model 700 oven (SciGene, Sunnyvale, CA, USA). The arrays were scanned with a DNA microarray scanner (Agilent Technologies, Inc.), and the data were feature-extracted using Feature Extraction software 10.5.1.1 (Agilent Technologies, Inc.) and statistically analyzed using the default settings for GeneSpring GX 11.0.1 software (Agilent Technologies, Inc.) (15).
RT-qPCR
PCR was performed using SYBR Premix Ex Taq II (Takara Bio, Inc., Otsu, Japan) under the following conditions: 95°C for 10 sec, and 40 cycles at 95°C for 5 sec and 60°C for 30 sec. The SYBR Green signal was detected in real time using a StepOne Plus Real-Time PCR system (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The PCR primers used to generate a 144-bp fragment of AKR1B10 were 5′-GTGGGGGAAGCCATCCAAGA-3′ (sense, exon 2) and 5′-CAGCTTCAGGTCCTTGAGGG-3′ (antisense, exon 3). The primers used to generate an 85-bp fragment of Ras-related protein Rab-25 (RAB25) were 5′-AAAGTGACCTCAGCCAGGCC-3′ (sense, exon 3) and 5′-GTCTCCAGGAAGAGCAGTCC-3′ (antisense, exon 4). The primers used to generate a 95-bp fragment of erythrocyte membrane protein band 4.1 like 4B (EPB41L4B) were 5′-AGCCTCTCACTGACCCTGGA-3′ (sense, exon 19) and 5′-GCAGGTGTTCCTGGACTCAG-3′ (antisense, exon 20). The primers used to generate a 145-bp fragment of lipoma HMGIC fusion partner-like 1 (LHFPL1) were 5′-GGCTGCTGATAAGCTCAGGC-3′ (sense, exon 3) and 5′-GCTCCACCTCCAGCACAGTA-3′ (antisense, exon 4). GAPDH expression was quantified in each sample for standardization purposes. The primers used to generate a 226-bp fragment of GAPDH were 5′-GAAGGTGAAGGTCGGAGTC-3′ (sense) and 5′-GAAGATGGTGATGGGATTTC-3′ (antisense). All RT-qPCR experiments were performed at least three times, including negative controls without a template. The absolute quantification method was used to determine input copy number, which is based on a standard curve, due to its advantages in studies with large sample numbers (16). The expression of each gene was calculated as follows: Value of the expressed gene/value of GAPDH × 103.
Statistical analysis
Continuous variables were expressed as median and range and compared using the Mann-Whitney U-test. Categorical variables were compared using the χ2 or Fisher's exact tests, as appropriate. Recurrence-free survival (RFS) and overall survival (OS) rates were estimated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional hazards models were used to determine the independent risk factors associated with RFS and OS. All statistical analyses were performed using JMP Pro software version 11.0.0 (SAS International Inc., Cary, NC, USA). P-values are two-tailed, and P<0.05 was considered to indicate a statistically significant difference.
Results
Expression profiling via microarray analysis
To identify novel tumor-related genes in the normal liver tissue surrounding an HCC, the gene expression profiles of a CN sample and the pooled SN samples were compared. Microarray analysis revealed that AKR1B10, RAB25, EPB41L4B and LHFPL1 were upregulated in the CN sample (Table II). We focused on AKR1B10 as a CN-expressed prognostic factor.
Table II.
Hepatocellular carcinoma-related genes identified in microarrays.
| Gene | RefSeq accession #a | Fold change CN vs. SN | Regulation CN vs. SN | CN value | SN value | Flagsb CN | Flags SN |
|---|---|---|---|---|---|---|---|
| AKR1B10 | NM_020299 | 55.37 | Up | 14609.94 | 218.97 | Detected | Detected |
| RAB25 | NM_020387 | 43.96 | Up | 527.62 | 9.96 | Detected | Not detected |
| EPB41L4B | NM_019114 | 36.51 | Up | 272.23 | 6.19 | Detected | Not detected |
| LHFPL1 | NM_178175 | 35.45 | Up | 290.66 | 6.80 | Detected | Not detected |
RefSeq accession numbers were obtained from the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/nuccore/NM_020299.3, https://www.ncbi.nlm.nih.gov/nuccore/NM_020387, https://www.ncbi.nlm.nih.gov/nuccore/NM_019114 and https://www.ncbi.nlm.nih.gov/nuccore/NM_178175).
