Abstract
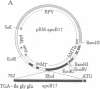
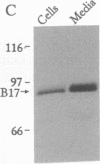
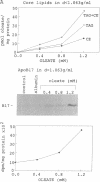
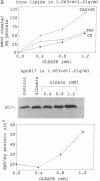
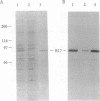
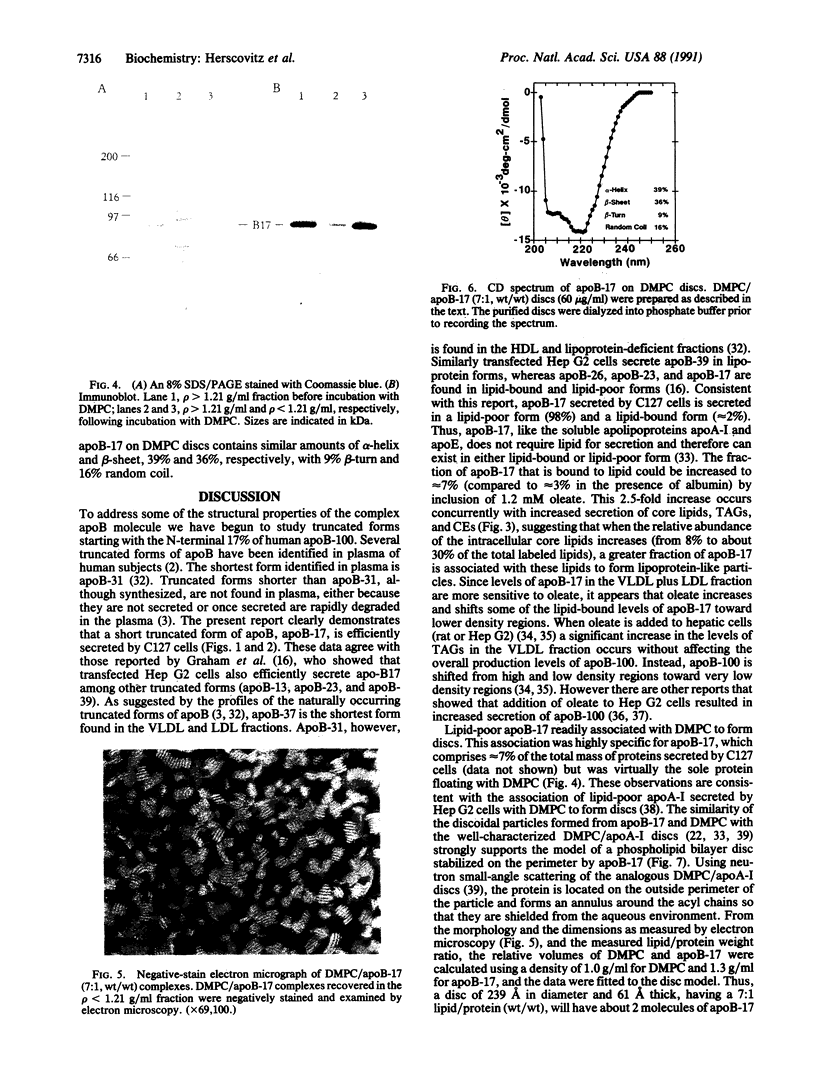
The N-terminal 17% of human apolipoprotein B (apoB-17) was expressed in murine C127 cells following transfection with a bovine papilloma virus-based expression vector. A permanent cell line overexpressing the expected 89-kDa protein was selected and characterized. Pulse-chase experiments showed that the depletion of intracellular apoB-17 follows an apparent first-order kinetics with t1/2 = 51 min. Under conditions of continuous labeling, greater than 60% of the total synthesized apoB-17 was secreted in a soluble form, approximately 98% lipid-poor and approximately 2% lipid-bound. Inclusion of 1.2 mM oleate resulted in 5- and 2.5-fold increases in the amount of labeled apoB-17 in the p less than 1.063 g/ml and 1.063 less than p less than 1.21 g/ml fractions, respectively, which was coordinated with increased secretion of radiolabeled core lipids, triacylglycerols, and cholesteryl esters. Thus under conditions in which lipid pools are enriched a greater fraction of apoB-17 may be secreted on lipoprotein-like particles. The lipid-poor apoB-17 present in p greater than 1.21 g/ml readily associates with exogenously added dimyristoylphosphatidylcholine (DMPC) multilamellar vesicles to form discoidal particles. Discs formed with DMPC/apoB-17, 7:1 (wt/wt), are 239 +/- 43 A in diameter and 61 +/- 4 A thick and contain approximately 2 molecules of apoB-17 and 2250 molecules of DMPC per disc. Based on volume calculations we conclude that apoB-17 forms an annulus about one bilayer high and 10 A thick surrounding the DMPC disc. Circular dichroic spectra of apoB-17 on DMPC discs showed apoB-17 to contain 39% alpha-helix, 36% beta-sheet, 9% beta-turn, and 16% random coil. To be consistent with this model greater than 70% of apoB-17 on DMPC discs must bind to lipid. These data suggest that the N-terminal 17% of apoB-100 can bind lipid and may contribute to some extent to the stabilization of triglyceride-rich lipoproteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D., Shipley G. G. Structural studies of plasma lipoproteins. Basic Life Sci. 1984;27:211–226. doi: 10.1007/978-1-4899-0375-4_13. [DOI] [PubMed] [Google Scholar]
- Atkinson D., Small D. M. Recombinant lipoproteins: implications for structure and assembly of native lipoproteins. Annu Rev Biophys Biophys Chem. 1986;15:403–456. doi: 10.1146/annurev.bb.15.060186.002155. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blackhart B. D., Yao Z. M., McCarthy B. J. An expression system for human apolipoprotein B100 in a rat hepatoma cell line. J Biol Chem. 1990 May 25;265(15):8358–8360. [PubMed] [Google Scholar]
- Boström K., Borén J., Wettesten M., Sjöberg A., Bondjers G., Wiklund O., Carlsson P., Olofsson S. O. Studies on the assembly of apo B-100-containing lipoproteins in HepG2 cells. J Biol Chem. 1988 Mar 25;263(9):4434–4442. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Chen G. C., Hardman D. A., Hamilton R. L., Mendel C. M., Schilling J. W., Zhu S., Lau K., Wong J. S., Kane J. P. Distribution of lipid-binding regions in human apolipoprotein B-100. Biochemistry. 1989 Mar 21;28(6):2477–2484. doi: 10.1021/bi00432a019. [DOI] [PubMed] [Google Scholar]
