Abstract
Introduction
The effect of various airway management strategies, such as the timing of tracheostomy, on liberation from mechanical ventilation (MV) is uncertain. We tested the hypothesis that tracheostomy, when performed prior to active weaning, does not influence the duration of weaning or of MV in comparison with a more selective use of tracheostomy.
Patients and methods
In this observational prospective cohort study, surgical patients requiring ≥ 72 hours of MV were followed prospectively. Patients undergoing tracheostomy prior to any active weaning attempts (early tracheostomy [ET]) were compared with patients in whom initial weaning attempts were made with the endotracheal tube in place (selective tracheostomy [ST]).
Results
We compared the duration of weaning, the total duration of MV and the frequency of fatigue and pneumonia. Seventy-four patients met inclusion criteria. Twenty-one patients in the ET group were compared with 53 patients in the ST group (47% of whom ultimately underwent tracheostomy). The median duration of weaning was shorter (3 days versus 6 days, P = 0.05) in patients in the ET group than in the ST group, but the duration of MV was not (median [interquartile range], 11 days [9–26 days] in the ET group versus 13 days [8–21 days] in the ST group). The frequencies of fatigue and pneumonia were lower in the ET group patients.
Discussion
Determining the ideal timing of tracheostomy in critically ill patients has been difficult and often subjective. To standardize this process, it is important to identify objective criteria to identify patients most likely to benefit from the procedure. Our data suggest that in surgical patients with resolving respiratory failure, a patient who meets typical criteria for a trial of spontaneous breathing but is not successfully extubated within 24 hours may benefit from a tracheostomy. Our data provide a framework for the conduct of a clinical trial in which tracheostomy timing can be assessed for its impact on the duration of weaning.
Conclusion
Tracheostomy prior to active weaning may hasten liberation from ventilation and reduce complications. However, this does not reduce the overall duration of MV.
Keywords: respiratory failure, tracheostomy, weaning
Introduction
The need for prolonged mechanical ventilation (MV) is considered the most common indication for tracheostomy in the intensive care unit. The decision to perform a tracheostomy is often based on the concern for airway injury secondary to extended periods of translaryngeal intubation [1]. The use of tracheostomy early in the course of respiratory failure may reduce the danger of premature extubation and the complications associated with reintubation [2,3]. Finally, the timing of tracheostomy has been thought to influence liberation from MV. Patients receiving early tracheostomy (ET) are reported to have an overall shorter duration of MV than patients who undergo late tracheostomy [4,5]. Other workers have found no benefit to early tracheostomy in critically ill surgical patients [6].
Taken together, the existing literature reflects the difficulty in conducting and analyzing studies of the potential benefits of tracheostomy in patients with acute respiratory failure. Physician belief in the utility of tracheostomy, patient selection and the lack of blinding may introduce bias, leading to difficulties in comparing patients receiving 'early' or 'late' tracheostomy. Moreover, it is not certain which end-points can be affected by the timing of tracheostomy, as it does not alter the course of respiratory failure. The availability of a percutaneous approach has potentially lowered the threshold for performing tracheostomy, yet there remains little evidence of a beneficial impact upon patient care and outcomes [7,8].
We reanalyzed a subgroup of patients from a previously reported prospective cohort study, initially designed to determine the utility of weaning parameters in patients requiring ≥ 72 hours of MV [9]. In the present review, we sought to determine whether tracheostomy, performed after readiness-to-wean criteria were met but prior to active weaning, when compared with a more selective and delayed use of tracheostomy (rather than after an arbitrary duration of endotracheal intubation), affected the duration of weaning and MV. We also sought to determine which clinical information was useful in identifying patients most likely to benefit from a tracheostomy. Finally, we wanted to identify which end-points or outcomes could potentially be influenced by performing a tracheostomy relatively early in patients with acute respiratory failure. We tested the hypothesis that tracheostomy, when performed prior to active weaning, does not influence the duration of weaning or of MV, and does not affect the incidence of clinical fatigue or complications such as pneumonia in comparison with a selective but delayed use of tracheostomy.
