Abstract
Introduction
Consistent data about the incidence and outcome of sepsis in Latin American intensive care units (ICUs), including Brazil, are lacking. This study was designed to verify the actual incidence density and outcome of sepsis in Brazilian ICUs. We also assessed the association between the Consensus Conference criteria and outcome
Methods
This is a multicenter observational cohort study performed in five private and public, mixed ICUs from two different regions of Brazil. We prospectively followed 1383 adult patients consecutively admitted to those ICUs from May 2001 to January 2002, until their discharge, 28th day of stay, or death. For all patients we collected the following data at ICU admission: age, gender, hospital and ICU admission diagnosis, APACHE II score, and associated underlying diseases. During the following days, we looked for systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock criteria, as well as recording the sequential organ failure assessment score. Infection was diagnosed according to CDC criteria for nosocomial infection, and for community-acquired infection, clinical, radiological and microbiological parameters were used.
Results
For the whole cohort, median age was 65.2 years (49–76), median length of stay was 2 days (1–6), and the overall 28-day mortality rate was 21.8%. Considering 1383 patients, the incidence density rates for sepsis, severe sepsis and septic shock were 61.4, 35.6 and 30.0 per 1000 patient-days, respectively. The mortality rate of patients with SIRS, sepsis, severe sepsis and septic shock increased progressively from 24.3% to 34.7%, 47.3% and 52.2%, respectively. For patients with SIRS without infection the mortality rate was 11.3%. The main source of infection was lung/respiratory tract.
Conclusion
Our preliminary data suggest that sepsis is a major public health problem in Brazilian ICUs, with an incidence density about 57 per 1000 patient-days. Moreover, there was a close association between ACCP/SCCM categories and mortality rate.
Keywords: epidemiology, incidence, outcome, sepsis
Introduction
Consistent data about the incidence and outcome of sepsis in Latin American intensive care units (ICUs), including Brazil, are lacking. In general, local registries consider sepsis as an admission diagnosis only, and do not identify those patients who develop sepsis during their ICU stay. Furthermore, the behavior of sepsis and sepsis-related organic dysfunction has not been established outside the developed countries. Mainly for economic reasons, there are no consistent epidemiological data that would allow adequate investigational, preventive or even corrective strategies with regard to this problem.
Brazil is a country of continental dimensions with a heterogeneous population and unequal access to health services. Private hospitals are usually better equipped and have more resources than public hospitals, with the exception of some public university hospitals. Measures to diminish the prevalence of sepsis and to reduce sepsis-related mortality driven by reliable data about sepsis in the population of Brazil should be included in any national health programs.
During the past decade there has been a host of discoveries about sepsis pathogenesis, prevention and therapeutic strategies [1-3]. However, sepsis prevalence and mortality rates remain extremely high [4-7]. Angus and colleagues [5] estimated the incidence of severe sepsis to be 751,000 cases per year in the USA, which corresponds to 3.0 cases per 1000 inhabitants and 2.26 cases per 100 hospital discharges. The observed overall mortality rate was 28.6%. In a large, prospective European epidemiological study, Alberti and colleagues [6] evaluated 14,364 patients admitted to 28 ICUs. Their main findings were a crude infection incidence of 21.1%, and in ICU patients hospitalized longer than 24 hours an infection incidence of 18.9%, including 45% of patients already infected at ICU admission. About 24% of infections were associated with severe sepsis and 30% with septic shock. Total hospital mortality rates ranged from 16.9% in non-infected patients to 53.6% in patients with hospital-acquired infections. More recently, a multicenter French study [7] has shown a high incidence of septic shock (8.2 per 100 admissions) among critically ill patients associated with a high crude mortality rate (60.1%).
Although some randomized clinical trials [2,8] have used the American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) Consensus Conference criteria [9] there are a few prospective cohort studies [6,10] addressing the actual incidence of sepsis, severe sepsis and septic shock based on those criteria. Furthermore, despite the putative capacity of ACCP/SCCM categories to discriminate the severity of illness and related mortality, they do not provide a dynamic measurement of organ dysfunction development and outcome. A simultaneous daily use of organ dysfunction descriptors might therefore be useful to better characterize in-ICU progress and estimate the severity of patients' condition daily.
In this study we prospectively analyzed all patients admitted to five mixed ICUs with different characteristics, located in two distinct Brazilian regions, to determine the actual incidence density of sepsis. Additionally, we assessed the association between the severity categories of the Consensus Conference criteria and outcomes.
Methods
Study design and setting
This was a multicenter observational cohort study conducted from May 2001 to January 2002, involving all adult patients admitted consecutively to five mixed ICUs in two different regions of Brazil: Hospital Israelita Albert Einstein (a private tertiary hospital), Hospital Geral do Grajaú (a public community hospital managed by a private university), Hospital Governador Celso Ramos (a public tertiary hospital and regional trauma center), University Hospital of the Federal University of Santa Catarina (a public university hospital), and Hospital do Servidor Público of the State of São Paulo (a public tertiary teaching hospital). The detailed characteristics of each hospital, such as number of hospital and ICU beds, mean monthly hospital and ICU admissions, and mean ICU length of stay (LOS) are shown in Table 1.
Table 1.
