Abstract
Changes in tumor metabolic activity have been shown to be an early indicator of treatment effectiveness for breast cancer, mainly in the neoadjuvant setting. The histopathologic response at the completion of chemotherapy has been used as the reference standard for assessment of the accuracy of 18F-FDG PET in predicting a response during systemic treatment. Although a pathologic complete response (pCR) remains an important positive prognostic factor for an individual patient, a recent metaanalysis could validate pCR as a surrogate marker for patient outcomes only in aggressive breast cancer subtypes. For establishment of the clinical application of metabolic treatment response studies, larger series of specific breast cancer subtypes—including hormone receptor–positive, human epidermal growth factor receptor 2–positive, and triple-negative breast cancers—are necessary. In addition, thresholds for relative changes in 18F-FDG uptake to distinguish between responding and nonresponding tumors need to be validated for different systemic treatment approaches, with progression-free survival and overall survival as references. A PET-based treatment stratification is applicable clinically only if valid alternative therapies are available. Of note, patients who do not achieve a pCR might still benefit from neoadjuvant therapy enabling breast-conserving surgery. In the metastatic setting, residual tumor metabolic activity after the initiation of systemic therapy is an indicator of active disease, whereas a complete resolution of metabolic activity is predictive of a successful treatment response.
Keywords: 18F-FDG, 18F-FDG PET, PET/CT, breast cancer, treatment response, therapy monitoring
Neoadjuvant systemic therapy is being used in women with large or locally advanced breast cancers and is considered a potential approach in patients requiring adjuvant chemotherapy (1). The administration of systemic therapy before surgery offers an increased rate of breast-conserving surgery and allows for assessment of a response in a resection specimen. Modern treatment strategies are tailored to molecular subtypes of breast cancer, allowing for a more individualized treatment approach. A pathologic complete response (pCR) is an important prognostic parameter and has commonly been used as a surrogate marker for a treatment response. Achievement of a pCR has been correlated with an improved long-term outcome, although only for aggressive breast cancer subtypes (1–3). Changes in tumor size represent an accepted endpoint for evaluating therapeutic effects in metastatic breast cancer. RECIST was established 15 y ago (4) and has been updated since then (5). However, several cycles of treatment are often required before CT or MRI can detect a measurable change in tumor size (6).
A decrease in tumor metabolic activity offers both assessment of a treatment response after the completion of therapy and early prediction of therapeutic effectiveness after the first or second cycle of chemotherapy. Identifying nonresponding patients on the basis of changes in tumor metabolic activity early during treatment could facilitate a change from an ineffective to a more effective treatment approach. To allow the use of 18F-FDG PET–based treatment stratification in clinical practice, several items need to be addressed; these include the best timing for measuring changes in tumor metabolic activity during treatment and defined cutoff values for changes in tumor metabolic activity.
18F-FDG PET FOR EARLY PREDICTION OF TREATMENT RESPONSE
The first observation of early changes in tumor glucose metabolism of breast cancer occurred in 1993, when Wahl et al. described an early decrease in the metabolic activity of tumors responding to a combination of chemotherapy and endocrine therapy (7). Similar results were found in further studies often including small sample sizes (8,9). Smith et al. (9) reported a significantly greater reduction in 18F-FDG uptake in patients who subsequently achieved a macroscopic pathologic response. Rousseau et al. studied 64 stage II and III breast cancer patients at multiple cycles during neoadjuvant chemotherapy and found a marked decrease in 18F-FDG uptake in nearly all patients who achieved a greater than 50% therapeutic effect (10). Schwarz-Dose et al. confirmed, in 104 patients, that the greater the reduction in tumor metabolic activity early during neoadjuvant treatment, the more likely the patients would achieve a pathologic response (11). After the first cycle of chemotherapy, tumor metabolic activity decreased by 50% ± 18% in pathologic responders; in comparison, the decrease in pathologic nonresponders was 36% ± 20%. Of note, all breast carcinomas (23%) with a baseline SUV of less than 3.0 did not respond to chemotherapy (11). In another study, in 126 patients, a significantly higher baseline SUV (mean, 10.5) was found in 41 patients who subsequently achieved a pathologic response (defined as a reduction in tumor cellularity of >90%); in comparison, the baseline SUVs in partial responders and nonresponders were 6.9 and 5.2, respectively (12). A cutoff of an SUV of greater than 5.9 at baseline predicted a pathologic response with 78% sensitivity and 65% specificity.