Patients and methods
Patient enrollment
The subjects in the present study are from a prospective cohort examining the utility of standard weaning parameters in surgical patients requiring ≥ 72 hours of MV. The methods have been previously published [9], so the important details are summarized. Ninety-five patients admitted to our surgical and trauma intensive care unit were followed once they had received MV for 72 hours and were not brain dead. Patients were screened for readiness-to-wean criteria daily at 5:00 am. These criteria included resolution of the underlying disease process, no inotropic or vasopressor support, PaO2/FiO2 > 150, FiO2 ≤ 50%, positive end-expiratory pressure ≤ 5 cmH2O and pH of 7.30–7.50. Once all criteria were met, the patient was considered for possible extubation. This study is based on data from the subgroup of patients who were not immediately extubated and underwent a period of gradual transition to unsupported spontaneous breathing.
Weaning followed an established protocol that was automatically instituted by the respiratory therapists. In this protocol, all patients were placed on pressure-support ventilation at a level to maintain a spontaneous respiratory rate of 20–28/min and involved a step-wise reduction in the level of pressure support. The protocol permitted overnight resting at the discretion of the attending intensivist. In addition to excluding patients who were immediately extubated once meeting criteria, we did not include patients who had received a tracheostomy for airway control as part of a surgical procedure or resuscitation.
Study group assignment and clinical definitions
Patients were classified into one of two study groups. The ET group included those who underwent tracheostomy prior to any attempts at weaning, and the selective tracheostomy (ST) group included patients in whom weaning attempts were made with the endotracheal tube in place. The ST group therefore includes patients who were successfully extubated and never underwent tracheostomy as well as patients who underwent tracheostomy after initial weaning efforts were unsuccessful. The decision to perform a tracheostomy was typically made by the attending surgeon in discussion with the respiratory therapists and the patient's family members. Figure 1 illustrates patient enrollment, exclusions and assignment to ET or ST groups.
Figure 1.
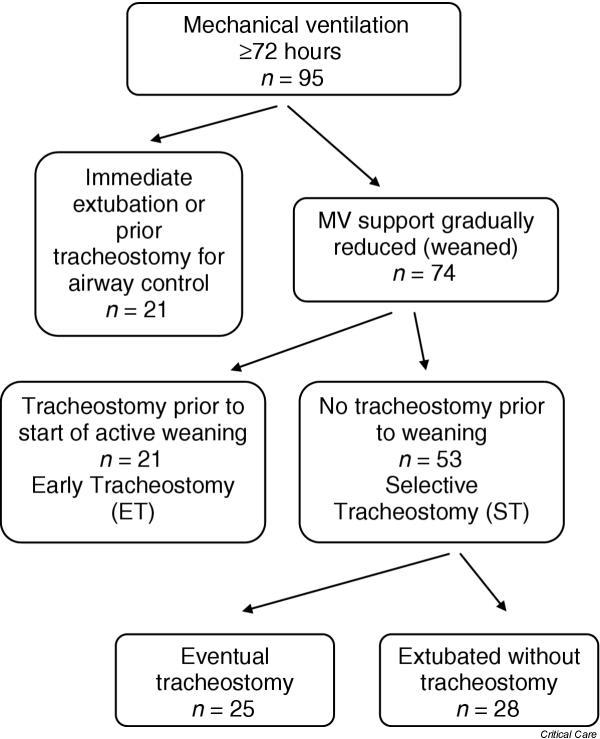
Of 95 subjects receiving mechanical ventilation (MV) for ≥ 72 hours and who were not brain dead, 74 were not immediately extubated once readiness-to-wean criteria were met (defined in Patients and methods). The early tracheostomy (ET) group consists of 21 patients who underwent tracheostomy prior to any active weaning attempts, and the selective tracheostomy (ST) group consists of all patients (n = 53) who were initially weaned with an endotracheal tube in place. Ultimately, of the ST group, 25 patients underwent tracheostomy and 28 patients were successfully liberated and extubated.
