Abstract
Visual recognition requires connecting perceptual information with contextual information and existing knowledge. The ventromedial temporal cortex (VTC), including the medial fusiform, has been linked with object recognition, paired associate learning, contextual processing, and episodic memory, suggesting that this area may be critical in connecting visual processing, context, knowledge and experience. However, evidence for the link between associative processing, episodic memory, and visual recognition in VTC is currently lacking. Using electrocorticography in a single human patient, medial regions of the left VTC were found to be sensitive to the contextual associations of objects. Electrical brain stimulation of this part of the left VTC of the patient, functionally defined as sensitive to associative processing, caused memory related, associative visual hallucinations. This provides evidence of a relationship between visual recognition, associative processing, and episodic memory. These results suggest a potential role for abnormalities of these processes as part of a mechanism that gives rise to some visual hallucinations.
Keywords: Associative processing, electrocorticography, fusiform, electrical brain stimulation, visual recognition
1. Introduction
Recognizing an object not only involves the perception of sensory input, but also relating the percept to an existing knowledgebase and past experiences to provide conceptual meaning and context (Biederman, Mezzanotte, & Rabinowitz, 1982; Martin, 2007; Oliva & Torralba, 2007). For example, recognizing an oven involves perceiving its form, identifying its cooking utility, and knowing that a refrigerator is likely nearby. Studies suggest that activation of this associated knowledge is automatic and obligatory, and these visual and memory processes may support and facilitate one another (Aminoff, Schacter, & Bar, 2008; Bar & Aminoff, 2003; Biederman et al., 1982; Wheatley, Weisberg, Beauchamp, & Martin, 2005). Based on its reported link to visual recognition (Grill-Spector et al., 1999), associative and contextual processing (Aminoff & Tarr, 2015; Bar & Aminoff, 2003; Miyashita, 1993), and episodic memory (Garoff, Slotnick, & Schacter, 2005), the ventromedial temporal cortex (VTC) is likely to play a key role in associating high-level visual representations with one's existing knowledgebase and past experiences. This theory was tested using both intracranial electrocorticography (ECoG) recording – to measure associative processing - and electrical brain stimulation (EBS) data – to examine what happens when this region is abnormal – was obtained from a neurosurgical patient with intracranial electrodes (see Figure 1). Data revealed that the function of the VTC underlies how humans seamlessly perceive sensory information from the environment and relate it to past experiences and knowledgebase. These results provide evidence of a link between visual recognition, associative processing, and episodic memory within this region.
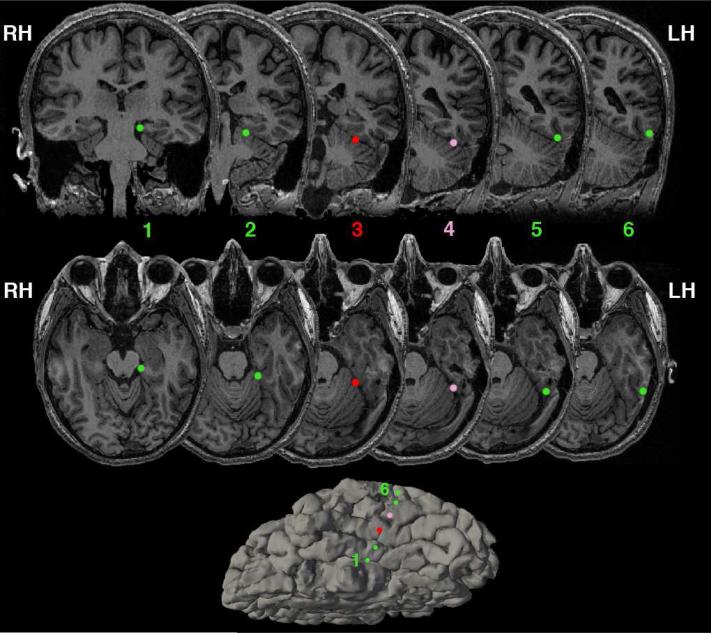
Figure 1.