Flags indicate detectability of signal intensity. CN, corresponding normal; SN, super normal; AKR1B10, aldo-keto reductase family 1, member B10; EPB41L4B, erythrocyte membrane protein band 4.1 like 4B; LHFPL1, lipoma HMGIC fusion partner-like 1; RAB25, Ras-related protein Rab-25.
RT-qPCR analysis of HCC and CN tissue
As determined via RT-qPCR, overall AKR1B10 expression (expression score/GAPDH × 1,000) was significantly higher in HCC tissue (median, 9.2200; range, 0.0003–611.0200; n=158) than in CN tissue (median, 0.5461; range, 0.0018–69.0300; n=158) tissues (P<0.001). However, there was no significant difference in expression between SN and CN tissue (Fig. 1).
Figure 1.
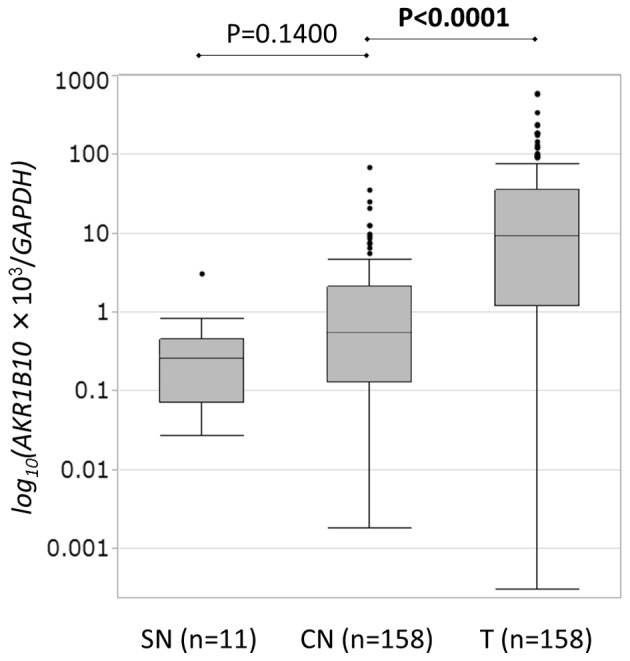
AKR1B10 messenger RNA levels in hepatocellular carcinoma and non-tumor tissue were quantified via reverse transcription-quantitative polymerase chain reaction. AKR1B10 expression (AKR1B10 score/GAPDH score × 1,000) was significantly higher in T tissue (median, 9.2200; range, 0.0003–611.0200; n=158) than in CN tissue (median, 0.5461; range, 0.0018–69.0300; n=158) (P<0.001). However, there was no significant difference between expression in CN tissue and SN tissue (median, 0.2616; range, 0.0272–3.1250; n=11 vs. median, 0.5461; range, 0.0018–69.0300; n=158, respectively) (P=0.1400). CN, corresponding normal; SN, super normal; T, tumor; AKR1B10, aldo-keto reductase family 1, member B10.
Correlation between AKR1B10 expression and the clinicopathological characteristics of HCC
AKR1B10 expression significantly correlated with liver damage (Child-Pugh score B or C vs. A) (P=0.035) and capsule infiltration (P=0.0284) (Table III). For example, 18 of 26 cases with liver damage scores of B or C had significantly greater amounts of AKR1B10 messenger RNA (mRNA) in CN tissue than in HCC tissue.
Table III.
Association between the clinicopathological characteristics of patients with hepatocellular carcinoma and AKR1B10 expression.