Fatigue while weaning was defined by a combination of clinical evidence of respiratory distress accompanied by an ordered increase in positive pressure support (increased synchronized intermittent mandatory ventilation rate, conversion to assist-control or an increase in pressure support). One or more of the following were considered as evidence of respiratory distress: PaCO2 > 50 mmHg or increase > 10 mmHg, SaO2 < 90% or PaO2 < 60 mmHg, pH < 7.30, heart rate > 120/min, systolic blood pressure > 180 mmHg or < 90 mmHg, respiratory rate > 30/min, and clinical distress (diaphoresis, agitation, accessory muscle use). Pneumonia was defined by the presence of all of the following: leukocytosis ≥ 10,000/ml, a new and persistent infiltrate on chest radiography, temperature ≥ 38.5°C and a positive nonbronchoscopic bronchoalveolar lavage culture (≥ 104 colony-forming units/ml).
Data presentation and statistical analysis
Categorical data are presented as percentages, and continuous data are presented as medians and associated interquartile ranges (IQR). The Mann–Whitney U test and the chi-square test were used to analyze differences between early and selective tracheostomy groups. Cox regression was used to compare the effect of ET on the duration of weaning and on the total duration of MV. Variables were included in the final models if they were associated with the duration of weaning or MV (adjusted P value ≤ 0.05), or if they affected the association between ET and outcome (i.e. a confounder). Actual P values are reported for all statistical comparisons. For the hazard ratios obtained from the Cox regression analysis, the 95% confidence intervals are also reported.
In addition to adjusting for covariates in multivariable regression, residual confounding and the effect of selection bias were addressed using propensity scores [10,11]. To calculate the propensity score, we included in a separate multivariable logistic regression analysis all factors that differed between the ET and ST groups. We fit a model predicting the likelihood of ET and incorporated this score as a covariate in the Cox regression model using duration of weaning as the dependent variable. Inclusion of the propensity score as a covariate theoretically adjusts for confounding and selection bias [10].
Results
Description of cohort
Overall demographic data and outcome information for the 74 patients are summarized in Table 1. There were 49 (66%) men, the median age was 47 years and 48 (65%) were trauma victims. Thirty (63%) of the 48 trauma patients had a traumatic brain injury (TBI) as their sole injury or in addition to torso and extremity injuries. The remaining patients had various surgical problems including intra-abdominal sepsis and ruptured abdominal aortic aneurysm.
Table 1.
Patient characteristics and clinical outcomes for study cohort (n = 74)
| Patient characteristic | |
| Gender, male | 49 (66%) |
| Age (years) | 47 (35–56) |
| Multiple trauma victim | 48 (66%) |
| Number of days from intubation to meeting readiness-to-wean criteria | 4 (3–6) |
| Number of days from meeting readiness-to-wean criteria to starting weaning | 1 (0–3) |
| Clinical outcomes | |
| Number of days of mechanical ventilation | 12 (8–21) |
| Number of days weaning | 4 (2–13) |
| Tracheostomy | 46 (62%) |
| Fatigue during weaning | 36 (49%) |
| Pneumonia | 20 (27%) |
| Successful liberation from mechanical ventilation | 68 (92%) |
| Lived | 69 (93%) |
Continuous data are presented as medians (interquartile ranges). Categorical data are presented as number (percentage). The large percentage of males and the relatively young age reflect the number of trauma victims cared for in our intensive care unit.
Patients had been intubated for a median duration of 4 days prior to meeting readiness-to-wean criteria. Overall, patients spent a considerable proportion of time on the ventilator after first meeting readiness-to-wean criteria. The median percentage of time a patient remained on the ventilator after the criteria were met was 42% (IQR, 25–69%) of the entire duration of MV. Tracheostomy was performed in 46 (62%) patients, and 36 (49%) patients met criteria for fatigue at least once after initially meeting readiness-to-wean criteria. Pneumonia developed in 20 (27%) patients. Most patients survived to be successfully liberated from MV.