Performing 18F-FDG PET after the second cycle of treatment potentially provides a more accurate prediction of a treatment response. Using a 40% decrease in the SUV, Rousseau et al. identified a negative predictive value of 68% for identifying nonresponders after the first cycle; this value increased to 85% after the second cycle (10). Schwarz-Dose et al. found, for histopathologic nonresponders, negative predictive values of 89.5% after the first cycle (cutoff: 45% decrease in the SUV) and 88.9% after the second cycle (cutoff: 55%); these findings indicated similar accuracies for predicting a nonresponse after the first and second cycles (11). A recent metaanalysis including 19 studies with more than 900 patients found that the best cutoff for a response was a decrease in 18F-FDG uptake ranging from 55% to 65% (13). Although the sensitivity and the specificity for identifying patients responding to treatment were limited (84% and 66%, respectively), the negative predictive value for identifying nonresponders was high (91%). Figure 1 shows a good treatment response after 2 cycles of chemotherapy in a right breast mass, whereas Figure 2 shows no metabolic response. A summary of these studies and the accuracy of the cutoff values are shown in Table 1.
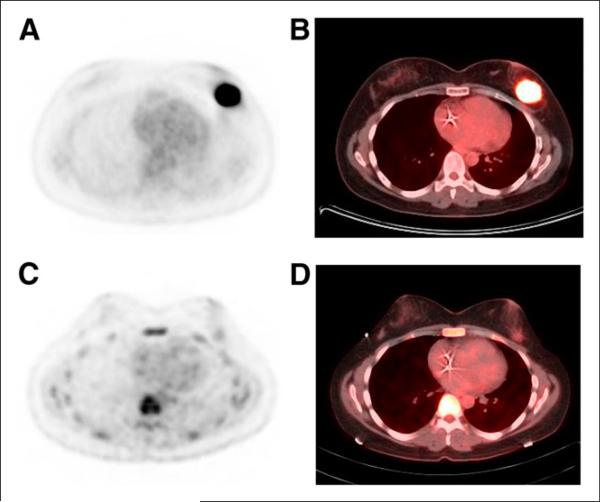
FIGURE 1.
37-y-old woman with HER2-positive ductal breast cancer (4.9 × 3.1 cm). (A and B) Baseline 18F-FDG PET (A) and fused 18F-FDG PET/CT (B). (C and D) After 2 cycles of carboplatin, docetaxel, trastuzumab, and pertuzumab treatment, significant reduction in tumor 18F-FDG uptake, from SUV of 16.1 to SUV of 1.6, was seen on 18F-FDG PET (C) and fused 18F-FDG PET/CT (D). Histopathology at completion of treatment showed minimal residual disease in tumor bed.
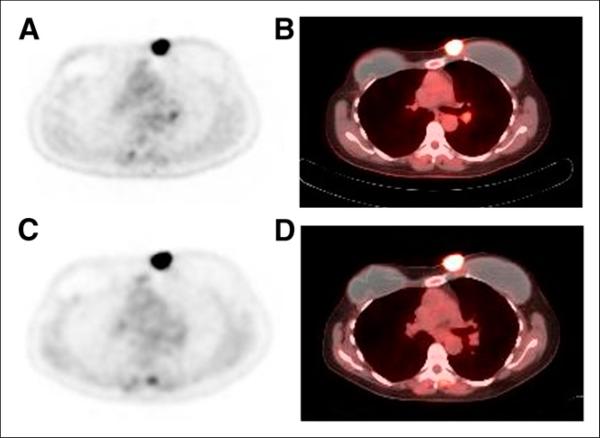
FIGURE 2.
60-y-old woman with recurrent HER2-positive breast cancer in medial left mastectomy site after left simple mastectomy and prophylactic right mastectomy. (A and B) Baseline 18F-FDG PET (A) and fused 18F-FDG PET/CT (B). (C and D) After 2 cycles of exemestane and trastuzumab treatment, slight increase in tumor metabolic activity, with SUV of 12.7 compared with SUV of 11.1 at baseline, was seen on 18F-FDG PET (C) and fused 18F-FDG PET/CT (D). In addition, a few mildly hypermetabolic left hilar lymph nodes, which suggested metastases (not histologically proven), showed decrease in metabolic activity after start of treatment. Because there was no response to treatment in recurrence at medial left mastectomy site, carboplatin treatment was initiated.