- Cladaras C., Hadzopoulou-Cladaras M., Felber B. K., Pavlakis G., Zannis V. I. The molecular basis of a familial apoE deficiency. An acceptor splice site mutation in the third intron of the deficient apoE gene. J Biol Chem. 1987 Feb 15;262(5):2310–2315. [PubMed] [Google Scholar]
- Cladaras C., Hadzopoulou-Cladaras M., Nolte R. T., Atkinson D., Zannis V. I. The complete sequence and structural analysis of human apolipoprotein B-100: relationship between apoB-100 and apoB-48 forms. EMBO J. 1986 Dec 20;5(13):3495–3507. doi: 10.1002/j.1460-2075.1986.tb04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D. R., Knott T. J., Pease R. J., Powell L. M., Wallis S. C., Robertson S., Pullinger C. R., Milne R. W., Marcel Y. L., Humphries S. E. Truncated variants of apolipoprotein B cause hypobetalipoproteinaemia. Nucleic Acids Res. 1988 Sep 12;16(17):8361–8375. doi: 10.1093/nar/16.17.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti N., Wolfbauer G. Secretion of lipids, apolipoproteins, and lipoproteins by human hepatoma cell line, HepG2: effects of oleic acid and insulin. J Lipid Res. 1987 Apr;28(4):423–436. [PubMed] [Google Scholar]
- Davis R. A., Boogaerts J. R. Intrahepatic assembly of very low density lipoproteins. Effect of fatty acids on triacylglycerol and apolipoprotein synthesis. J Biol Chem. 1982 Sep 25;257(18):10908–10913. [PubMed] [Google Scholar]
- Forte T. M., Nichols A. V., Selmek-Halsey J., Caylor L., Shore V. G. Lipid-poor apolipoprotein A-I in Hep G2 cells: formation of lipid-rich particles by incubation with dimyristoylphosphatidylcholine. Biochim Biophys Acta. 1987 Aug 15;920(3):185–194. doi: 10.1016/0005-2760(87)90094-4. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innerarity T. L., Weisgraber K. H., Rall S. C., Jr, Mahley R. W. Functional domains of apolipoprotein E and apolipoprotein B. Acta Med Scand Suppl. 1987;715:51–59. doi: 10.1111/j.0954-6820.1987.tb09903.x. [DOI] [PubMed] [Google Scholar]
- Kane J. P. Apolipoprotein B: structural and metabolic heterogeneity. Annu Rev Physiol. 1983;45:637–650. doi: 10.1146/annurev.ph.45.030183.003225. [DOI] [PubMed] [Google Scholar]
- Knott T. J., Pease R. J., Powell L. M., Wallis S. C., Rall S. C., Jr, Innerarity T. L., Blackhart B., Taylor W. H., Marcel Y., Milne R. Complete protein sequence and identification of structural domains of human apolipoprotein B. Nature. 1986 Oct 23;323(6090):734–738. doi: 10.1038/323734a0. [DOI] [PubMed] [Google Scholar]
- Krawczyk Z., Wu C. Isolation of RNA for dot hybridization by heparin-DNase I treatment of whole cell lysate. Anal Biochem. 1987 Aug 15;165(1):20–27. doi: 10.1016/0003-2697(87)90195-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. W., Grant S. M., Higuchi K., Hospattankar A., Lackner K., Lee N., Brewer H. B., Jr Human liver apolipoprotein B-100 cDNA: complete nucleic acid and derived amino acid sequence. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8142–8146. doi: 10.1073/pnas.83.21.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., Hirz R., Shrager R. I., Gotto A. M. The influence of lipid on the conformation of human plasma high density apolipoproteins. J Biol Chem. 1972 Apr 25;247(8):2598–2606. [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Milne R., Théolis R., Jr, Maurice R., Pease R. J., Weech P. K., Rassart E., Fruchart J. C., Scott J., Marcel Y. L. The use of monoclonal antibodies to localize the low density lipoprotein receptor-binding domain of apolipoprotein B. J Biol Chem. 1989 Nov 25;264(33):19754–19760. [PubMed] [Google Scholar]