Relationship between ET and duration of weaning and the total duration of MV
The 21 patients in the ET group were demographically similar to the 53 patients in the ST group, except for the greater number of patients with TBI in the ET group (Table 2). All 21 of the ET patients survived and were liberated from MV prior to discharge from the intensive care unit. Six patients in the ST group were not liberated from MV; five patients died and one patient was transferred to a long-term care hospital still requiring ventilator support. The median number of days of MV prior to meeting readiness-to-wean criteria was 6 days (IQR, 4–7 days) in the ET group and was 4 days (IQR, 3–7 days) in the ST group. The median Glasgow Coma Scale (GCS) score for the both the ET and ST patients was 11 (verbal score assigned '1') on the day the readiness-to-wean criteria were met. Twenty-five of the 53 ST patients ultimately received a tracheostomy, after a median of 14 days (IQR, 11–18 days) of MV. The 21 ET patients underwent tracheostomy after a median of 6 days (IQR, 5–9 days) of MV.
Table 2.
Comparison of early tracheostomy and selective tracheostomy groups
| Early tracheostomy (n = 21) | Selective tracheostomy (n = 53) | P value | |
| Clinical characteristics | |||
| Gender, male | 15 (71%) | 34 (64%) | 0.55 |
| Age (years) | 47 (33–58) | 47 (34–55) | 0.97 |
| Multiple trauma victim | 17 (81%) | 31 (59%) | 0.07 |
| Traumatic brain injury (trauma patients as denominator) | 14/17 (81%) | 15/31(52%) | 0.04 |
| Number of days from intubation to readiness-to-wean criteria | 6 (4–7) | 4 (3–7) | 0.39 |
| Number of days from readiness-to-wean criteria to starting weaning | 3 (1–4) | 1 (0–2) | 0.001 |
| Clinical outcomes | |||
| Number of days of mechanical ventilation | 11 (9–26) | 13 (8–21) | 0.86 |
| Number of days weaning | 3 (1–11) | 6 (3–14) | 0.06 |
| Tracheostomy | 21 (100%) | 25 (47%) | < 0.001 |
| Fatigue during weaning | 7 (33%) | 29 (55%) | 0.10 |
| Pneumonia | 3 (14%) | 17 (32%) | 0.12 |
| Successful liberation from mechanical ventilation | 21 (100%) | 47 (89%) | 0.11 |
| Lived | 21 (100%) | 48 (91%) | 0.15 |
Continuous data are presented as medians (interquartile ranges). Categorical data are presented as number (percentage). The large percentage of males and the relatively young age reflect the number of trauma victims cared for in our intensive care unit.
The median duration of weaning was 3 days (IQR, 1–11 days) in the ET group and was 6 days (IQR, 3–14 days) in the ST group (P = 0.05). Once readiness-to-wean criteria were met, active weaning commenced sooner in the patients in the ST group than those in the ET group (P = 0.001). Early tracheostomy was not associated with a shorter total duration of MV.
Figure 2 depicts the effect of ET on the duration of weaning, based upon Cox proportional hazards regression. Gender, age, diagnosis of trauma, duration of MV prior to meeting criteria, the GCS score and rapid shallow breathing index ≤ 105 on the day readiness criteria were met were tested in the initial model. Only rapid shallow breathing index ≤ 105 and ET were associated with more rapid liberation from MV. Patients receiving an ET had a hazard ratio of 2.1 (95% confidence interval, 1.2–3.8) for earlier liberation. A rapid shallow breathing index ≤ 105 had a hazard ratio of 2.4 (95% confidence interval, 1.4–4.4) for earlier liberation. The GCS score was not related to the duration of weaning and did not alter the estimates associated with the other variables in the model. In order to address residual confounding, we added the propensity score to the regression model. After this adjustment, ET had a hazard ratio of 2.2 (95% confidence interval, 1.2–3.7) for earlier liberation.
Figure 2.
