Abstract
Lactobacilli are important in intestinal homeostasis, which involves the regulation of immune function, digestive health, cholesterol absorption and intestinal tumor growth amongst others. Our previous investigations have suggested that oral intake of heat-killed Lactobacillus brevis (L. brevis) SBC8803 (SBL88™) suppresses dermatitis by modulating the immune function in an atopic dermatitis mouse model. The aim of the present study was to investigate the effect of heat-killed L. brevis SBC8803 intake on skin hydration conditions in humans. A randomized, double-blind, placebo-controlled study was conducted with volunteers with slightly higher levels of transepidermal water loss (TEWL) on the forearm. The subjects (126 people aged between 21 and 59 years) were randomly allocated to three groups so that the level of TEWL and the age were distributed equally among the groups. The subjects took placebo or heat-killed L. brevis SBC8803 at a daily dose of 25 or 50 mg for 12 weeks. Following the exclusion of eight subjects for plausible reasons (two withdrawals from the study, two for study violations, one for not meeting exclusion criteria and three due to their physical condition), 118 subjects were subjected to the analysis. The results of the present study revealed that following the analysis of the whole populations, marginal differences were observed in TEWL (for example, suppression of skin water loss) at the neck in the 25 mg/day group at week 8 and at the lower eye region in the 50 mg/day group at week 4 (P=0.05 and 0.09, respectively, compared with the placebo group analyzed by Dunnett's test). A significant increase in corneal hydration was also observed at the neck in the 25 mg/day group at week 12 (P=0.06, as compared with the placebo group as analyzed by Dunnett's test). In the analysis of the subpopulations whose habitual frequency of taking lactic fermentation products was less than once per week, the levels of corneal hydration at the neck (in the 50 mg/day group) and lower eye region (in the 25 mg/day group) were significantly increased at week 12 (P<0.05). In conclusion, the results of the present investigation suggest that oral intake of heat-killed L. brevis SBC8803 is effective at improving skin hydration conditions in populations with low habitual frequency of taking lactic fermentation products.
Keywords: Lactobacillus, skin care, transepidermal water loss, skin moisturizing, clinical study
Introduction
Lactic acid bacteria have long been taken not only as dairy fermented food but as various other traditionally fermented foods worldwide. A large variety of bacteria colonize the human gastrointestinal tract, and their interaction with the host maintains intestinal homeostasis that affects human health (1). Since intestinal homeostasis is maintained in a complex manner through the interaction between intestinal floras and the host, several lines of evidence suggest that probiotic intake of lactic bacteria leads to competitive and/or active exclusion of unfavorable bacteria (2–4), mucosal immune responses and a reduction in allergic responses (5), and digestive health (6). Among lactic bacteria, lactobacilli have additional functions such as lowering plasma cholesterol levels (7), suppressing intestinal tumor growth (8) and hypotension (9,10).
Previously, the intake of heat-killed Lactobacillus brevis (L. brevis) SBC8803 was reported to be effective at ameliorating alcoholic liver failure in mice and humans (11,12). It was also observed that heat-killed L. brevis SBC8803 intake resulted in beneficial effects in the T helper (Th)1/Th2 balance (13) and ameliorated dermatitis by reducing immunoglobulin E production in an atopic dermatitis mouse model (14). In addition, intake of heat-killed L. brevis SBC8803 was observed to reduce transepidermal water loss (TEWL), a parameter of the skin barrier function (15). Based on these observations, the present study aimed to investigate the effect of oral intake of L. brevis SBC8803 on skin hydration conditions in humans in a randomized, double-blind, placebo-controlled study. The strain used in the present study was L. brevis SBC8803, which was selected from in-house L. brevis isolated from barley that had preliminarily been tested in humans for its effectiveness at improving skin barrier functions.
Materials and methods
Test articles
Subjects consumed two capsules of the test articles per day. The composition of the capsulated test articles (per two capsules) was as follows: Heat-killed L. brevis SBC8803, of which 0 g was included for the placebo group (group P), 0.025 g for the 25 mg/day heat-killed L. brevis SBC8803 group (group L), and 0.050 g for the 50 mg/day heat-killed L. brevis SBC8803 group (group H). The carbohydrate content was 0.376 g for group P, 0.351 g for group L and 0.326 g for group H. There was also fat and sodium at trace amounts in all test articles. Heat-killed L. brevis SBC8803 was manufactured by Sapporo Breweries Ltd. (Tokyo, Japan). The ingredients of each test article are summarized in Table I.
Table I.
Compositions of the test article.
| Item (mg) | Group P | Group L | Group H |
|---|---|---|---|
| Heat-killed L. brevis SBC8803 | 0 | 25 | 50 |
| Caramel pigment | 100 | 50 | 0 |
| Finely powdered silica | 4 | 4 | 4 |
| Calcium stearate | 20 | 20 | 20 |
| Starch | 256 | 256 | 256 |
| Cellulose | 20 | 45 | 70 |
Group P, placebo group; group L, 25 mg/day heat-killed L. brevis SBC8803 group; group H, 50 mg/day heat-killed L. brevis SBC8803 group; L. brevis, Lactobacillus brevis.