- Pullinger C. R., North J. D., Teng B. B., Rifici V. A., Ronhild de Brito A. E., Scott J. The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin, and measurement of the mRNA half-life. J Lipid Res. 1989 Jul;30(7):1065–1077. [PubMed] [Google Scholar]
- Tall A. R., Small D. M., Deckelbaum R. J., Shipley G. G. Structure and thermodynamic properties of high density lipoprotein recombinants. J Biol Chem. 1977 Jul 10;252(13):4701–4711. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M. T., Atkinson D. Physical properties of apoprotein B in mixed micelles with sodium deoxycholate and in a vesicle with dimyristoyl phosphatidylcholine. J Lipid Res. 1986 Mar;27(3):316–325. [PubMed] [Google Scholar]
- Walsh M. T., Atkinson D. Solubilization of low-density lipoprotein with sodium deoxycholate and recombination of apoprotein B with dimyristoylphosphatidylcholine. Biochemistry. 1983 Jun 21;22(13):3170–3178. doi: 10.1021/bi00282a021. [DOI] [PubMed] [Google Scholar]
- Walsh M. T., Watzlawick H., Putnam F. W., Schmid K., Brossmer R. Effect of the carbohydrate moiety on the secondary structure of beta 2-glycoprotein. I. Implications for the biosynthesis and folding of glycoproteins. Biochemistry. 1990 Jul 3;29(26):6250–6257. doi: 10.1021/bi00478a020. [DOI] [PubMed] [Google Scholar]
- Yang C. Y., Chen S. H., Gianturco S. H., Bradley W. A., Sparrow J. T., Tanimura M., Li W. H., Sparrow D. A., DeLoof H., Rosseneu M. Sequence, structure, receptor-binding domains and internal repeats of human apolipoprotein B-100. Nature. 1986 Oct 23;323(6090):738–742. doi: 10.1038/323738a0. [DOI] [PubMed] [Google Scholar]
- Yang C. Y., Gu Z. W., Weng S. A., Kim T. W., Chen S. H., Pownall H. J., Sharp P. M., Liu S. W., Li W. H., Gotto A. M., Jr Structure of apolipoprotein B-100 of human low density lipoproteins. Arteriosclerosis. 1989 Jan-Feb;9(1):96–108. doi: 10.1161/01.atv.9.1.96. [DOI] [PubMed] [Google Scholar]
- Yao Z. M., Blackhart B. D., Linton M. F., Taylor S. M., Young S. G., McCarthy B. J. Expression of carboxyl-terminally truncated forms of human apolipoprotein B in rat hepatoma cells. Evidence that the length of apolipoprotein B has a major effect on the buoyant density of the secreted lipoproteins. J Biol Chem. 1991 Feb 15;266(5):3300–3308. [PubMed] [Google Scholar]
- Young S. G., Hubl S. T., Smith R. S., Snyder S. M., Terdiman J. F. Familial hypobetalipoproteinemia caused by a mutation in the apolipoprotein B gene that results in a truncated species of apolipoprotein B (B-31). A unique mutation that helps to define the portion of the apolipoprotein B molecule required for the formation of buoyant, triglyceride-rich lipoproteins. J Clin Invest. 1990 Mar;85(3):933–942. doi: 10.1172/JCI114522. [DOI] [PMC free article] [PubMed] [Google Scholar]