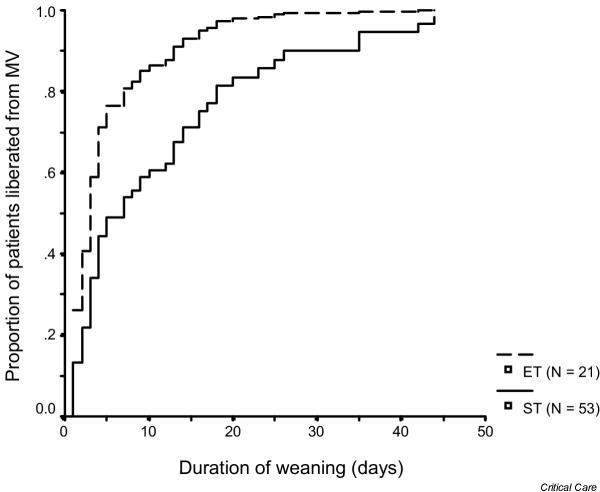
Curves showing the proportion of patients liberated from mechanical ventilation (MV) according to the time that readiness-to-wean criteria were met, based upon the Cox proportional hazards regression model (see 'Patients and methods' for details). The 21 early tracheostomy (ET) patients were liberated more rapidly (median duration of weaning, 3 days) than the selective tracheostomy (ST) group (median duration of weaning, 7 days).
Association among fatigue, pneumonia, ET and duration of weaning (Table 2)
The incidences of fatigue during weaning and pneumonia were higher in the ST group than in the ET group. The initial episode of fatigue occurred 3 days (IQR, 0–8 days) after meeting readiness criteria and a median of 9 days (IQR, 5–13 days) after intubation. Pneumonia was diagnosed a median of 5 days (IQR, 2–14 days) after initially meeting readiness criteria. Fatigue during weaning was strongly associated with the duration of weaning and overshadowed the effects of ET when included in a Cox regression model.
Discussion
Early and relatively nonselective use of tracheostomy in patients with severe acute respiratory failure has been proposed by a number of investigators [4,5]. However, this approach will commit a number of patients to the risks of a surgical procedure from which many will not benefit. Alternatively, limiting tracheostomy to the most difficult to wean patients may contribute to unnecessarily prolonged weaning, to more days of MV and to complications such as pneumonia and laryngeal injury, and possibly death [7]. The authors advocating ET only compared patients who underwent tracheostomy early with patients who underwent later tracheostomy, and did not consider patients who were extubated after the arbitrary definition of ET [4,5]. This leads to an important bias against late tracheostomy. In light of the difficulties presented by the existing literature, our objective was to define a reasonable, physiologically based and clinically relevant time point at which tracheostomy should be considered in critically ill patients requiring MV.
Before considering the implications of our observations, it is important to address the limitations of this study. First, this was a retrospective analysis of a dataset that, while prospectively collected, was used to address a separate research question. We were therefore cognizant of the potential pitfalls of overinterpreting our observations. In part, we selected a limited number of end-points in order to minimize falsely positive associations with tracheostomy. We were primarily interested in the potential influence of tracheostomy on the duration of weaning and of MV, as these periods are distinguished by a relatively clear onset (by criteria detailed in Patients and methods) and end (liberation from MV). The other end-points we examined, pneumonia and fatigue, were of secondary interest in this study, but nonetheless provided interesting and important observations. Fatigue, potentially the most subjective end-point, was assigned prospectively according to a predefined set of criteria, minimizing the bias in its assignment [9].
The outcomes we examined are arguably few of many end-points that a study such as this may address. There are, no doubt, other outcomes that are of equal relevance, such as mortality and the costs of care associated with tracheostomy. Relatively few patients in this series died (5/75 patients; 7%), and all were in the ST group. This low case-fatality rate will make it difficult to test the effect of tracheostomy on mortality in a clinical trial. Other complications of tracheostomy exist and it is possible that they would nullify any benefit of a more liberal use of tracheostomy. Although complication rates for surgical and percutaneous tracheostomy are low, it will be important to consider how this should influence the decision to perform these procedures [8,12]. Nevertheless, it reasonable to consider tracheostomy safe. When performed in patients who are hemodynamically stable and require minimal ventilator support (positive end-expiratory pressure ≤ 5 cmH2O, FiO2 ≤ 0.4, etc.), the perioperative complication rate has been reported to be 0–46% and the attributable mortality rate is not higher than 2% [8,13]. In our study, the majority of tracheostomies were performed by the open surgical technique in the operating room and there were no deaths attributable to the procedure. It is not clear whether the safety of the percutaneous approach should alter the decision regarding when to perform the procedure [12,14].