Study design
A double-blinded, placebo-controlled study was conducted with the aid of a fund from Sapporo Holdings Ltd. at the Kenshokai Fukushima Health Care Center (Osaka, Japan) and the Go Clinic (Osaka, Japan) under supervision by the principle investigator at the Kenshokai Fukushima Health Care Center. The study period lasted 12 weeks between February 28, 2014 and June 24, 2014. This study conformed to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the Incorporated Medical Institution of Kenshokai (Osaka, Japan) on the basis of the protocol and information on the test article. In addition, the present study was registered with the ID no. UMIN000014327 (A study on the effects of Lactobacillus brevis SBC8803 intake on skin in adults) at the UMIN Clinical Trials Registry in Japan. The study subjects were recruited between February and March 2014 and the details of the study were disclosed to the subjects prior to the start of the study. The investigators obtained informed consent from each subject.
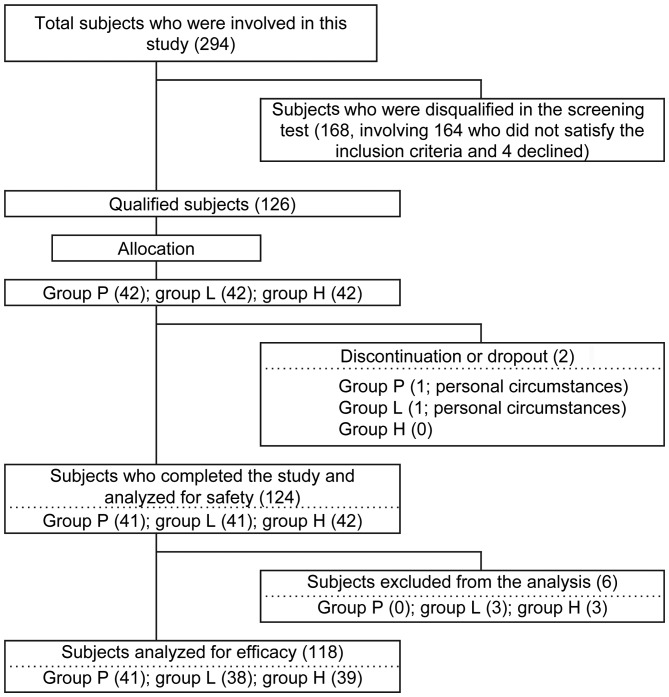
The TEWL value for the forearm at the screening test was used as one of the major inclusion criteria, and subjects whose TEWL value was >6.2 g/m2h were included (see below for measurement of TEWL). The inclusion and exclusion criteria are summarized in Table II. As shown in Fig. 1, the subjects (126 people) included 34 males and 92 females aged between 21 and 59 years (40.8±8.5) who fulfilled the eligibility criteria and were free of skin diseases, including atopic dermatitis, psoriasis and other systemic diseases that may contribute to skin conditions. The inclusion of the subjects was judged by the principle investigator (Dr Sumio Kondo). All subjects were sequentially assigned based on random number tables to one of the three masked products and randomly assigned (1:1:1) to group P (placebo; n=42), group L (25 mg heat-killed L. brevis SBC8803 per day; n=42) and group H (50 mg heat-killed L. brevis SBC8803 per day; n=42). Following randomization, it was confirmed that the subjects were distributed almost equally among the three groups in terms of their levels of TEWL and their age. Allocation was pre-assigned on the basis of randomization numbers and was concealed from the subjects, the investigators, and the researchers who recruited and assessed the participating subjects. The dose of L. brevis SBC8803 was defined based on our previous open-label clinical trial of its effect on skin conditions (unpublished observations). According to the schedule shown in Table III, various parameters on safety and efficacy were measured and are summarized in Table IV.
Table II.
Criteria for inclusion and exclusion.
| Inclusion criteria |
|---|
| 1) Male and female whose ages are between 20 and 59 |
| 2) Those with relatively higher level of TEWL at the forearm |
| Exclusion criteria |
| 1) Those who have been ingesting any article rich in lactic bacteria (such as yogurt, beverages, health foods, supplements, or pharmaceuticals that contained lactic acid bacteria) more than three times a week (or more than 13 times per month) |
| 2) Those who have been ingesting any article (such as health food, internal medicine, or quasi-pharmaceutical products) more than three times a week (or more than 13 times per month) |
| 3) Those who have experienced any cosmetic medical treatment (such as photofacial or injections of Botox, hyaluronic acid, or collagen) at the sites to be examined in this study |
| 4) Those who have experienced any cosmetic medical treatment or hormone replacement therapy (including taking oral contraceptives) at the site other than those to be examined in this study in the past one year |
| 5) Those who have experienced beauty treatment on the skin, scrubbing, or depilation at the site to be examined in this study in the past one month, or who are planning to conduct such during this study period |
| 6) Those who are using prescription drug regularly |
| 7) Those who have experienced sunburn during long outdoor work, recreation, or sports in the past one month, or who are planning to conduct such during this study period, excepting daily short exposure to sunshine |
| 8) Those who are habitually washing their body using materials (such as nylon towels excepting soft ones) that affect the skin conditions at the sites to be examined in this study |
| 9) Those who have skin failures (such as wound, inflammation, or pimples) that may affect examination in this study |
| 10) Those who are suffering from atopic dermatitis or skin failure that needs medical treatment |
| 11) Those who are suffering from asthma or who are to be liable to develop such during this study |
| 12) Those who are to be liable to develop pollinosis that needs medication or to emerge allergic skin diseases during this study |
| 13) Those who are to be liable, after menstruation, to develop rough skin conditions at the sites to be examined in this study |
| 14) Those who are taking shift work or who are going to take multiple night shift during this study |
| 15) Those who have suffering from serious diseases (such as diabetes mellitus or failure of the liver, kidney, or heart) or diseases that affect sex hormone secretion, or who with a case history of such |
| 16) Those who are participating in any other clinical trial or who are planning to do such after agreeing informed consent of joining this study |
| 17) Those who with values considerably different from reference ranges in the examination of the screening tests |
| 18) Those who are planning to get pregnant or nurse a baby during this study |
| 19) Those who are planning to make a foreign travel during this study |
| 20) Those who have experienced significant amounts of blood drawing or blood donation that exceed the level defined by the Japanese Red Cross in the past one year |
| 21) Those who are executing any matter that may affect the progress of this study or who may violate guideline of this study |
| 22) Those who are judged inappropriate as participants from answers to the lifestyle questionnaire |
| 23) Those who are judged inappropriate as participants from the view of principle investigator |
Figure 1.