Anatomical locations of the strip of electrodes on the VTC, going from most medial (electrode 1) to the most lateral (electrode 6). Note images are radiological convention – right hemisphere is presented on the left, and left hemisphere is presented on the right. The main electrode of interest is electrode 3 marked in red, residing over the medial fusiform. During EBS, current was passed between electrode 3 and electrode 4 (marked in pink). Bottom – Left hemisphere ventral view of the pial surface. See Figure 2 for complete coverage of electrodes beyond the VTC.
2. Materials and Methods
A brief overview of the Materials and Methods are presented here. Please refer to the Supplemental Materials for more detailed information.
2.1 Patient Medical History
The Institutional Review Board of the University of Pittsburgh approved the experimental protocols. Written informed consent was obtained from the patient and separate video consent was also obtained.
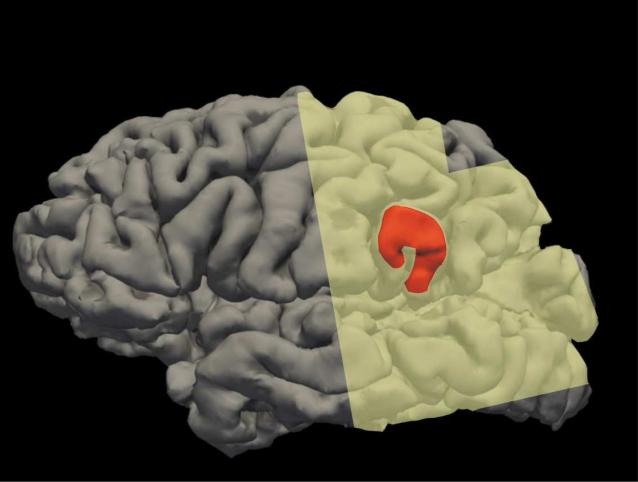
The patient was a 19-year-old male college student, with a history of medically intractable epilepsy since the age of 13. Preoperative neuropsychological testing demonstrated his performance IQ to be in the average range. Furthermore, all cognitive skills were in the average range, including verbal and visual memory. Ictal ECoG revealed that the patient's seizure onset zone comprised a portion of the lateral left supramarginal gyrus. After ECoG grid removal and all experimental studies reported here, the area subsequently was resected, and the patient has remained seizure free for 14 months (see Figure 2).
Figure 2. Lateral pial surface of the left hemisphere.
Resection is marked in red, and the electrode grid coverage is shaded in yellow. The lateral electrode grid coverage consisted of a dorsal 4×4 grid and a more ventral 8x8 grid. Locations based on anatomical landmarks.
2.2 Experimental Design
The data of this study was collected in two phases. The first phase was recording from the electrodes to determine whether any of the VTC electrodes were sensitive to the associative properties of the objects (contextual associative localizer). In a session after the ECoG recording session, for clinical purposes, EBS was administered to the patient while they performed a word naming and picture naming task. Resection of the supramarginal gyrus occurred after both these phases were completed.
Details of both phases, including stimuli, procedure, and data analysis can be found in the Supplemental Materials.