| AKR1B10 expression | |||
|---|---|---|---|
| Clinicopathological factor | T<CN | T≥CN | P-value |
| Age (years) | 0.8321 | ||
| ≥65 | 13 | 69 | |
| <65 | 13 | 63 | |
| Gender | 0.3191 | ||
| Male | 20 | 112 | |
| Female | 6 | 20 | |
| Virus infection | 0.9517 | ||
| HCV | 15 | 77 | |
| Others | 11 | 55 | |
| Albumin (mg/dl) | 0.1501 | ||
| <3.5 | 8 | 24 | |
| ≥3.5 | 18 | 107 | |
| PT (%) | 0.3187 | ||
| <70 | 5 | 14 | |
| ≥70 | 21 | 117 | |
| ICG-R15 (%) | 0.1183 | ||
| ≥15 | 7 | 22 | |
| <15 | 10 | 73 | |
| Liver cirrhosis | 0.1092 | ||
| (+) | 5 | 50 | |
| (−) | 20 | 80 | |
| Child-Pugh | 0.1705 | ||
| B | 3 | 6 | |
| A | 23 | 125 | |
| Liver damage | 0.0305 | ||
| B or C | 8 | 18 | |
| A | 17 | 109 | |
| Tumor number | 0.6017 | ||
| Multiple | 4 | 30 | |
| Solitary | 22 | 102 | |
| Tumor size (cm) | 0.1264 | ||
| ≥2 | 24 | 103 | |
| <2 | 1 | 22 | |
| AFP (ng/ml) | 0.0556 | ||
| ≥20 | 16 | 53 | |
| <20 | 10 | 76 | |
| Differentiation | 1.0000 | ||
| Poor | 2 | 10 | |
| Well/moderate | 23 | 119 | |
| Growth form | 0.5454 | ||
| Infiltrative | 5 | 18 | |
| Expansive | 21 | 111 | |
| Formation of capsule | 0.1036 | ||
| (−) | 4 | 42 | |
| (+) | 22 | 90 | |
| Infiltration to capsule | 0.0284 | ||
| (+) | 19 | 69 | |
| (−) | 6 | 63 | |
| Septal formation | 0.2419 | ||
| (−) | 5 | 44 | |
| (+) | 19 | 86 | |
| Serosal invasion | 0.2108 | ||
| (+) | 8 | 26 | |
| (−) | 16 | 95 | |
| Portal vein or hepatic vein invasion | 0.8830 | ||
| (+) | 7 | 36 | |
| (−) | 19 | 91 | |
| Surgical margin | 0.7663 | ||
| (+) | 3 | 21 | |
| (−) | 21 | 102 | |
| Japanese stage | 0.7670 | ||
| III/IV | 9 | 49 | |
| I/II | 17 | 81 | |
Fisher's exact test or χ2 test was applied as appropriate. T, tumor; CN, corresponding normal; HCV, hepatitis C virus; PT, prothrombin time; ICG-R15, retention rate of indocyanine green 15 min after administration; AFP, alpha fetoprotein; AKR1B10, aldo-keto reductase family 1, member B10.
Association between AKR1B10 expression and prognosis in 158 HCC cases
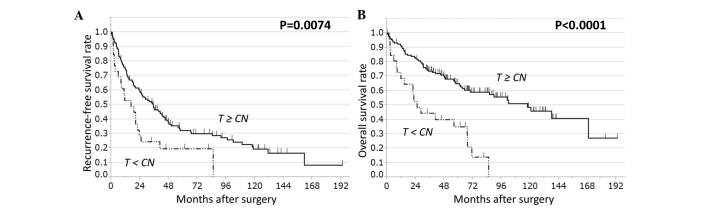
The clinical relevance of AKR1B10 expression was assessed in terms of its prognostic ability in HCC. The 158 HCC cases were divided into two groups based on AKR1B10 expression levels in HCC or CN tissue. Analysis of several pairings did not reveal any significant correlation between AKR1B10 expression and RFS or OS. The cases were also grouped as follows: i) AKR1B10 expression in HCC tissue was higher than or equal to AKR1B10 expression in CN tissue (HCC≥CN, n=132) and ii) AKR1B10 expression was lower in HCC tissue than in CN tissue (HCC<CN, n=26). The HCC<CN group had significantly worse RFS (P=0.022) and OS (P<0.0001) than the HCC≥CN group (Fig. 2).
Figure 2.
RFS and OS rates in patients with HCC based on AKR1B10 expression in T and CN tissue. HCC cases (n=158) were stratified on the basis of AKR1B10 expression in T tissue relative to CN tissue, either T≥CN (n=132) or T<CN (n=26). T<CN cases have significantly worse (A) RFS and (B) OS rates than T≥CN cases (log-rank test: RFS, P=0.0074; OS, P<0.0001). CN, corresponding normal; T, tumor; RFS, recurrence-free survival; OS, overall survival; AKR1B10, aldo-keto reductase family 1, member B10; HCC, hepatocellular carcinoma.