Procedure for the selection and the analysis of subjects. Group P, subjects who received a placebo; group L, subjects who received low-dose heat-killed L. brevis SBC8803; group H, subjects who received high-dose heat-killed L. brevis SBC8803. Values in parentheses denote the number of subjects. The reasons for subject exclusion were: Violation of the study restrictions (n=2 in group L); conformity to the exclusion criteria (n=1 in group H); and judgement by the principle investigator (Dr Sumio Kondo) based on the subjects' physical conditions (n=1 in group L and n=2 in group H). The subject allocation was performed by Yoshihisa Kibune (TTC Co., Ltd., Tokyo, Japan).
Table III.
Schedule for the study.
| Item | Screening | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|
| Informed consent | • | |||
| Questionnaire on life style | • | |||
| Selection and allocation | • | |||
| Medical interview | • | • | • | • |
| Somatometry | • | • | • | • |
| Physical examination | • | |||
| Corneal hydration | • | • | • | • |
| TEWL | • | • | • | • |
| Photography of the skin surface | • | • | • | |
| Evaluation of the skin surface | • | • | ||
| Questionnaire on skin conditions | • | • | • | • |
| Laboratory examination | • | |||
| Stool examination | • | • | ||
| Ingestion of test article | ↔ | |||
| Log | ↔ | |||
The symbol • denotes items tested; two-way arrow denotes the test period during which the subject should do the indicated task every day. TEWL, transepidermal water loss.
Table IV.
Details of the survey and testing.
| Item | Details |
|---|---|
| Informed consent | Informed consent was obtained by free will using a written form |
| Questionnaire on lifestyle | Anamnesis, intake of pharmaceutical products and health food, allergies, smoking, use of skin care products and topical agents, alcohol intake |
| Inclusion | Included subjects that provided written informed consent, fulfilled the incorporation criteria and did not conflict with the exclusion criteria |
| Medical interview | Confirmation of the physical conditions and the presence or absence of adverse events |
| Somatometry | Height (only in the screening test), body weight and body mass index |
| Physical examination | Systolic and diastolic blood pressure, pulse count |
| Corneal hydration | Measured on the left forearm, neck and lower eye region |
| Transepidermal water loss | Measured on the left forearm, neck and lower eye region |
| Photography of the skin surface | Taken for the left forearm and lower eye region |
| Evaluation of the skin surface | Examined by a responsible doctor based on the photographs taken as described above |
| Questionnaire on skin conditions | Subjective recognition of skin conditions |
| Laboratory examination | Fasting levels of white blood cells, red blood cells, hemoglobin, hematocrit, platelets, total protein, albumin, total billirubin, alkaline phosphatase, aspartate transaminase, alanine transaminase, lactate dehydrogenase, γ-glutamyltranspeptidase, total cholesterol, triglycerides, high-density lipoprotein-cholesterol, low density lipoprotein-cholesterol, urea nitrogen, creatinine, uric acid, Na+, K+, Cl, glucose |
| Stool examination | Testing of intestinal flora |
| Log | Status of test article ingestion, physical condition, medicine taking |
The primary and secondary endpoints of the present study were decreased in TEWL and increased in corneal hydration, respectively. Until the completion of the study, the subjects, care providers, doctors and the managing team were blinded. Data were analyzed regarding the whole subject groups as well as the subdivided subject populations whose habitual frequency of taking lactic fermentation products was less than once a week.
Measurement of TEWL
TEWL is recognized as one of the parameters that predicts skin barrier functions (16). The skin region of interest was cleansed with a cleansing agent (DRC cleansing liquid; DRC Co., Ltd., Osaka, Japan) and rinsed with warm water, prior to washing with another cleansing agent (DRC face wash; DRC Co., Ltd.) and rinsing with warm water. The TEWL was measured using a Tewameter TM300 (Courage + Khazaka Electronic GmbH, Köln, Germany) and measurements were taken after an acclimatization period of 20 min. The measurements were taken from the left forearm (inner side located 5 cm above the wrist), the neck (the intersection between the chin and the left earlobe) and the lower eye region (1 cm below the left eye). The measurements were performed under constant temperature (20.0±1°C) and humidity (relative humidity, 50.0±5%). Furthermore, a 1 min measurement was performed resulting in 7 reliable values. From the 7 values, the mid-5 values were used to calculate the mean value, which was expressed as g/m2h.
Measurement of the corneal hydration status
The level of corneal hydration was assessed as skin conductance using a Corneometer CM825 (Courage + Khazaka Electronic GmbH), which measures the reactive capacitance of the skin, using the stratum corneum as a dielectric membrane (17). The positions and conditions of the measurements were the same as those for the TEWL measurement. The measurement was repeated 7 times, and the mid-5 values obtained were used to calculate the mean value,. Each value was expressed as an arbitrary unit.