TABLE 1.
Summary of Studies of 18F-FDG PET for Early Prediction of Treatment Response
|
18F-FDG PET metabolic response |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Year | No. of patients included in 18F-FDG PET analysis | Pathologic response criteria* | Percentage of responders | After indicated treatment cycle | Decrease in SUV | Percentage sensitivity | Percentage specificity | Percentage negative predictive value |
| Schelling et al. (8) | 2000 | 22 | pCR + pMRD | 29 | First, second | > 45% after first or second | 100 (first), 83 (second) | 85 (first), 94 (second) | N/A |
| Smith et al. (9) | 2000 | 30 | pCR-macro | 38 | First | >20% | 90 | 74 | N/A |
| Rousseau et al. (10) | 2006 | 64 | >50% therapeutic effect | 56 | First, second | > 40% after first or second | 61 (first), 89 (second) | 96 (first), 95 (second) | 68 (first), 85 (second) |
| Schwarz-Dose (11) | 2009 | 104 | pCR + pMRD | 16 | First, second | > 45% after first, >55% after second | 73 | 63 | 90 |
| Humbert et al.† (32) | 2012 | 37 | pCR‡ | 38 | First | >75% | 64 | 83 | 79 |
| Groheux et al.† (35) | 2013 | 30 | pCR‡ | 53 | Second | >62% | 86 | 63 | N/A |
| SUV ≤ 3.0§ | 86 | 94 | 88 | ||||||
| Humbert et al.† (34) | 2014 | 54 | pCR‡ | 41 | First | >60% | 83 | 52 | 84 |
| SUV < 2.1§ | 59 | 88 | 76 | ||||||
pCR = pathologic complete response, defined as absence of residual invasive tumor in breast, irrespective of lymph node status [ypT0/is ypNX], unless otherwise indicated; pMRD = minimal residual disease, defined as a few scattered foci of microscopic residual invasive tumor (≤2 mm); pCR-macro = pathologic response, defined as absence of macroscopically visible tumor.
pCR was defined as absence of residual invasive tumor in both breast and axillary lymph nodes [ypT0/is ypN0].
Results were based on HER2-positive breast cancers only.
Absolute SUVs for defining cutoff values.
N/A = not available.
The fact that the histopathologic reference standards used in these studies were based on different criteria limits a direct comparison. Most used a pCR as the reference standard; 8 of 19 studies also included patients with minimal residual disease as histopathologic responders (13). To make matters more complex, previous clinical trials were heterogeneous with regard to systemic treatment approaches, which included combinations of various chemotherapeutic agents and possible additional targeted treatments. Most neoadjuvant chemotherapy regimens were based on a combination of anthracyclines and concurrent or sequential administration of taxanes, often with the addition of cyclophosphamide or fluorouracil as a third agent (14). There is little information about the relationship between potential changes in tumor metabolic activity and specific treatment regimens, a factor that should be considered if 18F-FDG PET is to be used for treatment stratification. Another important consideration is tumor shrinkage in patients without a pCR, which would allow for breast-conserving surgery. This endpoint was not addressed in previous studies.
Few studies have assessed early changes in metabolic activity in axillary lymph nodes (9,15,16). A marked decrease in the SUV was observed after the first cycle of treatment in 52 patients achieving a nodal pCR (17). When a cutoff of a 50% decrease was used, 18F-FDG PET predicted a lymph node response with a sensitivity of 96%, a specificity of 75%, and a negative predictive value of 95%. Not all patients had cytologically confirmed lymph node metastases at baseline, a fact that limited the calculation of true responders. Of note, there was no correlation between a histopathologic response of the primary tumor and axillary lymph node metastases (17).
Hybrid PET/MRI technology was recently introduced, appearing in the clinical setting in 2007 (18,19). There is little literature regarding the use of 18F-FDG PET/MRI in breast cancer (20,21), as no prospective study has addressed the potential role for treatment monitoring. It would be of interest to directly compare changes in tumor metabolic activity with dynamic contrast enhancement or diffusion-weighted imaging. Future applications might include a combination of such parameters, as shown for other tumor entities (22).