Hospital and demographic characteristics for participating centers
| Characteristic | Hospital Albert Einstein | Hospital Geral do Grajaú | Hospital Governador Celso Ramos | Hospital Universitário da UFSC | Hospital dos Servidores do Estado de SP | |
| (Center 1) | (Center 2) | (Center 3) | (Center 4) | (Center 5) | ||
| Type | Private tertiary hospital | Public community hospital managed by a private university | Public tertiary hospital with a regional trauma center | Public University hospital | Public tertiary teaching hospital | |
| Number of beds | 400 | 249 | 272 | 255 | 391 | |
| Number of ICU beds | 28 | 12 | 12 | 06 | 16 | |
| Stepdown unit | Yes | No | No | No | No | |
| Mean monthly hospital admissions | 1400 | 1050 | 503 | 624 | 2184 | |
| Mean monthly ICU admissions | 170 | 38 | 46 | 25 | 103 | |
| Enrollment period | 21 May to 10 October 2001 | 21 May to 10 October 2001 | 21 May to 10h October 2001 | 21 May to 10 October 2001 | 1 November to 31 January 2002 | Overall |
| Number of ICU admissions | 837 | 194 | 228 | 124 | 305 | 1688 |
| Number of excluded patients | 100 | 02 | 07 | 10 | 13 | 132 |
| Number of missed patients | 134 | 11 | 02 | 12 | 14 | 173 |
| Number of enrolled patients | 603 | 181 | 219 | 102 | 278 | 1383 |
| Ratio of >24 h patients to all patients | 0.59 | 0.80 | 0.57 | 0.75 | 0.64 | 0.64 |
| Mean ICU LOS, days | 1 (1–4) | 4 (2–9) | 2 (1–7) | 2 (1–6) | 2 (1–6) | 2 (1–6) |
| Overall ICU mortality rate,% | 11.6 | 38.25 | 27.7 | 28.7 | 26.1 | 21.8 |
ICU, intensive care unit; LOS, length of stay. Figures in parentheses are interquartile ranges.
Subjects
All patients were admitted to the ICUs during the study period. Patients less than 18 years old or transferred from other hospitals were excluded from the study. Patients without informed consent were not included.
Measurements and outcome evaluation
Complete data for all patients admitted to the ICUs were obtained until their ICU discharge, 28th day of stay, or death. Mortality status was obtained for all patients at the 28th day after inclusion in the study. For patients who stayed less than 24 hours in the ICU, we recorded only their demographic data, ICU diagnosis (according to admission categories defined in the Acute Physiology and Chronic Health Evaluation II [APACHE II], which included sepsis) and outcome. For patients with a LOS greater than 24 hours, on admission to the ICU (day zero) the age, gender, hospital and ICU admission diagnoses, APACHE II score, and associated underlying diseases were noted. Additionally, systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis and septic shock criteria, as well as sequential organ failure assessment (SOFA) score [11] were evaluated daily. For calculation of APACHE II and SOFA scores, all laboratory and clinical data not measured were considered to be within normal ranges. For those variables that do not change acutely (such as bilirubin and creatinine concentrations) and were not measured on a specific day, we assumed the closest value from the previous day. The neurological status of patients receiving sedative drugs was assessed by the Glasgow Coma Scale as measured or estimated before sedation.
A single patient might have contributed to more than one surveillance episode, if he or she were admitted more than once to the ICU during his or her hospitalization period. The onset of sepsis, severe sepsis or septic shock was defined as the time at which screening and confirmatory criteria were first documented.
Definitions
Sepsis and sepsis-related conditions were diagnosed in accordance with the criteria proposed by ACCP/SCCM [6], as follows.
SIRS was defined by two or more of the following criteria: temperature above 38°C or below 36°C, tachycardia (heart rate more than 90 beats/min), tachypnoea manifested by a raised respiratory rate (more than 20 breaths/min) or hyperventilation (partial pressure of CO2 in arterial blood < 32 mmHg) or mechanical ventilation, altered white blood cell count (more than 12,000/mm3, less than 4000/mm3 or more than 10% of band forms).
Sepsis was defined as a systemic inflammatory response due to infection.
Severe sepsis was defined as sepsis plus at least one organ dysfunction according to the SOFA score. Any value of the SOFA score ascribed for organ dysfunction defined a patient as having severe sepsis.
Septic shock was defined as severe sepsis and vasoactive drug requirement (SOFA 3–4).
The diagnosis of community-acquired infection was based on clinical, image and microbiological parameters. As directed by the attending physician, blood, tracheal secretion, urine, cerebrospinal fluid and wound/skin secretion samples were obtained for culture. For nosocomial infection, the diagnosis was based on the definitions of the Centers for Disease Control [12]. The infection source was classified by the attending physician as lung/respiratory tract, urine, primary bloodstream or wound/surgical site.
After the cohort had been identified, two study groups were determined: patients with an ICU LOS of less than 24 hours, and patients with an ICU LOS of more than 24 hours. We have included data from the first group to obtain an overview of ICU profile (Table 1). The incidence density and mortality rates of sepsis were calculated from the total number of patients enrolled in the study (n = 1383). We also calculated the incidence density of SIRS, sepsis, severe sepsis and septic shock in patients in the second group (n = 884). In addition, we compared epidemiological data between private and public hospitals (center 1 versus other centers).
Sepsis-related mortality was defined as death due to a septic event, in accordance with the investigator's judgment.
In each participating center, physicians and research nurses were trained to collect data; a coordinator research nurse validated those data. A coordinating center (Hospital Israelita Albert Einstein) supported data registration at each center, and all data were checked to be within acceptable ranges. Every clinical report form was therefore checked for blank fields or obvious errors. Whenever present, exception reports were returned to each center for immediate correction. A subset of about 10% (n = 130) of all clinical report forms were cross-checked with original patient charts to test data validity. Most of them were considered adequate. An operating manual defined all collected variables precisely.