Evaluation of subjective and objective recognition of skin conditions
Subjective overall skin conditions were evaluated based on a questionnaire on the skin conditions of the face and body for each subject prior to and following the intake of test articles (at weeks 4, 8 and 12). Data are expressed as a 7-grade score that represents 1 as being a poor or problematic skin condition and 7 as skin in good condition. In addition, an objective evaluation was performed based on a comparison of the photograph of the face and forearm skin surfaces taken prior to and following the intake of test articles (at weeks 8 and 12). The data are expressed as a 7-grade score that represents the changes from the initial status (prior to test article intake): −3, aggravated by >60%; −2, aggravated by 30–60%; −1, aggravated by <30%; 0, no change; +1, improved by <30%; +2, improved by 30–60% and +3, improved by >60%.
Statistical analysis
All of the measured values and changes are expressed as the mean ± standard deviation. The methods of statistical analysis are described in each Table. Statistical analyses were performed using SPSS version 18 (SPSS, Inc., Chicago, IL, USA) and Microsoft Excel (Microsoft Corporation, Tokyo, Japan). P<0.05 was used to indicate a statistically significant difference. Where indicated, P-values of statistical significance (P<0.01) are shown, which suggest the effect of the test article.
Results
Subjects
A total of 126 subjects, aged between 21 and 59 years, were enrolled and 42 subjects were randomly allocated to each of the 3 groups. It was confirmed that the level of TEWL and the age of the subjects were distributed almost equally among the groups. The subjects took the test article [placebo (group P) or heat-killed L. brevis SBC8803 at a daily dose of 25 mg (group L) or 50 mg (group H)] for a total of 12 weeks. Following the exclusion of 8 subjects for plausible reasons (Fig. 1), 118 subjects completed the trial and were subjected to analysis. The background data for each group are shown in Table V.
Table V.
Background data of the subject populations.
| Item | Group P | Group L | Group H | P-valuea |
|---|---|---|---|---|
| Number of subjects (genderb) | 41 (M 12, F 29) | 38 (M 12, F 26) | 39 (M 9, F 30) | 0.71 |
| Age (years) | 42.2±8.9 | 40.4±9.2 | 40.6±7.5 | 0.57 |
| Height (cm) | 162.1±6.2 | 163.4±8.2 | 163.2±6.7 | 0.65 |
| Body weight (kg) | 57.9±9.5 | 60.0±13.1 | 57.7±9.2 | 0.58 |
| Body mass index (kg/m2) | 22.0±3.3 | 22.3±3.2 | 21.6±2.8 | 0.64 |
| TEWL (g/m2h) | ||||
| Forearm | 9.4±2.1 | 9.4±2.1 | 9.5±1.7 | 0.93 |
| Neck | 9.6±4.0 | 10.6±7.0 | 10.0±4.1 | 0.69 |
| Lower eye region | 24.5±8.0 | 25.1±8.0 | 23.1±7.2 | 0.52 |
| Corneal hydration (a.u.) | ||||
| Forearm | 25.7±5.8 | 22.8±3.9 | 23.5±5.3 | 0.04c |
| Neck | 49.2±8.8 | 46.1±11.0 | 48.1±9.3 | 0.37 |
| Lower eye region | 46.5±11.7 | 44.9±12.6 | 49.9±11.7 | 0.19 |
P-values were obtained by comparison among the three groups using a χ2 test (for gender) or one-way analysis of variance (for the other parameters).
M, male; F, female.
Statistically significant difference. Values are presented as the mean ± standard deviation. TEWL, transepidermal water loss; group P, placebo group; group L, 25 mg/day heat-killed L. brevis SBC8803 group; group H, 50 mg/day heat-killed L. brevis SBC8803 group; L. brevis, Lactobacillus brevis; a.u., arbitrary unit.
Effects on TEWL and skin hydration status, as analyzed in the whole population
As shown in Table VI, there was no significant change in TEWL at the left forearm after 4, 8 and 12 weeks of the intake of heat-killed L. brevis SBC8803. However, there was a significant decrease (−15.1%) in TEWL (for instance suppression of water loss from the skin) at the neck after 8 weeks in group L (P=0.05 compared with the change in group P, as determined by Dunnett's test). In addition, at the lower eye region the decrease in TEWL at week 4 in group H was somewhat decreased compared with that in group P (P=0.09) as determined by Dunnett's test.
Table VI.
Effects of the intake of heat-killed L. brevis SBC8803 on the levels of TEWL.