HISTOPATHOLOGIC RESPONSE
There is strong evidence that a histopathologic assessment after the completion of neoadjuvant therapy is a surrogate marker for a treatment response. Multiple large neoadjuvant trials have demonstrated a significant correlation between pCR and improved patient outcomes (2,23–25). Various definitions of a histopathologic response, based on the extent of residual invasive carcinoma in the breast and regional lymph nodes, regardless of residual in situ carcinoma, have been used (26). Of note, these various definitions of a histopathologic response have been shown to be significantly associated with patient outcomes. Therefore, PET trials with different histopathologic response criteria remain valid, although a comparison with SUV cutoffs is limited.
The largest metaanalysis to date was published in 2014 and included almost 12,000 women (27). Despite the strong prognostic information provided by a pCR in an individual patient, a pCR is not sufficient to demonstrate the superiority of a given treatment regimen over another, and survival data are still necessary for such comparisons. A proposal by an international working group recommended that a pCR should be defined as the absence of residual invasive carcinoma, with or without residual in situ carcinoma in the breast [ypT0/is ypNX or ypT0/is ypN0] (26,28). The panel also recognized a need for classification of the amount of residual tumor burden, which allows the measurement of residual tumor as a continuous variable, in addition to the dichotomized distinction of a pCR versus no pCR. The MD Anderson Cancer Center group has published criteria for measuring the extent of residual breast cancer burden, which has been shown to be associated with survival (26,28).
PET THERAPY MONITORING FOR DIFFERENT BREAST CANCER SUBTYPES
Molecular studies have identified intrinsic breast cancer subtypes characterized by common gene expression profiles (29,30). Immunohistochemical analysis of the estrogen receptor, the progesterone receptor, and human epidermal growth factor receptor 2 (HER2) is often used instead of gene expression to define 3 major subtypes of breast cancer: luminal (estrogen receptor–positive, progesterone receptor–positive, or both, HER2-negative), HER2-positive, and triple-negative (estrogen receptor–negative/progesterone receptor–negative/HER2-negative) (31). HER2-positive and triple-negative tumors are generally more aggressive than luminal breast cancers.
An analysis of 115 women identified the highest SUVs (11.3 ± 8.5) in triple-negative tumors (32). The decrease in the SUV after the first cycle of systemic therapy was significantly higher in triple-negative and HER2-positive subtypes than in the luminal subtype. However, the decrease in the SUV was a predictor for a subsequent pCR only in HER2-positive tumors (accuracy, 76%). The molecular heterogeneity (33) of triple-negative tumors and their small number may partly explain the lack of a significant correlation with a pCR. Triple-negative breast cancers tend to have an aggressive clinical course but often respond to anthracycline- or taxane-based chemotherapy. Patients not achieving a pCR after neoadjuvant treatment have a higher risk for early recurrences and shorter survival. The addition of platinum-based chemotherapy is a potential option, but it involves significant additional toxicity, and it is unclear which patients would benefit the most. These circumstances present an exciting opportunity to assess the role of 18F-FDG PET imaging in the prediction of a response.
In 57 HER2-positive patients treated with chemotherapy and trastuzumab, an SUV of less than 2.1 after the first cycle was the best independent predictor of a pCR (34). A decrease in the SUV of greater than 60% had the highest negative predictive value for identifying nonresponding HER2-positive breast cancers. Groheux et al. found, in 30 HER2-positive patients, that low residual 18F-FDG uptake (SUV, <3.0) after the second cycle was the best predictor of a pCR (35). A decrease in 18F-FDG uptake of 62% or more was also predictive of a pCR, but at a lower overall accuracy than absolute SUVs (73% and 90%, respectively). There are 2 competing analysis approaches—measuring absolute SUVs and measuring relative changes in SUVs—and no conclusion regarding which approach offers better identification of nonresponding tumors can be made on the basis of current literature.
Luminal (hormone receptor–positive, HER2-negative) breast cancers are characterized by lower metabolic activity, and patients with these cancers rarely achieve a pCR. A recent study with patient outcomes as a reference found that a poor metabolic response (<16% decrease in the SUV) after the first cycle was associated with a shorter 5-y overall survival relative to the findings for metabolic responders (49% vs. 96%) (36). Only 42 of 61 tumors (69%) were hypermetabolic at baseline and could be assessed with 18F-FDG PET; this factor represents a distinct limitation for this tumor subtype. Of note, patients with low tumor glucose metabolism at baseline had the best 5-y survival (100%). Groheux et al. studied 82 patients with hormone receptor–positive (HER2-negative) breast cancer and found that a small decrease in the SUV (<12% after the second cycle) was significantly associated with short event-free survival (37).