The institutional review boards of all participating centers approved the study protocol. Informed consent was obtained from each patient or next of kin.
Statistical analysis
Results are expressed as means ± SD for variables that putatively exhibited a normal distribution. On rejection of the normality hypothesis or for ordinal variables, we used the median and interquartile range (IQR). Student's t-test for independent groups was applied to data with a normal distribution. When normality was rejected or ordinal variables were involved, the Mann–Whitney U-test was used for independent groups. For categorical variables the Pearson χ2 test or Fisher's exact test was applied as appropriate. Survival probabilities were estimated with the product-limit method (Kaplan–Meier algorithm). The log-rank test was used to compare patient survivals in different groups. All analyses were two-tailed. A 95% confidence interval (CI) was used for the incidence rates. Statistical significance was recognized at P < 0.05. The statistical analysis was performed with the Minitab software package for Windows (release 13.1, Minitab Inc., State College, PA, USA).
Results
General overview
There were five participating ICUs from two different States of Brazil. The mean monthly hospital admissions average about 190,000 for São Paulo State and 32,000 for Santa Catarina State (data from Ministry of Health; http://www.datasus.gov.br). Hospital and demographic characteristics of the participating centers are shown in Table 1. During the period from 21 May 2001 to 31 January 2002, 1688 patients were admitted to the ICUs of the participating centers. Of these, 132 (7.8%) were excluded and 173 (10.2%) were missed. The total number of enrolled patients was therefore 1383 (81.9%). Of these, 884 (63.9%) had an ICU LOS longer than 24 hours, and 499 (36.1%) had an ICU LOS less than 24 hours. Figure 1 depicts the whole process of patient screening and their respective mortality rates in accordance with Consensus Conference categories.
Figure 1.
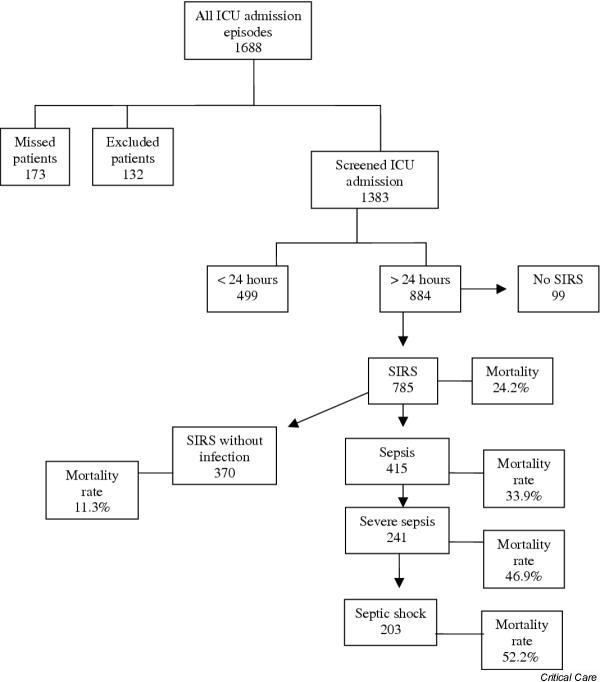
Flow diagram of enrolled patients and their mortality rate according to Consensus Conference categories. ICU, intensive care unit; SIRS, systemic inflammatory response syndrome.
Groups for LOS
For the whole cohort (n = 1383), the median age was 65.2 years (IQR 49–76); 700 (58.7%) were males, the median ICU LOS was 2 days (1–6), and the overall 28-day mortality rate was 21.8%. The main ICU admission diagnoses were as follows: lung/respiratory tract infection (8.5%), acute coronary syndrome (7.2%), gastrointestinal surgery (6.0%), sepsis (5.2%), cardiovascular surgery (4.6%), gastrointestinal surgery for malignancy (4.6%) and clinical neurological disorders (4.1%).
For the 1383 patients, the incidence density of sepsis was 57.9 (95% CI 51.5–65.3) per 1000 patient-days or 421 episodes of sepsis, corresponding to 30.5 (28.9–32.1) per 100 screened ICU admissions. In the same population, there were 241 episodes of severe sepsis and 203 episodes of septic shock, corresponding to 17.4 (16.5–18.6) and 14.7 (13.9–15.5) per 100 screened ICU admissions. For patients who stayed less than 24 hours in ICU, the median age was 64 years (48–74), 57.6% were male, main admission diagnoses were similar to those of the whole group, and 20.8% of them died.
A total of 884 patients with an ICU LOS longer than 24 hours were enrolled in all participating centers. The median age was 66.4 years (IQR 49.2–77.1), 522 (59%) were males, median ICU LOS was 4 days (2–9), median APACHE II score was 17 (11–22), median SOFA score on the first day was 4 (2–8) and the overall 28-day mortality rate was 22.3%. The main ICU admission diagnoses were as follows: lung/respiratory tract infection (13.9%), acute coronary syndrome (12%), gastrointestinal surgery (9.3%), sepsis (7.8%), gastrointestinal surgery for malignancy (7.0%), post-surgical cardiovascular vigilance (6.9%) and clinical neurological disorders (6.4%). Several other admission diagnostic categories, each amounting to less than 6% of enrolled patients, accounted for the remaining 30.9% of diagnoses (Table 2).