| Position | Group | Screening | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|---|
| Forearm | P | 9.4±2.1 | 8.8±2.3a | 8.6±2.8 | 9.2±2.1 |
| (−0.5±1.6) | (−0.7±2.4) | (−0.1±2.0) | |||
| L | 9.4±2.1 | 9.5±2.2 | 8.7±2.0a | 9.9±2.3 | |
| (0.1±2.2) | (−0.7±2.1) | (0.4±2.1) | |||
| H | 9.5±1.7 | 9.6±1.9 | 8.8±2.0b | 9.4±2.1 | |
| (0.1±1.5) | (−0.8±1.5) | (−0.1±1.8) | |||
| Neck | P | 9.6±4.0 | 10.0±3.7 | 9.9±4.8 | 9.5±3.1 |
| (0.3±2.7) | (0.3±3.8) | (−0.1±4.0) | |||
| L | 10.6±7.0 | 10.7±5.7 | 9.0±3.7a | 9.9±3.4 | |
| (0.1±4.7) | (−1.7±4.8c) | (−0.8±5.4) | |||
| H | 10.0±4.1 | 10.0±3.3 | 9.1±3.4 | 10.1±4.1 | |
| (0.0±3.2) | (−0.9±3.2) | (0.1±3.4) | |||
| Lower eye region | P | 24.5±8.0 | 20.8±5.3b | 20.4±4.8b | 20.0±5.2b |
| (−3.6±8.0) | (−4.1±7.8) | (−4.5±8.8) | |||
| L | 25.1±8.0 | 21.0±6.4b | 21.2±6.0b | 19.7±5.8b | |
| (−4.0±6.5) | (−3.8±6.7) | (−5.3±7.7) | |||
| H | 23.1±7.2 | 22.5±6.9 | 21.1±5.6 | 20.6±4.8a | |
| (−0.6±6.1c) | (−2.0±6.5) | (−2.5±6.1) |
Each value (g/m2h) represents the mean ± standard deviation, and the difference from the screening value is shown in parenthesis.
P<0.05
P<0.01 by intragroup comparison using paired Student's t-test.
P<0.1 by intergroup comparison using Dunnett's test using the value of group P as a reference. Group P, placebo group; group L, 25 mg/day heat-killed L. brevis SBC8803 group; group H, 50 mg/day heat-killed L. brevis SBC8803 group; L. brevis, Lactobacillus brevis.
The level of corneal hydration was measured based on the skin conductance. The hydration levels of the forearm and lower eye region remained unaffected by heat-killed L. brevis SBC8803 intake, whereas the levels increased by 24% at the neck in group L at week 12 (Table VII). This change was somewhat increased compared with that observed in group P (11%; P=0.06), as determined by Dunnett's test.
Table VII.
Effects of the intake of heat-killed L. brevis SBC8803 on the levels of corneal hydration.
| Position | Group | Screening | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|---|
| Forearm | P | 25.7±5.8 | 26.2±6.0 | 26.5±6.2 | 29.7±6.0b |
| (0.5±4.0) | (0.8±4.4) | (4.1±6.3) | |||
| L | 22.8±3.9 | 23.8±4.7 | 24.8±5.2b | 29.0±5.1b | |
| (1.1±3.3) | (2.1±4.2) | (6.2±5.4) | |||
| H | 23.5±5.3 | 23.7±5.2 | 24.3±5.5 | 27.8±6.1b | |
| (0.2±3.4) | (0.8±3.2) | (4.3±5.4) | |||
| Neck | P | 49.2±8.8 | 52.1±9.1a | 54.9±9.6b | 55.5±9.7b |
| (3.0±8.6) | (5.8±10.4) | (6.3±10.3) | |||
| L | 46.1±11.0 | 49.4±10.7 | 54.1±10.5b | 57.3±9.8b | |
| (3.3±10.6) | (8.0±9.0) | (11.2±10.1c) | |||
| H | 48.1±9.3 | 50.1±10.5 | 53.8±10.6b | 54.8±11.4b | |
| (2.0±8.1) | (5.7±8.3) | (6.7±9.8) | |||
| Lower eye region | P | 46.5±11.7 | 49.4±10.4a | 48.3±11.3 | 51.4±10.8a |
| (2.9±8.8) | (1.8±8.4) | (4.8±11.9) | |||
| L | 44.9±12.6 | 50.0±14.3b | 46.4±16.4 | 50.7±12.5b | |
| (5.1±6.1) | (1.5±8.9) | (5.7±6.1) | |||
| H | 49.9±11.7 | 52.0±9.5 | 52.4±10.7 | 53.0±11.4 | |
| (2.1±9.8) | (2.5±10.5) | (3.1±11.3) |
Each value represents the mean ± standard deviation, and the difference from the screening value is shown in parenthesis.
P<0.05
P<0.01 by intragroup comparison using paired Student's t-test.
P<0.1 by by intergroup comparison using Dunnett's test using the value of group P as a reference. Group P, placebo group; group L, 25 mg/day heat-killed L. brevis SBC8803 group; group H, 50 mg/day heat-killed L. brevis SBC8803 group; L. brevis, Lactobacillus brevis.
Effects on TEWL and skin hydration status, as analyzed in the subject populations with low habitual frequency of taking lactic fermentation products
Since the dose of heat-killed L. brevis SBC8803 was relatively low, the intake of lactic fermentation products during the test period was considered to be capable of affecting skin conditions. Therefore, the data for the subdivided subject populations (39 people) whose habitual frequency of taking lactic fermentation products was less than once a week (their background data is shown in Table VIII) were analyzed. As shown in Table IX, the level of TEWL did not improved in groups L and H. However, the levels of corneal hydration at the neck in group H and lower eye region in group L were observed to be significantly increased at week 12 (Table X).
Table VIII.