18F-FDG PET THERAPY MONITORING FOR METASTATIC BREAST CANCER
For patients with metastatic disease, there are several chemotherapy agents (including anthracyclines, taxanes, gemcitabine, and capecitabine) as well as targeted endocrine or anti-HER2 therapy. Treatment response is based on changes in tumor size, as a histopathologic assessment is often not practical or even possible. In accordance with RECIST (5), up to 5 measurable target lesions representative of involved organs are evaluated (5). An important limitation is that changes in tumor size often do not correlate with patient outcomes.
A pilot study enrolling 11 patients with 26 metastatic lesions revealed a statistically significant reduction in tumor metabolic activity after the first and second cycles of first-line chemotherapy in lesions that responded (38). The overall survival of nonresponding patients was significantly shorter than that of responding patients (8.8 vs. 19.2 mo). Patients not responding to treatment were identified several weeks earlier with 18F-FDG PET than with conventional imaging.
Eighty-two HER2-positive patients underwent dual targeted anti-HER2 therapy with lapatinib and trastuzumab (39). A metabolic nonresponse, defined as a decrease in the SUV of less than 25% after 1 wk, had a high negative predictive value (91%) for identifying patients who would not achieve an objective response according to RECIST. In addition, patients identified as metabolic nonresponders after week 1 had a shorter time to progression than responding patients. In 20 patients with metastatic breast cancer, a decrease in the SUV of greater than 45% after the third cycle was significantly associated with a clinical response at the completion of chemotherapy and a longer overall survival (40).
The use of early changes in tumor metabolic activity is more difficult for hormone receptor–positive breast cancer patients receiving antihormonal therapies. Studies revealed an increase in 18F-FDG uptake 7–10 d after the initiation of endocrine therapy. This “metabolic flare phenomenon” occurring within the first 1 or 2 wk of endocrine therapy was attributed to the initial agonist effect of tamoxifen and was found to be predictive of a positive response to therapy (41,42). Recently, 18F-FDG PET predicted progression-free survival in 22 patients with metastatic hormone receptor–positive breast cancer (43).
18F-FDG PET FOR RESPONSE ASSESSMENT AFTER COMPLETION OF THERAPY
Residual tumor metabolic activity after the completion of therapy is an indicator of residual viable tumor tissue, whereas a complete resolution of increased metabolic activity provides a high positive predictive value for a successful treatment response. In a neoadjuvant multicenter trial, 99 patients underwent 18F-FDG PET, mammography, ultrasound, and MRI before surgery (44). Patients who achieved a pCR had significantly lower 18F-FDG uptake than nonresponding patients. Nevertheless, the sensitivity of 18F-FDG PET for detecting residual tumor was only 32.9% when an SUV threshold of 2.0 was used; the sensitivity increased to 57.5% when a threshold of 1.5 was used. Conventional imaging modalities were more sensitive than 18F-FDG PET for identifying residual tumor but had lower specificity. Neither 18F-FDG PET nor conventional imaging could exclude the presence of residual viable tumor; this factor is an important limitation for all imaging modalities.
18F-FDG PET was performed after 3 cycles of high-dose chemotherapy in 47 metastatic breast cancer patients, and a negative post-treatment 18F-FDG PET result was the most powerful predictor of survival—superior to CT imaging (45). A total of 34 patients (72%) achieved a complete metabolic response and had a median survival of 24 mo; in comparison, the median survival of patients with a positive 18F-FDG PET result was 10 mo. According to multivariate analysis, the relative risk of death was highest in patients with 18F-FDG PET–positive disease (relative risk, 5.3).
Changes in the sizes of bone metastases are particularly difficult to evaluate with conventional imaging, as sclerotic lesions do not disappear and lytic lesions can show sclerotic changes as an indicator of a treatment response. Two studies demonstrated a high sensitivity of 18F-FDG PET/CT for the detection of osseous metastases in patients with newly diagnosed metastatic breast cancer, and the metabolic activity of osseous breast cancer metastases provided prognostic information (46,47). Stafford et al. studied 24 patients with bone-dominant metastatic breast cancer and reported a correlation between changes in 18F-FDG uptake and the overall clinical assessment of a response (48). The same group's subsequent study of 28 metastatic breast cancer patients revealed that patients with no change in 18F-FDG uptake were twice as likely to progress as those who showed a metabolic response (49). In a retrospective analysis, bone metastases in 102 patients were assessed with 18F-FDG PET/CT before and after treatment, and a decrease in 18F-FDG uptake was a significant predictor of the response duration in univariate and multivariate analyses (50). Treatment monitoring of bone metastases is discussed further in this supplement.