3. Results
3.1 Intracranial Electrocorticography Recording
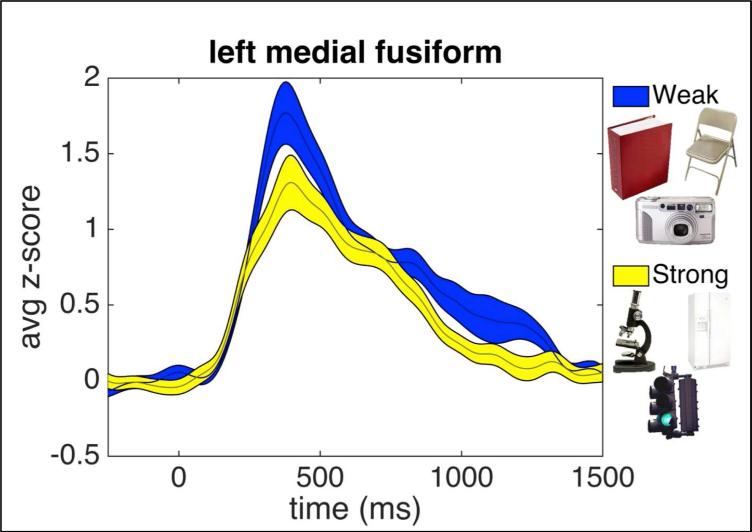
To examine the relationship between object recognition, episodic memory, and associative processing, electrical brain stimulation and intracranial electrocorticography recording was performed on one neurosurgical patient with epilepsy whose electrode coverage included a contact over left hemisphere VTC centered over the medial fusiform (Figure 1). Sensitivity of the VTC covered by the electrodes to the associative properties of objects during recognition was evaluated. Previously, sensitivity to associative properties in this part of the brain has been shown in functional MRI and magnetoencephalography by differential responses elicited to single isolated objects that are strongly associated with a single context (e.g., bathtub - bathroom) compared to objects that are weakly associated with many contexts (e.g., pen - many contexts) (Bar & Aminoff, 2003; Kveraga et al., 2011). These same stimuli were presented while using ECoG, and the medial fusiform (underneath electrode 3, marked in red in Figure 1) was found to be sensitive to the associative properties of objects being viewed (Figure 3; see Figure S1 for the time courses recorded from the other electrodes). Specifically, the broadband gamma activity (40-100 Hz), which previous studies have linked to the underlying population firing rates and fMRI BOLD response (Hermes et al., 2012; Manning, Jacobs, Fried, & Kahana, 2009; Ray, Crone, Niebur, Franaszczuk, & Hsiao, 2008), showed significantly different area-under-curve (AUC) for objects with strong compared with weak associations (Figure 3; mean AUC for weak associations 0.98 ± 0.08 (s.t.e.), mean AUC for strong associations 0.74 ± 0.06 (s.t.e.), t(165) = 2.33, p < 0.02, 150ms to 1500ms). Critically the neural activity that differentiated between strongly associative objects and weakly associative objects was found during a size judgment task - a task unrelated to associative processing. Thus, these functional results support that this electrode was located over a portion of the VTC that previous studies have shown to be involved in both object recognition and associative processing.
Figure 3. Broadband gamma activation (y-axis) recorded from electrode 3, within the left medial fusiform.
Stimulus onset is 0ms. Activity in response to weak associative objects are in blue, and activity in response to strong associative objects are in yellow. Width of shaded region represents standard error. Stimuli are displayed with a white background for figure display; in the experiment they were presented against a grey background. See Supplemental Figure S1 for results of the neighboring electrodes.
It is somewhat surprising that the broadband activity of the weakly associative objects were found to be greater than the strongly associative objects in the ECoG results, which is in the opposite direction as reported in the previous fMRI results. However, the nature of the relationship between the BOLD signal and local field potentials and electrophysiology is complex and somewhat heterogeneous in time and space with both positive and negative correlations found (Conner, Ellmore, Pieters, DiSano, & Tandon, 2011; Winterer, Carver, Musso, & Mattay, 2007). The directionality of the effect does not mitigate confirmation of the prediction that this region is sensitive to object associations. Future studies should be done to better clarify the relationship between electrophysiology and the BOLD signal, particularly at this cortical location.