Table 2.
Clinical and infectious characteristics of all patients with an ICU LOS of more than24 hours
| Characteristic | Center 1 | Center 2 | Center 3 | Center 4 | Center 5 | Total |
| Enrolled patients (n) | 359 | 146 | 125 | 76 | 178 | 884 |
| Median age (IQR) | 73.6 (58.5–81.5) | 55.4 (42.5–60.6) | 47.6 (32.0–66.9) | 61.4 (44.2–68.7) | 68.9 (57.8–77.1) | 66.4 (49.2–77.1) |
| Male/female (%) | 62/38 | 59/41 | 59/41 | 62/38 | 51/49 | 59/41 |
| Median APACHE II score (IQR) | 16 (11–21) | 17.5 (9.7–25.2) | 16 (12–20) | 16 (10–20) | 18 (14–21) | 17 (11–22) |
| First-day SOFA score (IQR) | 3 (1–5) | 6 (2.7–9) | 6 (3–8) | 4 (2–7) | 6 (4–9) | 4 (2–8) |
| Median ICU LOS, days (IQR) | 3 (2–7) | 5.5 (3–10.2) | 6 (3–12) | 3 (2–7.7) | 4 (2–10) | 4 (2–9) |
| Main ICU admission diagnosis | ||||||
| Respiratory infection, n (%) | 43 (11.9) | 16 (10.9) | 10 (8.0) | 12 (15.8) | 42 (23.6) | 123 (13.9) |
| Acute coronary syndrome, n (%) | 52 (14.5) | 24 (16.4) | 4 (3.2) | 25 (32.9) | 2 (1.1) | 107 (12.0) |
| GI postoperative patients, n (%) | 9 (2.5) | 11 (7.5) | 5 (4.0) | 7 (9.2) | 50 (28.1) | 82 (9.3) |
| Sepsis, n (%) | 34 (9.5) | 2 (1.4) | 17 (13.6) | 3 (3.9) | 13 (7.3) | 69 (7.8) |
| GI surgery for neoplasm, n (%) | 22 (6.1) | 4 (2.7) | 4 (3.2) | 7 (9.2) | 25 (14.0) | 62 (7.0) |
| Cardiovascular surgery, n (%) | 38 (10.6) | 1 (0.06) | 4 (3.2) | - | 18 (10.1) | 61 (6.9) |
| Clinical neurological disorders, n (%) | 36 (10.0) | 10 (6.8) | 6 (4.8) | - | 5 (02.8) | 57 (6.4) |
| Site of infection | ||||||
| Lung, n (%) | 86 (72.3) | 30 (43.5) | 52 (67.5) | 24 (68.5) | 75 (70.1) | 267 (65.6) |
| Urinary tract, n (%) | 9 (7.6) | 4 (5.7) | 5 (6.5) | - | 5 (4.7) | 23 (5.6) |
| Abdomen/surgical wound, n (%) | 4 (3.3) | 5 (7.3) | - | 5 (14.3) | 6 (5.6) | 20 (4.9) |
| Bloodstream, n (%) | 5 (4.2) | 1 (1.5) | 1 (1.3) | 3 (8.6) | 21 (19.6) | 10 (2.5) |
| Other/unknown, n (%) | 15 (12.6) | 29 (42.2) | 19 (24.7) | 3 (8.6) | 21 (19.6) | 87 (21.4) |
| Diagnosed infections, n (%) | 119 (100) | 69 (100) | 77 (100) | 35 (100) | 107 (100) | 407 (100) |
| Enrolled patients, n | ||||||
| Consensus Conference Diagnosis | 359 | 146 | 125 | 76 | 178 | 884 (100%) |
| SIRS | 291 | 130 | 122 | 74 | 168 | 785 (88.8%) |
| Sepsis | 121 | 72 | 79 | 34 | 109 | 415 (46.9%) |
| Severe sepsis | 57 | 38 | 46 | 191 | 81 | 241 (27.3%) |
| Septic shock | 42 | 34 | 39 | 16 | 72 | 203 (23.0%) |
| Overall mortality rate (%) | 12.5 | 32.2 | 23.7 | 24.8 | 31.4 | 22.3 |
GI, gastrointestinal; IQR, interquartile range; LOS, length of stay; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment.
Patients with SIRS, sepsis, severe sepsis and septic shock
Of 884 patients with an ICU LOS of more than 24 hours, 785 (88.8%) met SIRS criteria in at least 1 day during their ICU stay. In the same direction, sepsis criteria were fulfilled in 415 (46.9%), severe sepsis in 241 (27.3%) and septic shock in 203 (23%) patients. It is noteworthy that the same patient could be classified in more than one category if he or she fulfilled the criteria, because these categories represent evolving stages of the same process. The incidence density rates for sepsis, severe sepsis and septic shock were 61.4 (95% CI 55.5–67.3), 35.6 (31.1–40.1) and 30.0 (25.9–31.4) per 1000 patient-days, respectively. The mortality rates of patients with SIRS, sepsis, severe sepsis and septic shock were 24.2%, 33.9%, 46.9% and 52.2%, respectively (Fig. 2). For patients with SIRS without infection the mortality rate was 11.3%.
Figure 2.