CONCLUSION
On the basis of data published to date, the monitoring of systemic treatment by 18F-FDG PET is a powerful tool for the early prediction and assessment of a response in patients with newly diagnosed and in metastatic breast cancer. Although a histopathologic assessment is an established surrogate marker for a treatment response, approximately 13%–25% of patients showing a pCR develop a systemic recurrence after 5 y of follow-up and 60%–70% of patients not showing a pCR remain free of a recurrence. The strength of a histopathologic assessment is the identification of a pCR after the completion of systemic treatment, whereas the strength of 18F-FDG PET is the identification of nonresponders early during treatment.
The lack of clinical acceptance of 18F-FDG PET–based treatment stratification is driven by several factors. No SUV cutoffs for separating responders and nonresponders have been established or validated by patient outcome data. Baseline tumor metabolic activities and subsequent treatment-induced changes are different for distinct molecular subtypes, and specific SUV cutoffs are necessary for different breast cancer subtypes. Neoadjuvant treatment is generally based on aggressive chemotherapy regimens, and alternative treatment approaches—other than immediate surgery—are often not available. An important contribution of 18F-FDG PET could be directing the addition of platinum-based chemotherapy in poorly responding triple-negative breast cancer patients, but such an approach requires prospective validation. The possibility that tumor shrinkage in patients not achieving a pCR might enable breast-conserving surgery is important. The lack of standardization of clinical 18F-FDG PET imaging outside research protocols leads to high variations in 18F-FDG uptake in tissue and hinders clinical acceptance. Although less applicable for routine clinical use because of cost and availability, PET/MRI would allow for a direct comparison between changes in glucose metabolism and the accumulation and kinetic behavior of MRI contrast agents, and diffusion-weighted MRI could be used to examine changes in water restriction in the same patients. Such comparisons would be helpful for identifying the strengths and weaknesses of 18F-FDG PET versus or combined with MRI.
In the metastatic setting, the potential clinical application of 18F-FDG PET is based on no significant changes in tumor metabolic activity, indicating ineffective treatment. More alternative treatment options are available in this setting, and treatment could be adapted earlier than with conventional imaging. The number of prospective trials is still too small to support this approach in clinical practice.
Given the high cost of applying ineffective treatments in (breast) cancer patients and the cost of evaluating PET imaging, alternative national and international funding methods for performing the prospective clinical multicenter trials needed to establish PET treatment monitoring must be found.
Acknowledgments
This publication was made possible by the Clinical and Translational Science Collaborative of Cleveland, by UL1TR000439 and KL2TR000440 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH Roadmap for Medical Research, by Case Western Reserve University, and by Ohio Third Frontier Funding.
Footnotes
DISCLOSURE
No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 2.Bonadonna G, Valagussa P, Brambilla C, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16:93–100. doi: 10.1200/JCO.1998.16.1.93. [DOI] [PubMed] [Google Scholar]
- 3.Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–695. doi: 10.1002/cncr.10741. [DOI] [PubMed] [Google Scholar]
- 4.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Avril N, Sassen S, Roylance R. Response to therapy in breast cancer. J Nucl Med. 2009;50(suppl 1):55S–63S. doi: 10.2967/jnumed.108.057240. [DOI] [PubMed] [Google Scholar]
- 7.Wahl RL, Zasadny K, Helvie M, Hutchins GD, Weber B, Cody R. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol. 1993;11:2101–2111. doi: 10.1200/JCO.1993.11.11.2101. [DOI] [PubMed] [Google Scholar]
- 8.Schelling M, Avril N, Nährig J, et al. Positron emission tomography using [18F] fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689–1695. doi: 10.1200/JCO.2000.18.8.1689. [DOI] [PubMed] [Google Scholar]