3.2 Electrical Brain Stimulation
During an extra-operative functional mapping session (first single word reading, then picture naming), bipolar EBS was applied between the VTC electrode identified above and its neighboring lateral electrode (Figure 1). This stimulation caused the participant to be interrupted and distracted by additional mental processes. The participant seemed to perceive memory-like visual hallucinations that were linked to the current stimulus he was viewing (see Movie 1). On both of the two separate occasions when 5 mA EBS was delivered between this electrode pair during the word naming task the patient stopped and seemed distracted. When asked what happened, the patient responded: “It reminded me of something from my past, like I went through a bunch of pictures in my mind.” During picture naming approximately 5 minutes later, a similar effect was seen, again only once stimulation reached 5 mA between the same pair of electrodes noted above. In this case, the patient was visibly distracted and when describing what happened, he said: “It was more like... I was connected to Facebook; I saw a bunch of pictures from Facebook. I don't know – like old pictures.” We use the term hallucinations loosely because it is undetermined the extent to whether this involuntary visual episode was contained only within this mind, as in forced imagery, as indicated in the first stimulation event by “pictures in my mind,” or external to his mind as well, more akin to hallucinations, as indicated in the second stimulation event by seeing “a bunch of pictures.” Also, note that whether the content of these hallucinations was accurately recalled from memory was not confirmed; therefore they are termed “memory-like.” When asked whether the object (a lion) looked funny and if he could see it, the patient replied, “It was...trying to connect to a different picture that was like it. I knew what it was, but I really couldn't, like contact it, match it together.” This comment – connecting to a different picture that was like it – is indicative that the patient was processing associations of the picture as a result of the stimulation. It suggests that the stimulation resulted in associative processing superseding the act of recognition alone: the stimulation resulted in the patient activating the associations of the stimulus over the identity of the stimulus.
Additionally, the participant affirmed that this was similar to what happened during stimulation with word reading and that it was not like his seizure-related aura. Importantly, the stimulation location was well outside of the seizure onset zone in left supramarginal gyrus, see Methods - Figure 3, and no seizure-related afterdischarges were associated with the stimulation. When stimulating more lateral sites, no disruption occurred – therefore this type of hallucination of associated content was induced 3 out of 3 times when only a selective region, within the mid-to-medial portions of the fusiform, were stimulated at 5 mA, and not at lower amplitudes (0/28 trials) or other regions (0/774 trials). The effect of associating the presented object with information beyond what was perceived in the immediate environment was selectively a result of stimulating the same region of the brain that significantly differentiated between strong and weak associative objects.
The only other EBS effects seen during the session were involuntary arm movement during stimulation of the arm area in motor cortex and epileptic afterdischarges during stimulation near the patient's seizure network near the border of lateral temporal and parietal cortices in supramarginal gyrus (Figure 2). In addition, EBS to electrodes medial to electrode 3 (i.e., 1 & 2) were not possible as it caused discomfort, which occasionally occurs when one of the electrode pairs being stimulated makes contact with the membranes surrounding the brain. Indeed, the post-operative CT indicated that the most medial electrode on the strip was not well placed on the cortex and therefore, the medial extent of the effect could not be mapped. Finally, when asked at a later time, the patient did not consider the events during stimulation as aversive or affective.
4. Discussion
Complex visual hallucinations, déjà vu, and “experiential” and “interpretive” perceptual phenomena in response to EBS have been reported since the pioneering EBS studies of Penfield in the early 20th century (Penfield, 1963; Selimbeyoglu & Parvizi, 2010). These reports of complex visual phenomena in the literature have almost exclusively occurred in response to EBS to limbic structures and paralimbic cortex (Bancaud, Brunet-Bourgin, Chauvel, & Halgren, 1994; Gloor, Olivier, Luis, Andermann, & Horowitz, 1982; Selimbeyoglu & Parvizi, 2010; Vignal, Maillard, McGonigal, & Chauvel, 2007), and have thus generally been thought to relate to the episodic memory and familiarity processing functions of those areas (though see (Blanke, Landis, & Seeck, 2000; Jacobs, Lega, & Anderson, 2012; Mégevand et al., 2014). In addition, previous reports of complex visual hallucinations have generally occurred in or near the seizure network and sometimes are related to the patients’ aura and/or accompanied by epilepsy-related afterdischarges (Bancaud et al., 1994; Gloor et al., 1982; Jacobs et al., 2012; Selimbeyoglu & Parvizi, 2010; Vignal et al., 2007). In contrast, in the current report EBS was delivered to high-level visual association cortex functionally localized from ECoG testing, far from the patient's seizure network, and caused complex visual phenomena unlike his somatosensory-based seizure-related auras. Specifically, this EBS caused him to contextualize the presented stimulus within a hallucination comprised of memory-like images from the past and aspects of his stored knowledge. The hallucinations were associating the stimulus (words and pictures) within a broader context (e.g., viewing Facebook pages). Recent theories on object processing have emphasized the importance of reciprocal interactions between visual decoding and associated knowledge (Aminoff & Tarr, 2015; Kveraga et al., 2011; Martin, 2007). The combination of EBS and ECoG results reported here provide evidence that the VTC underpins the intrinsic processes that connect visual object information to associated information stored in memory.