A multivariate analysis with those factors that displayed significant difference in an univariate analysis was next performed. The risk associated with this approach is that certain variables that were not significant in univariate analysis may have the potential to be significant in multivariate analysis. However, in situations when there is not enough information about the importance of each factor, this approach seems to be feasible. In the multivariate analysis, the finding for OS was confirmed (P=0.0011) (Table IV), whereas the finding for RFS was not (P=0.1884) (Table V). Multivariate analysis also revealed significant associations between survival (both OS and RFS) and serosal invasion (P=0.0407), and between OS and vascular invasion (P=0.0104) (Tables IV and V). Our findings suggest that the ratio of AKR1B10 mRNA levels in HCC and CN tissues predicts prognosis after curative hepatectomy, with low expression in HCC tissue relative to normal liver tissue being indicative of poor prognosis.
Table IV.
Univariate and multivariate analysis of overall survival.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Clinicopathological factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age (years) | ||||||
| ≥65 vs. <65 | 1.55 | 0.98–2.48 | 0.0585 | |||
| Gender | ||||||
| Male vs. female | 1.25 | 0.69–2.52 | 0.4709 | |||
| Virus infection | ||||||
| HCV vs. others | 1.50 | 0.94–2.46 | 0.0848 | |||
| Albumin (mg/dl) | ||||||
| <3.5 vs. ≥3.5 | 1.65 | 0.95–2.75 | 0.0731 | |||
| PT (%) | ||||||
| <70 vs. ≥70 | 1.75 | 0.90–3.12 | 0.0914 | |||
| ICG-R15 (%) | ||||||
| ≥15 vs. <15 | 1.74 | 0.92–3.19 | 0.0836 | |||
| Liver cirrhosis | ||||||
| (+) vs. (−) | 1.29 | 0.80–2.06 | 0.2876 | |||
| Child-Pugh | ||||||
| B vs. A | 1.63 | 0.63–3.48 | 0.2824 | |||
| Liver damage | ||||||
| B or C vs. A | 2.07 | 1.16–3.50 | 0.0149 | |||
| Tumor number | ||||||
| Multiple vs. single | 1.68 | 0.99–2.75 | 0.0534 | |||
| Tumor size (cm) | ||||||
| ≥2 vs. <2 | 2.02 | 0.95–5.23 | 0.0681 | |||
| AFP (ng/ml) | ||||||
| ≥20 vs. <20 | 2.06 | 1.30–3.30 | 0.0022 | 1.40 | 0.79–2.46 | 0.2368 |
| Differentiation | ||||||
| Poor vs. well/moderate | 2.29 | 1.05–4.38 | 0.0365 | 1.35 | 0.43–3.46 | 0.5732 |
| Growth form | ||||||
| Infiltrative vs. expansive | 1.57 | 0.86–2.71 | 0.1334 | |||
| Formation of capsule | ||||||
| (−) vs. (+) | 0.91 | 0.54–1.49 | 0.7282 | |||
| Infiltration to capsule | ||||||
| (+) vs. (−) | 0.97 | 0.61–1.54 | 0.9168 | |||
| Septal formation | ||||||
| (−) vs. (+) | 1.05 | 0.64–1.70 | 0.8221 | |||
| Serosal invasion | ||||||
| (+) vs. (−) | 2.51 | 1.48–4.17 | 0.0009 | 1.86 | 1.02–3.28 | 0.0407 |
| Portal vein or hepatic vein invasion | ||||||
| (+) vs. (−) | 2.25 | 1.38–3.62 | 0.0014 | 2.15 | 1.20–3.76 | 0.0104 |
| Surgical margin | ||||||
| (+) vs. (−) | 1.84 | 1.00–3.18 | 0.0498 | 1.37 | 0.63–2.71 | 0.4034 |
| Japanese stage | ||||||
| III/IV vs. I/II | 1.56 | 0.97–2.47 | 0.0622 | |||
| AKR1B10 expression | ||||||
| T<CN vs. T≥CN | 3.14 | 1.81–5.23 | <0.0001 | 3.06 | 1.58–5.71 | 0.0011 |
A multivariate Cox proportional hazard model was used to investigate independent risk factors for overall survival. HR, hazard ratio; CI, confidence interval; HCV, hepatitis C virus; PT, prothrombin time; ICG-R15, retention rate of indocyanine green 15 min after administration; AFP, alpha fetoprotein; T, tumor; CN, corresponding normal; AKR1B10, aldo-keto reductase family 1, member B10.