- 9.Smith IC, Welch AE, Hutcheon AW, et al. Positron emission tomography using [ 18F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol. 2000;18:1676–1688. doi: 10.1200/JCO.2000.18.8.1676. [DOI] [PubMed] [Google Scholar]
- 10.Rousseau C, Devillers A, Sagan C, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24:5366–5372. doi: 10.1200/JCO.2006.05.7406. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz-Dose J, Untch M, Tiling R, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27:535–541. doi: 10.1200/JCO.2008.17.2650. [DOI] [PubMed] [Google Scholar]
- 12.García Vicente AM, Cruz Mora MA, Leon Martin AA, et al. Glycolytic activity with 18F-FDG PET/CT predicts final neoadjuvant chemotherapy response in breast cancer. Tumour Biol. 2014;35:11613–11620. doi: 10.1007/s13277-014-2495-7. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhang C, Liu J, Huang G. Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast Cancer Res Treat. 2012;131:357–369. doi: 10.1007/s10549-011-1780-z. [DOI] [PubMed] [Google Scholar]
- 14.Thompson AM, Moulder-Thompson SL. Neoadjuvant treatment of breast cancer. Ann Oncol. 2012;23(suppl 10):x231–x236. doi: 10.1093/annonc/mds324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassa P, Kim EE, Inoue T, et al. Evaluation of preoperative chemotherapy using PET with fluorine-18-fluorodeoxyglucose in breast cancer. J Nucl Med. 1996;37:931–938. [PubMed] [Google Scholar]
- 16.Mankoff DA, Dunnwald LK, Gralow JR, et al. Changes in blood flow and metabolism in locally advanced breast cancer treated with neoadjuvant chemotherapy. J Nucl Med. 2003;44:1806–1814. [PubMed] [Google Scholar]
- 17.Rousseau C, Devillers A, Campone M, et al. FDG PET evaluation of early axillary lymph node response to neoadjuvant chemotherapy in stage II and III breast cancer patients. Eur J Nucl Med Mol Imaging. 2011;38:1029–1036. doi: 10.1007/s00259-011-1735-y. [DOI] [PubMed] [Google Scholar]
- 18.Pichler BJ, Judenhofer MS, Wehrl HF. PET/MRI hybrid imaging: devices and initial results. Eur Radiol. 2008;18:1077–1086. doi: 10.1007/s00330-008-0857-5. [DOI] [PubMed] [Google Scholar]
- 19.Schlemmer HP, Pichler BJ, Schmand M, et al. Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology. 2008;248:1028–1035. doi: 10.1148/radiol.2483071927. [DOI] [PubMed] [Google Scholar]
- 20.Sher A, Valls L, Muzic RF, Jr, Plecha D, Avril N. Whole-body positron emission tomography-magnetic resonance in breast cancer. Semin Roentgenol. 2014;49:313–320. doi: 10.1053/j.ro.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Sher A, Vercher-Conejero JL, Muzic RF, Jr, Avril N, Plecha D. Positron emission tomography/magnetic resonance imaging of the breast. Semin Roentgenol. 2014;49:304–312. doi: 10.1053/j.ro.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Valls L, Hoimes C, Sher A, et al. Early response monitoring of receptor tyrosine kinase inhibitor therapy in metastatic renal cell carcinoma using [F-18]fluorothymi-dine-positron emission tomography-magnetic resonance. Semin Roentgenol. 2014;49:238–241. doi: 10.1053/j.ro.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 24.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 25.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;2001:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 26.Provenzano E, Bossuyt V, Viale G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol. 2015;28:1185–1201. doi: 10.1038/modpathol.2015.74. [DOI] [PubMed] [Google Scholar]
- 27.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 28.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 29.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 30.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes: dealing with the diversity of breast cancer—highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humbert O, Berriolo-Riedinger A, Riedinger JM, et al. Changes in 18F-FDG tumor metabolism after a first course of neoadjuvant chemotherapy in breast cancer: influence of tumor subtypes. Ann Oncol. 2012;23:2572–2577. doi: 10.1093/annonc/mds071. [DOI] [PubMed] [Google Scholar]
- 33.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humbert O, Cochet A, Riedinger JM, et al. HER2-positive breast cancer: 18F-FDG PET for early prediction of response to trastuzumab plus taxane-based neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41:1525–1533. doi: 10.1007/s00259-014-2739-1. [DOI] [PubMed] [Google Scholar]