The results of this study suggest that associated content of our environment is automatically linked to an object upon recognition through mechanisms mediated by the VTC. If so, we can speculate what may happen when this process is abnormal: If VTC is responsible for connecting visual processing to long-term associated information, as implicated with the current results, then when this function is abnormal, associative processing may become disruptive. The current results may suggest a potential mechanism that contributes to pathological forced imagery or hallucinations. Although it is unclear the extent that the visual episode resulting from stimulation was experienced internally in the mind of the patient, or in external space, it is conceivable that abnormal associative processing may lead to both kinds of experiences. Individuals with temporal lobe epilepsy, particularly those with seizures that involve VTC, often report with their seizures and seizure-related aura complex visual hallucinations of a type similar to those seen by the current patient (Manford & Andermann, 1998). A recent case study reported an individual who began having complex visual hallucinations following a stroke that selectively damaged left hemisphere medial VTC (Tombini et al., 2012). Furthermore, structural and functional studies in individuals with Parkinson's disease with visual hallucinations show that the VTC, including medial fusiform, is abnormal (Goldman et al., 2014; Lenka, Jhunjhunwala, Saini, & Pal, 2015; Watanabe et al., 2013). It is notable that in these examples, as well as the results of the current report, the individuals are usually aware that their visual hallucinations are not real. However, this is typically not the case in psychotic conditions. In terms of psychosis, a recent fMRI study showed that the medial parts of VTC are more active in adolescents with a “brief psychotic disorder” during visual hallucinations compared to auditory hallucinations (Jardri, Thomas, Delmaire, Delion, & Pins, 2013). Thus, aberrant processing in the VTC may represent a partially unifying pathology for cross-diagnostic visual hallucinations. Disruptive associative processing due to VTC abnormalities could be interpreted as unwanted hallucinations and may further contribute to psychosis if a person also has deficits in processes related to cognitive or executive control or attention. Indeed, a “loosening” of associations has often been described in psychosis (Meadow, Greenblatt, & Solomon, 1953; Modestin, Huber, Satirli, Malti, & Hell, 2003). In fact, in schizophrenia correlations across positive symptoms have linked overactive spreading of semantic associations (Kerns, Berenbaum, Barch, Banich, & Stolar, 1999) to hallucinations and to fusiform activity (Sass et al., 2014). Future studies are necessary to clarify the extent to which the episode resulting from the stimulation was forced imagery, or occurred in the external environment, as well as how these two experiences may or may not be linked to the same underlying mechanism.
The combined EBS and ECoG results from the study provide support for a link between visual object recognition and associative processing and episodic memory in VTC. However, there are a few limitations to highlight when interpreting the results. These results should ideally be replicated in other patients who have the same electrode placement within the VTC and could be extended by examining how stimulation of VTC impacts objective performance (reaction time, accuracy, etc.) on visual associative memory tasks. Furthermore, the extent of neurons affected by the stimulation may lie beyond the site of the electrode itself. And therefore, the resulting imagery experienced by the patient may be a result of the stimulation reaching neighboring regions or pathways, such as the inferior longitudinal fasciculus. However, the result found in the a priori motivated ECoG investigation of associative processing in this region provides corroborative evidence that the source of the imagery is likely from stimulation to the VTC. Lastly, to make a direct link to episodic memory, the details of the hallucination should be verified and quantified.
In conclusion, EBS delivered to a region in left VTC caused complex, memory-like visual hallucinations that the patient associated with the image he was viewing at the time. This particular region of the VTC was sensitive to the associative content of an image, as determined through ECoG testing. This provides evidence of a link between visual object recognition and associative processing and episodic memory in VTC. These results reveal how interpreting our visual world may employ an interaction between perception of the stimulus in our environment and contextualizing the stimulus with our past experiences and knowledgebase.