Perhaps the greatest limitation of our study is the inclusion of heterogeneous patients with regard to the presence of a TBI. Nevertheless, because all patients were cared for by the same critical care team and according to the same respiratory care protocols, we chose to include all patients rather than an arbitrary subgroup based upon the presence or absence of TBI. We have attempted to address this issue in our analyses, but recognize that our conclusions must be tempered by the baseline differences between the two groups.
In some patients who are otherwise able to breathe spontaneously, liberation from MV may be prevented by concerns over the ability to protect against pharyngeotracheal aspiration and to clear pulmonary secretions. Tracheostomy may facilitate liberation in such circumstances, although the benefits here are not clear. It is often difficult to objectively determine which patients will be able to protect their airway after extubation. For example, neurosurgical patients with GCS score < 8 are more likely to require reintubation than patients with GCS score ≥ 8 [15]. However, extubation is often possible in patients with GCS score < 8 [16]. A number of patients in our study had TBI and many were in the ET group (14/30 patients with TBI had an ET). It is probable that concerns over the ability of patients with depressed consciousness to protect their airway contributed to the decision to perform tracheostomy earlier in patients with TBI. Nevertheless, the frequency of altered mental status at the time readiness-to-wean criteria were met was similar in the ET group (14/21 patients with GCS score ≤ 8) and in the ST group (41/53 patients with GCS ≤ 8), suggesting that differences in mental status were not the primary factor in deciding for tracheostomy.
In addition, calculation of the propensity score is meant to address unmeasured bias and confounding that may exist in the decision to perform tracheostomy. We observed that after this adjustment the association between ET and a shorter duration or weaning remained unchanged, suggesting no important residual confounding or bias. However, in order to further explore the potential role of altered mental status and TBI in the process of liberation from MV, we re-examined the relationship between ET and the duration of weaning separately for patients with and without TBI, controlling for the same factors in each analysis. We observed that the association between ET and a shorter period of weaning was similar in patients with and without TBI. Thus, while the inclusion of patients with TBI did not appear to bias our results, it will be necessary to consider the importance of a depressed level of consciousness and to determine other objective measures of a patient's ability to protect their airway in our decisions about tracheostomy and weaning and in future studies of the role of tracheostomy. It will be important to consider the observations of Coplin and colleagues, which indicate that a reduced level of consciousness should not be the primary factor in deciding for or against tracheostomy [16].
It is not clear from previous reports what the correct reference time point should be for defining ET. This was a primary objective of the present report. A threshold based upon an arbitrary number of days after intubation is problematic, given that many patients may not safely undergo tracheostomy due to hemodynamic, neurological or respiratory instability for a number of days. In our study, 20% of patients did not meet readiness-to-wean criteria until after more than 8 days of MV. For many of these patients it may have been unsafe to perform a tracheostomy at a point that has been defined by other investigators as 'early'. Nonetheless, the findings of our study provide the basis for re-examining whether the timing of tracheostomy may impact clinical outcomes. In patients with resolving respiratory failure, most studies have found that tracheostomy results in small changes in dead space ventilation, work of breathing or other objective parameters that may aid in liberation from MV [17,18]. These small changes probably benefit the few patients with borderline respiratory muscle function or relatively large percentage of alveolar dead space. Both circumstances are not typically encountered in postoperative or patients or trauma victims [17,18]. Therefore, potential benefits would be related to less easily quantifiable measures, such as improvements in patient comfort, reductions in anxiety, changes in physician behavior or the minimization of aspiration related to translaryngeal intubation. These may translate into a measurable decrease in the duration of weaning.