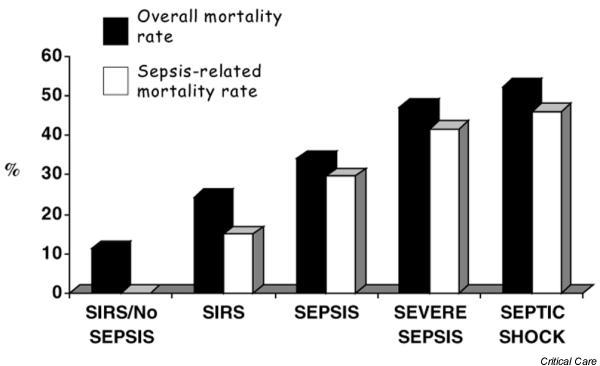
Overall mortality rate and sepsis-related mortality rate according to American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions. A progressively higher mortality was observed as systemic inflammatory response syndrome (SIRS) was due to infection, namely sepsis, and as more severe organ dysfunctions were present, namely severe sepsis and septic shock. The first column shows mortality from the group of patients who met SIRS criteria but who had no infection; in that case there was no sepsis-related death.
For a better understanding of the relationship between ACCP/SCCM definitions and outcome, we calculated the first-day SOFA score for each category. The median scores were 3 (IQR 1–5.2), 4 (3–6), 7.5 (5–10) and 8 (6–11) for SIRS, sepsis, severe sepsis and septic shock, respectively. Among septic patients (n = 415), the medians of the maximum SOFA scores for each category were 5.0 (4–7), 8.8 (7–11), 11.0 (9–13) for sepsis, severe sepsis and septic shock, respectively. There was a significant positive correlation between the maximum SOFA score and each category (rs = 0.72, P < 0.0005).
Sepsis was diagnosed in 415 patients during their ICU stay, with 281 (67.7%) meeting sepsis diagnostic criteria on admission and 134 (32.3%) on the following days. Among patients with sepsis diagnosed after admission to ICU, the main primary admission diagnoses were as follows: neurological disorders (20.1%), head trauma (14.2%), gastrointestinal surgery (13.4%) and lung/respiratory tract infection (8.2%). For all septic patients, the source of infection in each septic episode was lung/respiratory tract in 65.6%, urinary tract in 5.6%, abdomen/surgical wound in 4.9%, bloodstream in 2.5%, and other/unknown sites in 21.4%.
Septic versus nonseptic patients
We prospectively divided the patients with an ICU LOS of more than 24 hours into septic and nonseptic groups. The median ages were similar for septic (66.2 years [IQR 48.2–78.3]) and nonseptic (66.4 years [50–76]) patients, respectively. The median APACHE II score was higher in septic patients (19 [14-24]) than in nonseptic patients (15 [10-19]) (P < 0.0005).
We assessed the SOFA scores for all patients to identify the severity of organ dysfunction in septic and nonseptic patients. The median SOFA score on the first sepsis day (or admission day for nonseptic patients) was higher in septic patients (6 [IQR 4–9]) than in nonseptic patients (3 [1-5]) (P < 0.0005). In addition, survivors in both groups had significantly lower SOFA scores than non-survivors.
However, some chronic diseases were more prevalent in septic patients, such as congestive heart failure (4.1% versus 1.5%; P < 0.05), malignancy (18.3% versus 12.1%; P < 0.05) and chronic obstructive pulmonary disease (14.0% versus 9.4%; P < 0.05). Hypertension (38.1% versus 38.6%), diabetes mellitus (21.7% versus 22.2%), chronic renal failure (7.5% versus 6.8%) and liver cirrhosis (4.3% versus 2.9%) were equally distributed between the two groups. Finally, Fig. 3 depicts the 28-day Kaplan–Meyer survival curve of septic and nonseptic patients, showing the impact of a septic episode on their outcome. The 28-day survival rates were 66% and 88% for the septic and nonseptic groups, respectively (P < 0.001).
Figure 3.
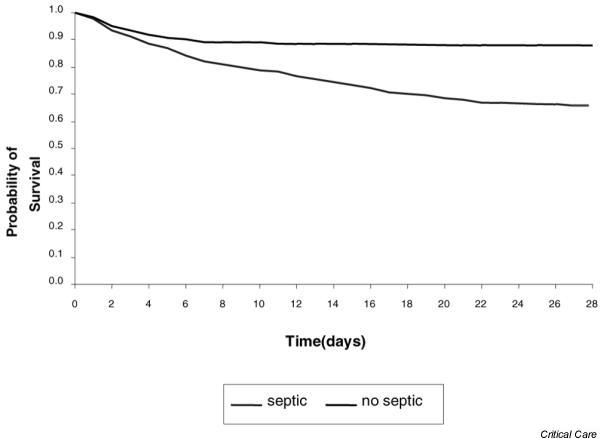
Kaplan–Meier survival curve for intensive care unit admissions with sepsis and without sepsis.
Private versus public hospitals
The median age was higher for patients from the private hospital (73.6 [IQR 58.5–81.5]) than for those from public hospitals (60.9 [44.7–72.2]) (P < 0.0005). There was no difference in median first-day APACHE II score between patients from the private hospital (16 [11-21]) and those from public hospitals (17 [12-22]) (P < 0.0005). The median first-day SOFA score was lower for patients from the private hospital (3 [1-5]) than for those from public hospitals (6 [3–8.5]) (P < 0.0005). The overall mortality rate was lower for patients from the private hospital than for those from public hospitals (12.5% versus 28.9%; P < 0.0005).