Supplementary Material
Highlights.
Ventromedial temporal cortex supports visual recognition and associative processing
Direct neural stimulation of the area resulted in associative visual hallucinations
Provides evidence of a link between visual, associative and memory processes
Supports a mechanism associating visual perception to experience and knowledge
Acknowledgments
We thank the patient and his family for their time and participation and the epilepsy monitoring unit staff, Cheryl Plummer, Gena Ghearing, and administration for their assistance and cooperation with our research, and Robert Sweet and Dean Salisbury for their valuable comments. This research was supported by the National Institute of Mental Health under award number P50 MH103204-01 (PI: David Lewis; Co-I A.S.G.), the National Science Foundation 1439237 (to E.A.), and the Office of Naval Research - MURI N000141010934 (to E.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Movie 1: Patient response to stimulation of electrode 3 during word and picture naming.
The authors declare no competing financial interests.
References
- Aminoff EM, Tarr MJ. Associative Processing Is Inherent in Scene Perception. PLoS ONE. 2015;10(6):e0128840. doi: 10.1371/journal.pone.0128840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E, Schacter DL, Bar M. The cortical underpinnings of context-based memory distortion. Journal of Cognitive Neuroscience. 2008;20(12):2226–2237. doi: 10.1162/jocn.2008.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancaud J, Brunet-Bourgin F, Chauvel P, Halgren E. Anatomical origin of déjà vu and vivid “memories” in human temporal lobe epilepsy. Brain. 1994;117:71–90. doi: 10.1093/brain/117.1.71. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38(2):347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Biederman I, Mezzanotte RJ, Rabinowitz JC. Scene perception: detecting and judging objects undergoing relational violations. Cognitive Psychology. 1982;14(2):143–177. doi: 10.1016/0010-0285(82)90007-x. [DOI] [PubMed] [Google Scholar]
- Blanke O, Landis T, Seeck M. Electrical cortical stimulation of the human prefrontal cortex evokes complex visual hallucinations. Epilepsy & Behavior : E&B. 2000;1(5):356–361. doi: 10.1006/ebeh.2000.0109. [DOI] [PubMed] [Google Scholar]
- Conner CR, Ellmore TM, Pieters TA, DiSano MA, Tandon N. Variability of the Relationship between Electrophysiology and BOLD-fMRI across Cortical Regions in Humans. Journal of Neuroscience. 2011;31(36):12855–12865. doi: 10.1523/JNEUROSCI.1457-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff RJ, Slotnick SD, Schacter DL. The neural origins of specific and general memory: the role of the fusiform cortex. Neuropsychologia. 2005;43(6):847–859. doi: 10.1016/j.neuropsychologia.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Gloor P, Olivier A, Luis F, Andermann F, Horowitz S. The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Annals of Neurology. 1982;12:129–144. doi: 10.1002/ana.410120203. [DOI] [PubMed] [Google Scholar]
- Goldman JG, Stebbins GT, Dinh V, Bernard B, Merkitch D, deToledo-Morrell L, Goetz CG. Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson's disease with hallucinations. Brain. 2014;137(3):849–859. doi: 10.1093/brain/awt360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24(1):187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Hermes D, Miller KJ, Vansteensel MJ, Aarnoutse EJ, Leijten FSS, Ramsey NF. Neurophysiologic correlates of fMRI in human motor cortex. Human Brain Mapping. 2012;33(7):1689–1699. doi: 10.1002/hbm.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Lega B, Anderson C. Explaining how brain stimulation can evoke memories. Journal of Cognitive Neuroscience. 2012;24(3):553–563. doi: 10.1162/jocn_a_00170. [DOI] [PubMed] [Google Scholar]