Prolonged translaryngeal intubation is associated with a number of complications potentially leading to permanent damage to the laryngeal complex. While the incidence of vocal cord injury is associated with increased length of intubation and certain medical conditions such as diabetes mellitus, conversion from a translaryngeal airway to a tracheostomy may not reduce anatomical airway complications [19]. In general, long-term airway complications such as laryngeal stenosis are uncommon enough to be considered reportable events, and often occur in the presence of additional airway insults, such as inhalation injury [20]. Because laryngeal stenosis is uncommon (2–6%) it would be necessary to study over 1000 patients with adequate follow-up in order to demonstrate a 50% reduction in laryngeal stenosis [21]. However, while most of the acute laryngeal changes (inflammation, ulceration, and edema) resolve without long-term sequelae, translaryngeal intubation leads to transient vocal cord dysfunction that may cause microaspiration and pneumonia [22]. We observed a higher incidence of pneumonia in the ST group, and the majority were diagnosed after weaning criteria were met (15/20 patients; 75%). Given that the assignment of pneumonia is potentially biased in this nonblinded observational study, it is impossible to attribute the increased pneumonia risk to prolonged translaryngeal intubation. Nevertheless, this possibility should be addressed as an important end-point in future clinical trials of the effects of ET.
Conclusions
Our data suggest that ET in patients with respiratory failure may reduce the duration of weaning, the frequency of fatigue and complications such as pneumonia. This association is independent of whether the patient had a TBI or altered mental status at the time of meeting readiness-to-wean criteria. These observations should be confirmed by an appropriately designed clinical trial using entry criteria based on respiratory function not an arbitrary period of endotracheal intubation, and also incorporating appropriate outcome criteria such as the frequency of complications, duration of weaning and mortality.
Key messages
• Tracheostomy performed early, prior to prolonged attempts at weaning may hasten liberation from mechanical ventilation.
• Early tracheostomy appears to have no effect on the total duration of mechanical ventilation.
Competing interests
None declared.
Abbreviations
ET = early tracheostomy; FiO2 = Fraction of Inspired Oxygen; GCS = Glasgow Coma Scale; IQR = interquartile range; MV = mechanical ventilation; PaO2 = Arterial Partial Perssure of Oxygen; ST = selective tracheostomy; TBI = traumatic brain injury.
References
- Whited RE. A prospective study of laryngotracheal sequelae in long-term intubation. Laryngoscope. 1984;94:367–377. doi: 10.1288/00005537-198403000-00014. [DOI] [PubMed] [Google Scholar]
- Torres A, Gatell JM, Aznar E, el Ebiary M, Puig dlB, Gonzalez J, Ferrer M, Rodriguez-Roisin R. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152:137–141. doi: 10.1164/ajrccm.152.1.7599812. [DOI] [PubMed] [Google Scholar]
- Beckmann U, Gillies DM. Factors associated with reintubation in intensive care: an analysis of causes and outcomes. Chest. 2001;120:538–542. doi: 10.1378/chest.120.2.538. [DOI] [PubMed] [Google Scholar]
- Rodriguez JL, Steinberg SM, Luchetti FA, Gibbons KJ, Taheri PA, Flint LM. Early tracheostomy for primary airway management in the surgical critical care setting. Surgery. 1990;108:655–659. [PubMed] [Google Scholar]
- Armstrong PA, McCarthy MC, Peoples JB. Reduced use of resources by early tracheostomy in ventilator-dependent patients with blunt trauma. Surgery. 1998;124:763–766. doi: 10.1067/msy.1998.91224. [DOI] [PubMed] [Google Scholar]