In comparison with the other centers, the private hospital had 47.3% versus 38.1% patients with SIRS, 18.4% versus 20.9% with sepsis, 15.3% versus 35% with severe sepsis and 11.7% versus 30.7% with septic shock. The 28-day mortality rates for SIRS, sepsis, severe sepsis and septic shock patients in the private hospital and the other centers were, respectively, 8.8% versus 17.5%, 16.7% versus 15.4%, 32.7% versus 51.6% (P = 0.08) and 33.3% versus 57.1% (P = 0.006). Because mortality rate for septic shock was greatly different between the private and public hospitals we also calculated a first-day SOFA score for these patients. For 'private' septic shock patients the score was 8 (IQR 4–10), whereas for 'public' septic shock patients it was 9 (6–11) (P = 0.085.
Discussion
This is the first prospective cohort epidemiological study of sepsis performed in Brazil, the largest country in South America, and to our knowledge it is the first large cohort of this kind performed in an underdeveloped country. Brazil is heterogeneous in many aspects such as income distribution, educational profiles and use of technology. Even within a particular State, those characteristics can vary significantly. We have initially included five ICUs belonging to the recently created Brazilian Critical Care Clinical Trials Network, because these ICUs already have all personnel and infrastructure for the study. A second phase of our BASES study has already been started in five other ICUs. We have therefore prospectively included 1383 patients admitted to five mixed ICUs from São Paulo and Santa Catarina States, and followed all of them daily, searching for the ACCP/SCCM criteria of SIRS, sepsis, severe sepsis and septic shock, and also for organ dysfunction development (SOFA score). This study design allowed the identification of septic episodes at admission and those beginning during the course of ICU stay. This information can be used to identify risk factors associated with sepsis in patients admitted to the ICU without infection, and also to verify differences in outcome for patients admitted with and without infection.
In general, we found a higher incidence density for sepsis, severe sepsis and septic shock than those reported by other studies [13,14]; this could have been due to a more severely ill cohort of patients, resulting in a greater proportional use of invasive devices such as central venous lines, urinary catheters and mechanical ventilation. In addition, our patients had a higher LOS in ICU. Different types of hospital (teaching versus non-teaching), types of ICU (mixed versus surgical), antibiotic use patterns and frequency of antimicrobial-resistant pathogens might also have been factors.
In comparison with the classical study of Rangel-Frausto [10], we have found a higher severe sepsis occurrence rate (27.3% versus 11.5%), which could be ascribed to the organ dysfunction criteria used in our study and to the fact that we did not enroll ward patients. We defined severe sepsis as any patient with at least one organ dysfunction identified by the SOFA score. In another prospective study, Sands and colleagues [13] documented an incidence density of sepsis of 2.8 ± 0.17 per 1000 patient-days. This study also included ICU and ward patients. Even in a study that followed only ICU patients [15], the occurrence rate of clinically suspected sepsis and confirmed severe sepsis was 9.0% and 6.3% of ICU admissions, respectively. In contrast and despite the smaller number of patients in our cohort, we have found a similar occurrence rate of severe sepsis (27.3%) to that found in the larger study of Alberti and colleagues (22.7%) [6]. This is important for validation of our data. It is noteworthy that both studies included only ICU patients.
There has been great variability in outcome reported in several studies in septic patients. In 1998, Friedman and colleagues published data from 131 studies [4] involving only septic shock patients. In that study they found a mortality rate of about 50%, with no major change in the previous 20 years. Other studies [10,13,16] reported mortality rates for severe sepsis and septic shock patients ranging from 20% to 81%. One of the major challenges in sepsis studies is to standardize the enrolled population to allow adequate comparison, principally in epidemiologic and therapeutic trials. The severe sepsis mortality rate in our study was similar to those in studies that used the same (ACCP/SCCM Consensus) criteria [6,16,17], but was higher than those found by Rangel-Frausto and colleagues [10] and Angus and colleagues [5]. Many factors can explain those differences, for example patient age, associated comorbidities and severity of sepsis (APACHE II score), source and type of infection (community, nosocomial or ICU-acquired), and number and severity of organ dysfunctions. In addition, we must consider access to the best standard of care [18], which can easily be appraised from the outcomes of private versus public hospitals in our cohort.
Although the ACCP/SCCM Consensus Conference definition categories have received severe criticisms, some authors [10,16] have found a close relationship between these categories and outcome. This relationship could be explained by the organ dysfunction presence implicit in this categorization. Thus, to clarify this matter, we measured the SOFA score daily and observed that as the sepsis-related conditions increased from sepsis to septic shock, the first-day and maximum SOFA scores also increased in parallel. This probably suggests that when using the ACCP/SCCM definitions it would be very useful to measure organ dysfunction by means of an organ dysfunction descriptor, to improve the characterization of the progress of the septic patient in ICU.
The lungs and respiratory tract was the main source of infection in our group of septic patients. This finding has been reported by others [6,19] and highlights the major role of respiratory infection in ICUs and the need for its prevention. Many of these episodes have been related to ventilator-associated pneumonia [20,21]. In our study, the diagnosis of community-acquired or nosocomial respiratory infection was made on the basis of clinical, laboratory and radiographic data. We did not routinely use any kind of tool-based diagnosis, such as protected brush-specimen or broncho-alveolar lavage. The high incidence of lung/respiratory infection in our study might therefore be due, at least in part, to the broad definition criteria we used.