Background of the subdivided subject populations whose habitual frequency of taking lactic fermentation products was less than once a week.
| Item | Group P | Group L | Group H | P-valuea |
|---|---|---|---|---|
| Number of subjects (genderb) | 13 (M 5, F 8) | 15 (M 5, F 10) | 11 (M 3, F 8) | 0.91 |
| Age (years) | 41.1±10.9 | 42.6±8.3 | 38.9±9.2 | 0.62 |
| Height (cm) | 163.0±4.6 | 163.1±8.9 | 165.3±6.7 | 0.68 |
| Body weight (kg) | 57.6±10.3 | 62.0±12.9 | 61.3±10.1 | 0.56 |
| Body mass index (kg/m2) | 21.6±3.1 | 23.1±3.3 | 22.3±2.6 | 0.43 |
| TEWL (g/m2h) | ||||
| Forearm | 10.3±2.8 | 9.7±1.4 | 10.0±0.8 | 0.72 |
| Neck | 11.2±5.7 | 11.4±5.3 | 10.4±3.7 | 0.89 |
| Lower eye region | 24.8±8.2 | 26.4±6.8 | 24.6±8.5 | 0.81 |
| Corneal hydration (a.u.) | ||||
| Forearm | 25.4±7.2 | 23.4±5.1 | 23.1±5.3 | 0.58 |
| Neck | 47.5±7.2 | 45.0±8.9 | 45.6±9.8 | 0.74 |
| Lower eye region | 48.3±11.4 | 40.2±10.9 | 53.2±10.2 | 0.01c |
P-values were obtained by comparison among the three groups using a χ2 test (for gender) or one-way analysis of variance (for other parameters).
M, male; F, female.
Statistically significant differences. Values are presented as the mean ± standard deviation. TEWL, transepidermal water loss; group P, placebo group; group L, 25 mg/day heat-killed L. brevis SBC8803 group; group H, 50 mg/day heat-killed L. brevis SBC8803 group; a.u., arbitrary unit.
Table IX.
Analysis of transepidermal water loss levels in subdivided subject populations whose habitual frequency of taking lactic fermentation products was less than once a week.
| Position | Group | Screening | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|---|
| Forearm | P | 10.3±2.8 | 9.7±3.2 | 9.8±3.8 | 9.8±2.5 |
| (−0.5±1.8) | (−0.4±3.5) | (−0.5±2.1) | |||
| L | 9.7±1.4 | 10.0±2.0 | 9.1±2.2 | 10.8±2.5a | |
| (0.3±1.5) | (−0.6±1.7) | (1.1±1.9c) | |||
| H | 10.0±0.8 | 9.5±1.6 | 8.9±1.8a | 10.6±2.3 | |
| (−0.5±1.4) | (−1.1±1.6) | (0.6±2.0) | |||
| Neck | P | 11.2±5.7 | 11.7±4.6 | 12.0±7.5 | 9.9±3.8 |
| (0.4±3.2) | (0.8±5.1) | (−1.4±6.3) | |||
| L | 11.4±5.3 | 12.5±7.0 | 9.7±3.9a | 11.4±3.6 | |
| (1.1±3.7) | (−1.7±2.9) | (0.0±3.5) | |||
| H | 10.4±3.7 | 9.6±2.2 | 9.9±3.5 | 11.0±3.8 | |
| (−0.8±2.7) | (−0.5±3.9) | (0.6±3.4) | |||
| Lower eye region | P | 24.8±8.2 | 23.2±7.0 | 22.1±6.9 | 21.9±7.1 |
| (−1.7±7.1) | (−2.7±6.7) | (−2.9±9.5) | |||
| L | 26.4±6.8 | 22.5±4.8b | 22.6±6.5b | 19.9±4.0b | |
| (−3.9±4.4) | (−3.8±4.3) | (−6.5±5.5) | |||
| H | 24.6±8.5 | 24.6±8.0 | 22.3±3.6 | 24.0±5.6 | |
| (0.0±7.1) | (−2.3±7.2) | (−0.5±7.6) |
Each value (g/m2h) represents the mean ± standard deviation, and the difference from the screening value is shown in parenthesis.
P<0.05
P<0.01 by intragroup comparison using paired Student's t-test.
P<0.1 by by intergroup comparison using Dunnett's test using the value of group P as a reference. Group P, placebo group; group L, 25 mg/day heat-killed L. brevis SBC8803 group; group H, 50 mg/day heat-killed L. brevis SBC8803 group; L. brevis, Lactobacillus brevis.
Table X.
Analysis of corneal hydration levels in subdivided subject populations whose habitual frequency of taking lactic fermentation products was less than once a week.
| Position | Group | Screening | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|---|
| Forearm | P | 25.4±7.2 | 24.0±6.2 | 24.3±7.6 | 27.6±5.6 |
| (−1.32±4.8) | (−1.0±5.6) | (2.2±7.5) | |||
| L | 23.4±5.1 | 23.1±4.6 | 24.3±4.1 | 29.8±5.2b | |
| (−0.3±2.7) | (0.9±3.3) | (6.5±6.5) | |||
| H | 23.1±5.3 | 22.5±4.8 | 22.4±5.8 | 26.7±5.5 | |
| (−0.6±3.4) | (−0.7±2.6) | (3.5±5.9) | |||
| Neck | P | 47.5±7.2 | 48.2±6.8 | 50.9±10.3 | 53.2±7.7a |
| (0.7±7.1) | (3.4±10.8) | (5.7±7.1) | |||
| L | 45.0±8.9 | 48.6±7.8 | 53.1±9.5b | 56.1±8.3 | |
| (3.7±7.6) | (8.1±10.4) | (11.1±6.3) | |||
| H | 45.6±9.8 | 50.3±10.5a | 52.9±12.8b | 58.4±11.8b | |
| (4.7±6.7) | (7.3±5.5) | (12.8±9.3c) | |||
| Lower eye region | P | 48.3±11.4 | 48.0±12.3 | 45.7±12.0 | 45.7±13.3 |
| (−0.2±9.1) | (−2.6±8.4) | (−2.6±11.6) | |||
| L | 40.2±10.9 | 44.4±14.6 | 41.6±16.4 | 46.7±11.5b | |
| (4.2±8.1) | (1.4±9.8) | (6.5±7.1c) | |||
| H | 53.2±10.2 | 54.7±10.3 | 54.6±8.2 | 54.8±10.4 | |
| (1.5±9.3) | (1.4±10.5) | (1.5±8.1) |
Each value (arbitrary unit) represents the mean ± standard deviation, and the difference between the screening value is shown in parenthesis.