- 35.Groheux D, Giacchetti S, Hatt M, et al. HER2-overexpressing breast cancer: FDG uptake after two cycles of chemotherapy predicts the outcome of neoadjuvant treatment. Br J Cancer. 2013;109:1157–1164. doi: 10.1038/bjc.2013.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Humbert O, Berriolo-Riedinger A, Cochet A, et al. Prognostic relevance at 5 years of the early monitoring of neoadjuvant chemotherapy using 18F-FDG PET in luminal HER2-negative breast cancer. Eur J Nucl Med Mol Imaging. 2014;41:416–427. doi: 10.1007/s00259-013-2616-3. [DOI] [PubMed] [Google Scholar]
- 37.Groheux D, Sanna A, Majdoub M, et al. Baseline tumor 18F-FDG uptake and modifications after 2 cycles of neoadjuvant chemotherapy are prognostic of outcome in ER1+/HER22− breast cancer. J Nucl Med. 2015;56:824–831. doi: 10.2967/jnumed.115.154138. [DOI] [PubMed] [Google Scholar]
- 38.Dose Schwarz J, Bader M, Jenicke L, Hemminger G, Janicke F, Avril N. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG PET. J Nucl Med. 2005;46:1144–1150. [PubMed] [Google Scholar]
- 39.Lin NU, Guo H, Yap JT, et al. Phase II study of lapatinib in combination with trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: clinical outcomes and predictive value of early [18F] fluorodeoxyglucose positron emission tomography imaging (TBCRC 003). J Clin Oncol. 2015;33:2623–2631. doi: 10.1200/JCO.2014.60.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Couturier O, Jerusalem G, N'Guyen JM, Hustinx R. Sequential positron emission tomography using [18F]fluorodeoxyglucose for monitoring response to chemotherapy in metastatic breast cancer. Clin Cancer Res. 2006;12:6437–6443. doi: 10.1158/1078-0432.CCR-06-0383. [DOI] [PubMed] [Google Scholar]
- 41.Dehdashti F, Mortimer JE, Trinkaus K, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res Treat. 2009;113:509–517. doi: 10.1007/s10549-008-9953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797–2803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 43.Mortazavi-Jehanno N, Giraudet AL, Champion L, et al. Assessment of response to endocrine therapy using FDG PET/CT in metastatic breast cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2012;39:450–460. doi: 10.1007/s00259-011-1981-z. [DOI] [PubMed] [Google Scholar]
- 44.Dose-Schwarz J, Tiling R, Avril-Sassen S, et al. Assessment of residual tumour by FDG-PET: conventional imaging and clinical examination following primary chemotherapy of large and locally advanced breast cancer. Br J Cancer. 2010;102:35–41. doi: 10.1038/sj.bjc.6605427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cachin F, Prince HM, Hogg A, Ware RE, Hicks RJ. Powerful prognostic stratification by [18F]fluorodeoxyglucose positron emission tomography in patients with metastatic breast cancer treated with high-dose chemotherapy. J Clin Oncol. 2006;24:3026–3031. doi: 10.1200/JCO.2005.04.6326. [DOI] [PubMed] [Google Scholar]
- 46.Morris PG, Lynch C, Feeney JN, et al. Integrated positron emission tomography/computed tomography may render bone scintigraphy unnecessary to investigate suspected metastatic breast cancer. J Clin Oncol. 2010;28:3154–3159. doi: 10.1200/JCO.2009.27.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris PG, Ulaner GA, Eaton A, et al. Standardized uptake value by positron emission tomography/computed tomography as a prognostic variable in metastatic breast cancer. Cancer. 2012;118:5454–5462. doi: 10.1002/cncr.27579. [DOI] [PubMed] [Google Scholar]
- 48.Stafford SE, Gralow JR, Schubert EK, et al. Use of serial FDG PET to measure the response of bone-dominant breast cancer to therapy. Acad Radiol. 2002;9:913–921. doi: 10.1016/s1076-6332(03)80461-0. [DOI] [PubMed] [Google Scholar]
- 49.Specht JM, Tam SL, Kurland BF, et al. Serial 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) to monitor treatment of bone-dominant metastatic breast cancer predicts time to progression (TTP). Breast Cancer Res Treat. 2007;105:87–94. doi: 10.1007/s10549-006-9435-1. [DOI] [PubMed] [Google Scholar]
- 50.Tateishi U, Gamez C, Dawood S, Yeung HW, Cristofanilli M, Macapinlac HA. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology. 2008;247:189–196. doi: 10.1148/radiol.2471070567. [DOI] [PubMed] [Google Scholar]