Variability in the time course of sepsis can introduce difficulties in case definition and might consequently explain some discrepancies in incidence rates between studies. In our study, sepsis was diagnosed in 415 patients during their ICU stay, with 281 (67.7%) meeting sepsis diagnostic criteria on admission and 134 (32.3%) on the following days. Similarly, Knaus and colleagues [22] found that 18% of patients did not meet case definition criteria for sepsis at the time of admission but did meet them within the first week of ICU stay. It is therefore very important to be aware of the case definition criteria used in each study, because the incidence of sepsis can vary according to the follow-up period.
The impact of sepsis in critically ill patients has been underestimated by governmental health services. Quartin and colleagues [23] showed in their large study that sepsis could jeopardize patients for up to 5 years after a septic episode. In addition, in their model they evaluated the impact of sepsis and common associated comorbidities. They generated a model of how comorbidities could affect survival by studying a large cohort of nonseptic patients. Application of this model to the septic population yielded a prediction of death rates from causes other than sepsis, and mortality beyond this prediction was considered 'sepsis-associated'. They found that although septic patients have many associated comorbidities, sepsis is the cause of many deaths that occur outside the time frame normally associated with this acute disease. In our population, we found that septic patients had higher APACHE II and SOFA scores and a similar number of comorbidities, but higher rates of malignancy, congestive heart failure and chronic obstructive pulmonary disease. Actually, septic patients have severe acute physiological disturbances, as shown by the SOFA score, and probably have chronic diseases, as shown by the APACHE II score, decreasing the possibility of a more rapid recovery free of sequels. In general, acute organ dysfunction is related to early outcome and associated comorbidities are related to late outcome. However, this apparent acute disease could interfere long after its identification. It is therefore possible that septic patients suffer tissue organ derangements leading to prolonged occult risks for mortality. Although this interesting hypothesis has not yet been explored, recent clinical trials in sepsis [2,24,25] have included long-term follow-up.
When comparing data from the private hospital with those from the public hospitals (Table 3), we found an incidence of severe sepsis of 16% versus 35%. This difference can be explained by a higher incidence of infection in public hospitals, where patients have a poorer nutritional status and are admitted later in the course of their disease to the ICU. Frequently, studies from developed countries correlate the incidence of severe sepsis with more advanced age and a greater number of underlying diseases and invasive procedures [15]. Although the private hospital presented all these factors in our study, it showed an incidence of severe sepsis similar to that reported in the literature and lower than that for the public hospitals. We suggest that in Brazil, social and economical factors have a greater influence on the incidence of infection than those classically demonstrated factors. In addition, among septic shock patients we also observed a higher mortality rate in the public hospitals, although the first-day SOFA scores were similar in both groups, namely private and public hospitals. Factors including care provided by the hospitals, delay between hospital (emergency room) and ICU admissions, quality of the multiprofessional ICU team, and access to the best standard of care could explain those discrepant mortality rates. Nevertheless, these data need to be confirmed by specifically designed trials, because we had data from only one private hospital and it could be an outlier institution. Caution should therefore be exercised before drawing definitive conclusions.
Table 3.
Incidence of sepsis and outcome in private and public hospitals
| Parameter | Private hospital | Public hospitals | P |
| Age, years | 73.6 (58.5–81.5) | 60.9 (44.7–72.2) | <0.0005 |
| APACHE II score | 16 (11–21) | 17 (12–22) | 0.162 |
| First-day SOFA score | 3 (1–5) | 6 (3–8.5) | <0.0005 |
| Severe sepsis rate,% | 15.3 | 35 | <0.05 |
| Septic shock rate,% | 11.7 | 30.7 | <0.05 |
| Overall mortality rate,% | 12.5 | 29.0 | <0.05 |
| Severe sepsis mortality rate,% | 32.7 | 51.6 | <0.05 |
| Septic shock mortality rate,% | 33.3 | 57.1 | <0.05 |
APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, sequential organ failure assessment. Figures in parentheses are interquartile ranges.
In conclusion, sepsis is an emergent public health problem with a high incidence density and high mortality rates. In our study, the incidence density of sepsis was about 57 per 1000 patient-days, with a high overall mortality rate and a sharp contrast between private and public hospitals. Moreover, there was a close relationship between ACCP/SCCM categories, organ dysfunction development and mortality rate.
Key messages
• For the 1383 patients, the incidence density of sepsis was 57.9 (95% CI 51.5–65.3) per 1000 patient-days or 421 episodes of sepsis, corresponding to 30.5 (28.9–32.1) per screened ICU admissions.
• The mortality rate of patients with SIRS, sepsis, severe sepsis and septic shock were 24.2%, 33.9%, 46.9% and 52.2%, respectively (Fig. 2). For patients with SIRS without infection the mortality rate was 11.3%.
• Among septic patients (n = 415), the medians for the maximum SOFA scores for each category were 5.0 (IQR 4–7), 8.8 (7–11) and 11.0 (9–13) for sepsis, severe sepsis and septic shock, respectively. There was a significant positive correlation between the maximum SOFA score and each category (rs = 0.72, P < 0.0005).
Competing interests
This study was supported by Eli Lilly Brazil.
Abbreviations
ACCP/SCCM = American College of Chest Physicians/Society of Critical Care Medicine; APACHE = Acute Physiology and Chronic Health Evaluation; CI = confidence interval; ICU = intensive care unit; IQR = interquartile range; LOS = length of stay; SIRS = systemic inflammatory response syndrome; SOFA = sequential organ failure assessment.