- Sugerman HJ, Wolfe L, Pasquale MD, Rogers FB, O'Malley KF, Knudson M, DiNardo L, Gordon M, Schaffer S. Multicenter, randomized, prospective trial of early tracheostomy. J Trauma. 1997;43:741–747. doi: 10.1097/00005373-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Kollef MH, Ahrens TS, Shannon W. Clinical predictors and outcomes for patients requiring tracheostomy in the intensive care unit. Crit Care Med. 1999;27:1714–1720. doi: 10.1097/00003246-199909000-00003. [DOI] [PubMed] [Google Scholar]
- Freeman BD, Isabella K, Cobb JP, Boyle WA, III, Schmieg RE, Jr, Kolleff MH, Lin N, Saak T, Thompson EC, Buchman TG. A prospective, randomized study comparing percutaneous with surgical tracheostomy in critically ill patients. Crit Care Med. 2001;29:926–930. doi: 10.1097/00003246-200105000-00002. [DOI] [PubMed] [Google Scholar]
- O'Keefe GE, Hawkins K, Boynton J, Burns D. Indicators of fatigue and of prolonged weaning from mechanical ventilation in surgical patients. World J Surg. 2001;25:98–103. doi: 10.1007/s002680020371. [DOI] [PubMed] [Google Scholar]
- Mehta RL, Pascual MT, Soroko S, Chertow GM. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288:2547–2553. doi: 10.1001/jama.288.20.2547. [DOI] [PubMed] [Google Scholar]
- Connors AF, Jr, Speroff T, Dawson NV, Thomas C, Harrell FE, Jr, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ, Jr, Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus WA. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276:889–897. doi: 10.1001/jama.276.11.889. [DOI] [PubMed] [Google Scholar]
- Holdgaard HO, Pedersen J, Jensen RH, Outzen KE, Midtgaard T, Johansen LV, Moller J, Paaske PB. Percutaneous dilatational tracheostomy versus conventional surgical tracheostomy. A clinical randomised study. Acta Anaesthesiol Scand. 1998;42:545–550. doi: 10.1111/j.1399-6576.1998.tb05164.x. [DOI] [PubMed] [Google Scholar]
- Freeman BD, Isabella K, Lin N, Buchman TG. A meta-analysis of prospective trials comparing percutaneous and surgical tracheostomy in critically ill patients. Chest. 2000;118:1412–1418. doi: 10.1378/chest.118.5.1412. [DOI] [PubMed] [Google Scholar]
- Lim JW, Friedman M, Tanyeri H, Lazar A, Caldarelli DD. Experience with percutaneous dilational tracheostomy. Ann Otol Rhinol Laryngol. 2000;109:791–796. doi: 10.1177/000348940010900901. [DOI] [PubMed] [Google Scholar]
- Namen AM, Ely EW, Tatter SB, Case LD, Lucia MA, Smith A, Landry S, Wilson JA, Glazier SS, Branch CL, Kelly DL, Bowton DL, Haponik EF. Predictors of successful extubation in neurosurgical patients. Am J Respir Crit Care Med. 2001;163:658–664. doi: 10.1164/ajrccm.163.3.2003060. [DOI] [PubMed] [Google Scholar]
- Coplin WM, Pierson DJ, Cooley KD, Newell DW, Rubenfeld GD. Implications of extubation delay in brain-injured patients meeting standard weaning criteria. Am J Respir Crit Care Med. 2000;161:1530–1536. doi: 10.1164/ajrccm.161.5.9905102. [DOI] [PubMed] [Google Scholar]
- Mohr AM, Rutherford EJ, Cairns BA, Boysen PG. The role of dead space ventilation in predicting outcome of successful weaning from mechanical ventilation. J Trauma. 2001;51:843–848. doi: 10.1097/00005373-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Davis K, Jr, Campbell RS, Johannigman JA, Valente JF, Branson RD. Changes in respiratory mechanics after tracheostomy. Arch Surg. 1999;134:59–62. doi: 10.1001/archsurg.134.1.59. [DOI] [PubMed] [Google Scholar]
- Deeb ZE, Williams JB, Campbell TE. Early diagnosis and treatment of laryngeal injuries from prolonged intubation in adults. Otolaryngol Head Neck Surg. 1999;120:25–29. doi: 10.1016/S0194-5998(99)70365-7. [DOI] [PubMed] [Google Scholar]
- Cobley TD, Hart WJ, Baldwin DL, Burd DA. Complete fusion of the vocal cords; an unusual case. Burns. 1999;25:361–363. doi: 10.1016/S0305-4179(98)00185-5. [DOI] [PubMed] [Google Scholar]
- Briche T, Le Manach Y, Pats B. Complications of percutaneous tracheostomy. Chest. 2001;119:1282–1283. doi: 10.1378/chest.119.4.1282. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Bauer TT, Torres A, Hernandez C, Piera C. Effect of nasogastric tube size on gastroesophageal reflux and microaspiration in intubated patients. Ann Intern Med. 1999;130:991–994. doi: 10.7326/0003-4819-130-12-199906150-00007. [DOI] [PubMed] [Google Scholar]