Table V.
Univariate and multivariate analysis of recurrence-free survival.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Clinicopathological factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age (years) | ||||||
| ≥65 vs. <65 | 1.11 | 0.77–1.61 | 0.5539 | |||
| Gender | ||||||
| Male vs. female | 1.57 | 0.95–2.78 | 0.0755 | |||
| Virus infection | ||||||
| HCV vs. others | 1.45 | 1.00–2.15 | 0.0496 | 1.14 | 0.65–2.02 | 0.6440 |
| Albumin (mg/dl) | ||||||
| <3.5 vs. ≥3.5 | 1.74 | 1.10–2.68 | 0.0189 | 1.48 | 0.76–2.80 | 0.2373 |
| PT (%) | ||||||
| <70 vs. ≥70 | 1.33 | 0.75–2.20 | 0.3092 | |||
| ICG-R15 (%) | ||||||
| ≥15 vs. <15 | 2.31 | 1.40–3.71 | 0.0012 | 1.72 | 0.90–3.19 | 0.0939 |
| Liver cirrhosis | ||||||
| (+) vs. (−) | 1.23 | 0.84–1.80 | 0.2742 | |||
| Child-Pugh | ||||||
| B vs. A | 1.66 | 0.74–3.20 | 0.1981 | |||
| Liver damage | ||||||
| B or C vs. A | 1.87 | 1.15–2.92 | 0.0121 | |||
| Tumor number | ||||||
| Multiple vs. single | 1.65 | 1.05–2.49 | 0.0277 | 1.41 | 0.66–2.72 | 0.3432 |
| Tumor size (cm) | ||||||
| ≥2 vs. <2 | 1.81 | 1.03–3.49 | 0.0352 | 0.88 | 0.42–1.97 | 0.7536 |
| AFP (ng/ml) | ||||||
| ≥20 vs. <20 | 1.43 | 0.98–2.08 | 0.0614 | |||
| Differentiation | ||||||
| Poor vs. well/moderate | 1.44 | 0.70–2.64 | 0.2899 | |||
| Growth form | ||||||
| Infiltrative vs. expansive | 1.18 | 0.68–1.93 | 0.5158 | |||
| Formation of capsule | ||||||
| (−) vs. (+) | 0.67 | 0.43–1.01 | 0.0577 | |||
| Infiltration to capsule | ||||||
| (+) vs. (−) | 1.18 | 0.82–1.72 | 0.3606 | |||
| Septal formation | ||||||
| (−) vs. (+) | 1.01 | 0.67–1.50 | 0.9240 | |||
| Serosal invasion | ||||||
| (+) vs. (−) | 2.75 | 1.76–4.19 | <0.0001 | 2.23 | 1.17–4.14 | 0.0151 |
| Portal vein or hepatic vein invasion | ||||||
| (+) vs. (−) | 1.94 | 1.28–2.88 | 0.0019 | 1.56 | 0.84–2.79 | 0.1481 |
| Surgical margin | ||||||
| (+) vs. (−) | 1.16 | 0.68–1.86 | 0.5608 | |||
| Japanese stage | ||||||
| III/IV vs. I/II | 1.37 | 0.93–1.99 | 0.1012 | |||
| AKR1B10 expression | ||||||
| T<CN vs. T≥CN | 1.90 | 1.14–3.01 | 0.0138 | 1.59 | 0.78–3.04 | 0.1884 |
A multivariate Cox proportional hazard model was used to investigate independent risk factors of recurrence-free survival. HR, hazard ratio; CI, confidence interval; HCV, hepatitis C virus; PT, prothrombin; ICG-R15, retention rate of indocyanine green 15 min after administration; AFP, alpha fetoprotein; AKR1B10, aldo-keto reductase family 1, member B10; T, tumor; CN, corresponding normal.