P<0.05
P<0.01 by intragroup comparison using paired Student's t-test.
P<0.05 by intergroup comparison using Dunnett's test using the value of group P as a reference. Group P, placebo group; group L, 25 mg/day heat-killed L. brevis SBC8803 group; group H, 50 mg/day heat-killed L. brevis SBC8803 group; L. brevis, Lactobacillus brevis.
Effects on subjective and objective recognition of skin conditions
Based on a questionnaire on the skin conditions, there was no improvement of subjective recognitions observed on the face and body. The objective examinations of skin surface on the face and forearm did not identify any differences among the three groups.
Adverse effects
There was no adverse effect that was considered to be caused by the intake of heat-killed L. brevis SBC8803 based on the judgment of the principal investigator of the present clinical study.
Discussion
The present study was conducted in order to investigate whether oral intake of heat-killed L. brevis SBC8803 is effective at improving skin conditions in human subjects with slightly higher TEWL levels. A randomized, double-blind, placebo-controlled protocol was used in which the subjects' level of TEWL and their age was distributed almost equally among the groups. The subjects received either a placebo or heat-killed L. brevis SBC8803 at a daily dose of 25 or 50 mg/day for 12 weeks. No clear statistically significant differences were observed between the objective or subjective recognition of skin conditions, although marginal decreases in TEWL at the neck and lower eye region (Table VI) and an increase in corneal hydration at the neck (Table VII) were observed. These results suggested that the intake of heat-killed L. brevis SBC8803 has some beneficial effects on the skin.
The subjects enrolled in the present study were males and females with a wide age range and the initial skin hydration levels were determined using TEWL and corneal hydration. In addition, the subjects' background of habitual intake of lactic fermentation products differed (79 of the 118 analyzed subjects consumed lactic fermentation products more than twice a week). Since the dose of heat-killed L. brevis SBC8803 was relatively small (25 and 50 mg/day), the intake of lactic fermentation products during the test period may bias the results. Therefore, 39 subjects were selected whose habitual frequency of taking lactic fermentation products was less than once a week for additional analysis. The results indicated that the levels of corneal hydration at the neck in the 50 mg/day group and lower eye region in the 25 mg/day group were significantly increased following 12 weeks of heat-killed L. brevis SBC8803 intake. Therefore, the intake of heat-killed L. brevis SBC8803 may be beneficial to skin moisturization in subjects who do not habitually consume lactic fermentation foods.
In experiments with rats, we previously reported that the administration of L. brevis SBC8803 suppressed cutaneous arterial sympathetic nerve activity, and thereby increased cutaneous blood flow, which led to the amelioration of dry skin (15). The mechanism underlying this effect is suggested to be the following: i) L. brevis SBC8803 stimulates serotonin release from intestinal cells such as enterochromaffin cells (18); ii) the resulting serotonin elevates afferent intestinal vagal nerve activity (19), which in turn iii) enhances efferent gastric vagal nerve activity (19) and iv) cutaneous arterial sympathetic nerve activity (15). Therefore, in addition to the habitual consumption of lactic fermentation foods, the use of cutaneous blood flow as a marker for normalization of subject populations may help design future studies that test skin moisturization conditions.
Studies by other groups, as well as ourselves, suggest that probiotic intake of lactobacilli alleviates allergic dermatitis or reactive skin in animals and humans (5,14,20–24). Furthermore, modulation of the immune reaction is suggested to be involved in these activities in inflammatory skin failures. Regarding skin moisturization however, intestinal serotonin release and the neurotransmission system may be important. Such phenomena are only reported with heat-killed L. brevis SBC8803.
In conclusion, the results of the present study demonstrated that intake of heat-killed L. brevis SBC8803 is safe and is suggested to be effective at improving skin hydration conditions. In particular, this effect is prominent in populations with low habitual frequency of taking lactic fermentation products. Further investigations are required in order to determine the appropriate dose that is applicable to a variety of subjects who have varying backgrounds with regard to skin conditions and consumption of lactic fermentation foods.