See related commentary: http://ccforum.com/content/8/4/222
Contributor Information
Eliézer Silva, Email: eliezer@einstein.br.
Marcelo de Almeida Pedro, Email: marcpedro@terra.com.br.
Ana Cristina Beltrami Sogayar, Email: sogayar@einstein.br.
Tatiana Mohovic, Email: mohovic@uol.com.br.
Carla Lika de Oliveira Silva, Email: carla@sepse.com.br.
Mariano Janiszewski, Email: mjaniszewski@einstein.br.
Ruy Guilherme Rodrigues Cal, Email: ruycal@uol.com.br.
Érica Fernandes de Sousa, Email: eliezer@einstein.br.
Thereza Phitoe Abe, Email: abe@einstein.br.
Joel de Andrade, Email: joel@hu.ufsc.br.
Jorge Dias de Matos, Email: joel@hu.ufsc.br.
Ederlon Rezende, Email: ederlon@uti-servidorsp.com.br.
Murillo Assunção, Email: ederlon@uti-servidorsp.com.br.
Álvaro Avezum, Email: avezum@yahoo.com.
Patrícia CS Rocha, Email: eliezer@einstein.br.
Gustavo Faissol Janot de Matos, Email: gustavofm@einstein.br.
André Moreira Bento, Email: eliezer@einstein.br.
Alice Danielli Corrêa, Email: joel@hu.ufsc.br.
Paulo Cesar Bastos Vieira, Email: eliezer@einstein.br.
Elias Knobel, Email: knobel@einstein.br.
References
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Recombinant human Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Friedman G, Silva E, Vincent JL. Has the mortality of septic shock changed with time? Crit Care Med. 1998;26:2078–2086. doi: 10.1097/00003246-199812000-00045. [DOI] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulme R, Lepage E, Le Gall R. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–121. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- Annane D, Aegerter P, Jars-Guincestre MC, Guidetfor B. Current epidemiology of septic shock: The CUB-Réa Network. Am J Respir Crit Care Med. 2003;168:165–172. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, Beale R, Svoboda P, Laterre PF, Simon S, Light B, Spapen H, Stone J, Seibert A, Peckelsen C, De Deyne C, Postier R, Pettila V, Artigas A, Percell SR, Shu V, Zwingelstein C, Tobias J, Poole L, Stolzenbach JC, Creasey AA, (OPTIMIST Trial Study Group) Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–247. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117–123. doi: 10.1001/jama.273.2.117. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/s001340050156. [DOI] [PubMed] [Google Scholar]
- Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Khan KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR, Black E, Scwartz JS, Moore R, Johnson BL, Platt R. Epidemiology of sepsis syndrome in 8 academic medical centers. Academic Medical Center Consortium Sepsis Project Working Group. JAMA. 1997;278:234–240. doi: 10.1001/jama.278.3.234. [DOI] [PubMed] [Google Scholar]
- Banerjee SN, Emori TG, Culver DH, Gaynes RP, Jarvis WR. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am J Med. 1991;91(3B):86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, Regnier B. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–974. doi: 10.1001/jama.274.12.968. [DOI] [PubMed] [Google Scholar]
- Salvo I, de Cian W, Musicco M, Langer M, Piadena R, Wolfler A, Montani C, Magni E. The Italian SEPSIS study: preliminary results on the incidence and evolution of SIRS, sepsis, severe sepsis and septic shock. Intensive Care Med. 1995;21:S244–S249. doi: 10.1007/BF01740762. [DOI] [PubMed] [Google Scholar]
- Jones GR, Lowes JA. The systemic inflammatory response syndrome as a predictor of bacteraemia and outcome from sepsis. Q J Med. 1996;89:515–522. doi: 10.1093/qjmed/89.7.515. [DOI] [PubMed] [Google Scholar]
- Yu DT, Black E, Sands KE, Schwartz JS, Hibberd PL, Graman PS, Lanken PN, Kahn KL, Snydman DR, Parsonnet J, Moore R, Platt R, Bates DW, for the Academic Medical Center Consortium Sepsis Project Working Group Severe sepsis: variation in resource and therapeutic modality use among academic centers. Crit Care. 2003;7:R24–R34. doi: 10.1186/cc2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, Wolff M, Spencer RC, Hemmer M. The prevalence of nosocomial infection in intensive care units in Europe: results of the European Prevalence of infection in intensive care (EPIC) Study. JAMA. 1995;274:639–644. doi: 10.1001/jama.274.8.639. [DOI] [PubMed] [Google Scholar]
- Ewig S, Torres A. Prevention and management of ventilator-associated pneumonia. Curr Opin Crit Care. 2002;8:58–69. doi: 10.1097/00075198-200202000-00010. [DOI] [PubMed] [Google Scholar]
- Curtis JR. The long-term outcomes of mechanical ventilation: what are they and how should they be used? Respir Care. 2002;47:496–505. [PubMed] [Google Scholar]
- Knaus WA, Sun X, Nystrom O, Wagner DP. Evaluation of definitions for sepsis. Chest. 1992;101:1656–1662. doi: 10.1378/chest.101.6.1656. [DOI] [PubMed] [Google Scholar]
- Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. JAMA. 1997;277:1058–1063. doi: 10.1001/jama.277.13.1058. [DOI] [PubMed] [Google Scholar]
- Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Penzes I, Kubler A, Knaub S, Keinecke HO, Heinrichs H, Schindel F, Juers M, Bone RC, Opal SM, for the KyberSept Trial Study Group Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]