Discussion
A major obstacle in HCC treatment is the high frequency of tumor recurrence even after curative resection and liver transplantation (17), and even in cases of small, well-differentiated tumors (18). We previously reported that MO was more common than IM in HCC (12,13). Accordingly, the detection of metachronous multicentric recurrent carcinoma at an early stage and the instigation of appropriate therapy may prolong survival (14). Furthermore, evaluation of CN liver tissue may provide useful information regarding MO risk along with the evaluation of the cancer tissue.
In the present study, microarray analysis revealed that four genes, including AKR1B10, were more highly expressed in CN tissue than in SN tissue. We decided to further investigate AKR1B10 as a potential non-tumor prognostic predictor of HCC outcome.
NAD(P)H-dependent oxidoreductases catalyze the reduction of a variety of carbonyl compounds, and AKR1B10, a member of this superfamily, efficiently reduces aliphatic and aromatic aldehydes (19). AKR1B10 is expressed in the kidney, nasal epithelium, liver and cervical epithelium, according to GeneCards (http://www.genecards.org/), and in several cancer cell lines, including liver, kidney, lung, colon, brain, prostate, cervix and breast (20). In non-small cell lung cancers, especially squamous cell carcinomas, AKR1B10 expression highly correlates with smoking (21). As shown by Zhang et al (22), AKR1B10 promotes pancreatic carcinogenesis via modulation of the K-RAS/E-cadherin pathway. Several studies demonstrated AKR1B10 expression in HCC via immunohistochemistry (23–26). To the best of our knowledge, however, there are no reports comparing AKR1B10 expression in HCC and CN tissue or determining its association with HCC prognosis.
In this study, and as determined by RT-qPCR, AKR1B10 expression was significantly higher in HCC than in CN samples, but not significantly different in SN and CN samples. Although the result of microarray analysis demonstrated higher AKR1B10 expression in CN than in SN, it was only one typical HCC case that was used to compare. Therefore, AKR1B10 expression is not an HCC marker specific to CN tissue. There was no significant correlation between AKR1B10 expression and HCC prognosis when these parameters were compared in 158 HCC surgical samples. However, when samples were grouped according to AKR1B10 expression in HCC tissue relative to CN tissue (expression in HCC≥CN or expression in HCC<CN), it was observed that HCC<CN was associated with significantly worse RFS and OS rates, and that AKR1B10 was an independent risk factor for OS in a multivariate analysis using a Cox hazard model.
Via immunohistochemistry, a previous study reported that AKR1B10 levels were higher in HCC than in surrounding tissue, and suggested that this enzyme may be useful for distinguishing HCC from benign hepatic tumors (25). Other studies found that HCCs with low AKR1B10 levels were highly proliferative, poorly differentiated and had a poor prognosis (23,24). In agreement with these studies, the present study identified that AKR1B10 mRNA levels were elevated in HCCs, and that prognosis (RFS and OS) was worse in cases in which the amounts of AKR1B10 mRNA were lower in HCC tissue than in CN tissue. AKR1B10 expression also correlated with capsule infiltration and liver damage. Capsule infiltration may cause genetic changes in non-tumorous liver tissue adjacent to HCCs and may be related to cell invasiveness. Liver damage may increase AKR1B10 expression in CN tissue. Sato et al (27) demonstrated that chronic hepatitis C-mediated AKR1B10 upregulation correlated with serum alpha-fetoprotein levels and HCC recurrence. Higher AKR1B10 expression in CN tissue may be associated with the oncogenic status of the background liver tissue, while lower AKR1B10 expression in HCC tissue may indicate HCC malignancy. Interestingly, factors considered to be prognostic such as vascular invasion (28–30) were unrelated to AKR1B10 expression. Therefore, changes in AKR1B10 expression may be worth considering as prognostic predictors, even if other prognostic factors are not observed.
HCC-resected patients with a low tumor-to-CN ratio of AKR1B10 mRNA could be offered a more intense follow-up program consisting of frequent examinations with ultrasonography or computed tomography, and adjuvant therapy should be considered if possible in the future. Further study is required to elucidate how genes such as AKR1B10 in tumor-adjacent normal liver tissue respond to HCC development and recurrence. Such knowledge would facilitate the design of novel approaches for prediction, prevention and treatment of HCC.