- Jardri R, Thomas P, Delmaire C, Delion P, Pins D. The Neurodynamic Organization of Modality-Dependent Hallucinations. Cerebral Cortex. 2013;23(5):1108–1117. doi: 10.1093/cercor/bhs082. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Berenbaum H, Barch DM, Banich MT, Stolar N. Word production in schizophrenia and its relationship to positive symptoms. Psychiatry Research. 1999;87(1):29–37. doi: 10.1016/s0165-1781(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Kveraga K, Ghuman AS, Kassam KS, Aminoff EA, Hämäläinen MS, Chaumon M, Bar M. Early onset of neural synchronization in the contextual associations network. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(8):3389–3394. doi: 10.1073/pnas.1013760108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenka A, Jhunjhunwala KR, Saini J, Pal PK. Parkinsonism and Related Disorders. Parkinsonism and Related Disorders. 2015;21(7):683–691. doi: 10.1016/j.parkreldis.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819–1840. doi: 10.1093/brain/121.10.1819. [DOI] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. Journal of Neuroscience. 2009;29(43):13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Meadow A, Greenblatt M, Solomon H. Looseness of association” and impairement in abstraction in schizophrenia. Journal of Nervous and Mental Disease. 1953;118:27–35. doi: 10.1097/00005053-195307000-00003. [DOI] [PubMed] [Google Scholar]
- Mégevand P, Groppe DM, Goldfinger MS, Hwang ST, Kingsley PB, Davidesco I, Mehta AD. Seeing scenes: topographic visual hallucinations evoked by direct electrical stimulation of the parahippocampal place area. Journal of Neuroscience. 2014;34(16):5399–5405. doi: 10.1523/JNEUROSCI.5202-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita Y. Inferior temporal cortex: where visual perception meets memory. Annual Review of Neuroscience. 1993;16:245–263. doi: 10.1146/annurev.ne.16.030193.001333. [DOI] [PubMed] [Google Scholar]
- Modestin J, Huber A, Satirli E, Malti T, Hell D. Long-term course of schizophrenic illness: Bleuler's study reconsidered. The American Journal of Psychiatry. 2003;160(12):2202–2208. doi: 10.1176/appi.ajp.160.12.2202. [DOI] [PubMed] [Google Scholar]
- Oliva A, Torralba A. The role of context in object recognition. Trends in Cognitive Sciences. 2007;11(12):520–527. doi: 10.1016/j.tics.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Penfield W. The brain's record of auditory and visual experience. A final summary and discussion. Brain. 1963;86:595–696. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60-200 Hz) in macaque local field potentials and their potential implications in electrocorticography. Journal of Neuroscience. 2008;28(45):11526–11536. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass K, Heim S, Sachs O, Straube B, Schneider F, Habel U, Kircher T. Neural correlates of semantic associations in patients with schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2014;264(2):143–154. doi: 10.1007/s00406-013-0425-0. [DOI] [PubMed] [Google Scholar]
- Selimbeyoglu A, Parvizi J. Electrical stimulation of the human brain: perceptual and behavioral phenomena reported in the old and new literature. Frontiers in Human Neuroscience. 2010;4:46. doi: 10.3389/fnhum.2010.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombini M, Pellegrino G, Zappasodi F, Quattrocchi CC, Assenza G, Melgari JM, et al. Complex visual hallucinations after occipital extrastriate ischemic stroke. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2012;48(6):774–777. doi: 10.1016/j.cortex.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Vignal J-P, Maillard L, McGonigal A, Chauvel P. The dreamy state: hallucinations of autobiographic memory evoked by temporal lobe stimulations and seizures. Brain. 2007;130:88–99. doi: 10.1093/brain/awl329. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Senda J, Kato S, Ito M, Atsuta N, Hara K, et al. Cortical and subcortical brain atrophy in Parkinson's disease with visual hallucination. Movement Disorders. 2013;28(12):1732–1736. doi: 10.1002/mds.25641. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Weisberg J, Beauchamp MS, Martin A. Automatic priming of semantically related words reduces activity in the fusiform gyrus. Journal of Cognitive Neuroscience. 2005;17(12):1871–1885. doi: 10.1162/089892905775008689. [DOI] [PubMed] [Google Scholar]
- Winterer G, Carver FW, Musso F, Mattay V. Complex relationship between BOLD signal and synchronization/desynchronization of human brain MEG oscillations. Human Brain Mapping. 2007;28(9):805–816. doi: 10.1002/hbm.20322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.