References
- 1.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 2.De Keersmaecker SC, Verhoeven TL, Desair J, Marchal K, Vanderleyden J, Nagy I. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett. 2006;259:89–96. doi: 10.1111/j.1574-6968.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 3.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 4.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkaid Y, Oldenhove G. Tuning microenvironments: Induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: A randomized controlled trial. Eur J Clin Nutr. 2012;66:1234–1241. doi: 10.1038/ejcn.2012.126. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Fruehauf J, Goldsmith JD, Xu H, Katchar KK, Koon HW, Zhao D, Kokkotou EG, Pothoulakis C, Kelly CP. Saccharomyces boulardii inhibits EGF receptor signaling and intestinal tumor growth in Apc(min) mice. Gastroenterology. 2009;137:914–923. doi: 10.1053/j.gastro.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aihara K, Kajimoto O, Hirata H, Takahashi R, Nakamura Y. Effect of powdered fermented milk with Lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. J Am Coll Nutr. 2005;24:257–265. doi: 10.1080/07315724.2005.10719473. [DOI] [PubMed] [Google Scholar]
- 10.Jauhiainen T, Vapaatalo H, Poussa T, Kyrönpalo S, Rasmussen M, Korpela R. Lactobacillus helveticus fermented milk lowers blood pressure in hypertensive subjects in 24-h ambulatory blood pressure measurement. Am J Hypertens. 2005;18:1600–1605. doi: 10.1016/j.amjhyper.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Segawa S, Wakita Y, Hirata H, Watari J. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates alcoholic liver disease in ethanol-containing diet-fed C57BL/6N mice. Int J Food Microbiol. 2008;128:371–377. doi: 10.1016/j.ijfoodmicro.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Wakita Y, Kanda H, Shimizu C, Nakakita Y, Kaneda H, Segawa S, Ozaki M, Shigyo T, Ohtake T, Fujiya M, Kohgo Y. Effect of Lactobacillus brevis SBC8803 on gamma-glutamyl transferase in Japanese habitual drinkers: A double-blind, placebo-controlled study. Food Nutr Sci. 2012;3:678–684. doi: 10.4236/fns.2012.35092. [DOI] [Google Scholar]
- 13.Segawa S, Nakakita Y, Takata Y, Wakita Y, Kaneko T, Kaneda H, Watari J, Yasui H. Effect of oral administration of heat-killed Lactobacillus brevis SBC8803 on total and ovalbumin-specific immunoglobulin E production through the improvement of Th1/Th2 balance. Int J Food Microbiol. 2008;121:1–10. doi: 10.1016/j.ijfoodmicro.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Segawa S, Hayashi A, Nakakita Y, Kaneda H, Watari J, Yasui H. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates the development of dermatitis and inhibits immunoglobulin E production in atopic dermatitis model NC/Nga mice. Biol Pharm Bull. 2008;31:884–889. doi: 10.1248/bpb.31.884. [DOI] [PubMed] [Google Scholar]
- 15.Horii Y, Kaneda H, Fujisaki Y, Fuyuki R, Nakakita Y, Shigyo T, Nagai K. Effect of heat-killed Lactobacillus brevis SBC8803 on cutaneous arterial sympathetic nerve activity, cutaneous blood flow and transepidermal water loss in rats. J Appl Microbiol. 2014;116:1274–1281. doi: 10.1111/jam.12435. [DOI] [PubMed] [Google Scholar]
- 16.Lehman PA, Franz TJ. Assessing the bioequivalence of topical retinoid products by pharmacodynamic assay. Skin Pharmacol Physiol. 2012;25:269–280. doi: 10.1159/000339899. [DOI] [PubMed] [Google Scholar]
- 17.Clarys P, Clijsen R, Barel AO. Influence of probe application pressure on in vitro and in vivo capacitance (Corneometer CM 825 (®)) and conductance (Skicon 200 EX (®)) measurements. Skin Res Technol. 2011;17:445–450. doi: 10.1111/j.1600-0846.2011.00516.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakakita Y, Kaneda H, Shigyo T. Heat-killed Lactobacillus brevis SBC8803 induces serotonin release from intestinal cells. Food Nutr Sci. 2013;4:767–771. doi: 10.4236/fns.2013.48099. [DOI] [Google Scholar]
- 19.Horii Y, Nakakita Y, Fujisaki Y, Yamamoto S, Itoh N, Miyazaki K, Kaneda H, Oishi K, Shigyo T, Nagai K. Effects of intraduodenal injection of Lactobacillus brevis SBC8803 on autonomic neurotransmission and appetite in rodents. Neurosci Lett. 2013;539:32–37. doi: 10.1016/j.neulet.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Chapat L, Chemin K, Dubois B, Bourdet-Sicard R, Kaiserlian D. Lactobacillus casei reduces CD8+ T cell-mediated skin inflammation. Eur J Immunol. 2004;34:2520–2528. doi: 10.1002/eji.200425139. [DOI] [PubMed] [Google Scholar]
- 21.Gueniche A, Benyacoub J, Philippe D, Bastien P, Kusy N, Breton L, Blum S, Castiel-Higounenc I. Lactobacillus paracasei CNCM I-2116 (ST11) inhibits substance P-induced skin inflammation and accelerates skin barrier function recovery in vitro. Eur J Dermatol. 2010;20:731–737. doi: 10.1684/ejd.2010.1108. [DOI] [PubMed] [Google Scholar]
- 22.Guéniche A, Bastien P, Ovigne JM, Kermici M, Courchay G, Chevalier V, Breton L, Castiel-Higounenc I. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp Dermatol. 2010;19:e1–e8. doi: 10.1111/j.1600-0625.2009.00932.x. [DOI] [PubMed] [Google Scholar]
- 23.Gueniche A, Philippe D, Bastien P, Reuteler G, Blum S, Castiel-Higounenc I, Breton L, Benyacoub J. Randomised double-blind placebo-controlled study of the effect of Lactobacillus paracasei NCC 2461 on skin reactivity. Benef Microbes. 2014;5:137–145. doi: 10.3920/BM2013.0001. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Kim Y, Kang M, Seo J, Kim H, Yu J, Hong S. Preventive effects of Lactobacillus rhamnosus (Lcr35) through the suppression of inflammatory cytokines in mouse model with atopic dermatitis. J Allergy Clin Immunol. 2012;129:AB51. doi: 10.1016/j.jaci.2011.12.853. (Suppl) [DOI] [Google Scholar]