In conclusion, our findings suggest that the ratio of AKR1B10 expression in HCC tissue and background non-tumorous liver tissue may be a prognostic indicator in patients receiving curative hepatectomies. Its combination with other prognostic factors may more accurately predict HCC prognosis.
Acknowledgements
This work was supported by the Japanese Society for the Promotion of Science (Tokyo, Japan) through a KAKENHI Grant-in-Aid for Scientific Research (C) (grant number 25461979).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Rahbari NN, Mehrabi A, Mollberg NM, Müller SA, Koch M, Büchler MW, Weitz J. Hepatocellular carcinoma: Current management and perspectives for the future. Ann Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi A, Kawasaki S, Miyagawa S, Miwa S, Noike T, Takagi S, Iijima S, Miyagawa Y. Results of 404 hepatic resections including 80 repeat hepatectomies for hepatocellular carcinoma. Hepatogastroenterology. 2006;53:736–741. [PubMed] [Google Scholar]
- 4.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taura K, Ikai I, Hatano E, Fujii H, Uyama N, Shimahara Y. Implication of frequent local ablation therapy for intrahepatic recurrence in prolonged survival of patients with hepatocellular carcinoma undergoing hepatic resection: An analysis of 610 patients over 16 years old. Ann Surg. 2006;244:265–273. doi: 10.1097/01.sla.0000217921.28563.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen PJ, Chen DS, Lai MY, Chang MH, Huang GT, Yang PM, Sheu JC, Lee SC, Hsu HC, Sung JL. Clonal origin of recurrent hepatocellular carcinomas. Gastroenterology. 1989;96:527–529. doi: 10.1016/0016-5085(89)91581-3. [DOI] [PubMed] [Google Scholar]
- 7.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/S0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 8.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: Prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cucchetti A, Piscaglia F, Caturelli E, Benvegnù L, Vivarelli M, Ercolani G, Cescon M, Ravaioli M, Grazi GL, Bolondi L, Pinna AD. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. 2009;16:413–422. doi: 10.1245/s10434-008-0232-4. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M, Fujii H, Kono H, Matsumoto Y. Surgical treatment of recurrent hepatocellular carcinoma based on the mode of recurrence: Repeat hepatic resection or ablation are good choices for patients with recurrent multicentric cancer. J Hepatobiliary Pancreat Surg. 2001;8:353–359. doi: 10.1007/s005340170008. [DOI] [PubMed] [Google Scholar]
- 11.Morimoto O, Nagano H, Sakon M, Fujiwara Y, Yamada T, Nakagawa H, Miyamoto A, Kondo M, Arai I, Yamamoto T, et al. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J Hepatol. 2003;39:215–221. doi: 10.1016/S0168-8278(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 12.Nomoto S, Yamashita K, Koshikawa K, Nakao A, Sidransky D. Mitochondrial D-loop mutations as clonal markers in multicentric hepatocellular carcinoma and plasma. Clin Cancer Res. 2002;8:481–487. [PubMed] [Google Scholar]
- 13.Nomoto S, Kinoshita T, Kato K, Otani S, Kasuya H, Takeda S, Kanazumi N, Sugimoto H, Nakao A. Hypermethylation of multiple genes as clonal markers in multicentric hepatocellular carcinoma. Br J Cancer. 2007;97:1260–1265. doi: 10.1038/sj.bjc.6604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M, Sassa T. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87–92. doi: 10.1002/hep.510250116. [DOI] [PubMed] [Google Scholar]
- 15.Stangegaard M. Gene expression analysis using agilent DNA microarrays. Methods Mol Biol. 2009;529:133–145. doi: 10.1007/978-1-59745-538-1_9. [DOI] [PubMed] [Google Scholar]
- 16.Tsai SJ, Wiltbank MC. Quantification of mRNA using competitive RT-PCR with standard-curve methodology. Biotechniques. 1996;21:862–866. doi: 10.2144/96215st04. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Zhang G, Wu J, Jia M. Adjuvant therapy for hepatocellular carcinoma: Current situation and prospect. Drug Discov Ther. 2013;7:137–143. [PubMed] [Google Scholar]
- 18.Takenaka K, Adachi E, Nishizaki T, Hiroshige K, Ikeda T, Tsuneyoshi M, Sugimachi K. Possible multicentric occurrence of hepatocellular carcinoma: A clinicopathological study. Hepatology. 1994;19:889–894. doi: 10.1002/hep.1840190414. [DOI] [PubMed] [Google Scholar]
- 19.Cao D, Fan ST, Chung SS. Identification and characterization of a novel human aldose reductase-like gene. J Biol Chem. 1998;273:11429–11435. doi: 10.1074/jbc.273.19.11429. [DOI] [PubMed] [Google Scholar]
- 20.Endo H, Shiroki T, Nakagawa T, Yokoyama M, Tamai K, Yamanami H, Fujiya T, Sato I, Yamaguchi K, Tanaka N, et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8:e77070. doi: 10.1371/journal.pone.0077070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang MW, Lee ES, Yoon SY, Jo J, Lee J, Kim HK, Choi YS, Kim K, Shim YM, Kim J, Kim H. AKR1B10 is associated with smoking and smoking-related non-small-cell lung cancer. J Int Med Res. 2011;39:78–85. doi: 10.1177/147323001103900110. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Li H, Yang Y, Liao J, Yang GY. Knockdown or inhibition of aldo-keto reductase 1B10 inhibits pancreatic carcinoma growth via modulating Kras-E-cadherin pathway. Cancer Lett. 2014;355:273–280. doi: 10.1016/j.canlet.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heringlake S, Hofdmann M, Fiebeler A, Manns MP, Schmiegel W, Tannapfel A. Identification and expression analysis of the aldo-ketoreductase1-B10 gene in primary malignant liver tumours. J Hepatol. 2010;52:220–227. doi: 10.1016/j.jhep.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz KJ, Sotiropoulos GC, Baba HA, Schmid KW, Müller D, Paul A, Auer T, Gamerith G, Loeffler-Ragg J. AKR1B10 expression is associated with less aggressive hepatocellular carcinoma: A clinicopathological study of 168 cases. Liver Int. 2011;31:810–816. doi: 10.1111/j.1478-3231.2011.02511.x. [DOI] [PubMed] [Google Scholar]
- 25.Matkowskyj KA, Bai H, Liao J, Zhang W, Li H, Rao S, Omary R, Yang GY. Aldoketoreductase family 1B10 (AKR1B10) as a biomarker to distinguish hepatocellular carcinoma from benign liver lesions. Hum Pathol. 2014;45:834–843. doi: 10.1016/j.humpath.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuzura H, Genda T, Sato S, Murata A, Kanemitsu Y, Narita Y, Ishikawa S, Kikuchi T, Mori M, Hirano K, et al. Expression of aldo-keto reductase family 1 member b10 in the early stages of human hepatocarcinogenesis. Int J Mol Sci. 2014;15:6556–6568. doi: 10.3390/ijms15046556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato S, Genda T, Hirano K, Tsuzura H, Narita Y, Kanemitsu Y, Kikuchi T, Iijima K, Wada R, Ichida T. Up-regulated aldo-keto reductase family 1 member B10 in chronic hepatitis C: Association with serum alpha-fetoprotein and hepatocellular carcinoma. Liver Int. 2012;32:1382–1390. doi: 10.1111/j.1478-3231.2012.02827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosuge T, Makuuchi M, Takayama T, Yamamoto J, Shimada K, Yamasaki S. Long-term results after resection of hepatocellular carcinoma: Experience of 480 cases. Hepatogastroenterology. 1993;40:328–332. [PubMed] [Google Scholar]
- 29.Izumi R, Shimizu K, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–727. doi: 10.1016/0016-5085(94)90707-2. [DOI] [PubMed] [Google Scholar]
- 30.Hanazaki K, Kajikawa S, Koide N, Adachi W, Amano J. Prognostic factors after hepatic resection for hepatocellular carcinoma with hepatitis C viral infection: Univariate and multivariate analysis. Am J Gastroenterol. 2001;96:1243–1250. doi: 10.1111/j.1572-0241.2001.03634.x. [DOI] [PubMed] [Google Scholar]