Abstract
Objective:
(1) Compare rates of abnormal screening electrocardiograms (ECGs) using updated criteria compared with older criteria. (2) Compare rates of abnormal ECGs by ethnicity. (3) Evaluate ability of ECG criteria to detect the predicted number of athletes with previously undetected cardiovascular abnormalities.
Design:
Prospective and retrospective review of ECGs. During the prospective portion of the study, the 2005 European Society of Cardiology criteria were used from 2008 to July 2011 and the 2011 Stanford criteria were used from August 2011 to 2013. Retrospectively, all ECGs were reevaluated using the 2011 Stanford criteria, 2013 Seattle criteria, and 2014 Sharma Refined criteria.
Setting:
Division I National Collegiate Athletic Association University.
Participants:
874 incoming athletes over a 5-year period.
Interventions:
ECG screening program.
Main Outcome Measures:
Number of abnormal ECGs and number of athletes with newly discovered cardiac abnormalities.
Results:
Abnormal ECG rates were the 2005 European criteria 10.7%, 2011 Stanford criteria 6.6%, 2013 Seattle criteria 2.8%, and 2014 Sharma Refined criteria 2.8%. In black athletes, the Stanford criteria resulted in more abnormal ECGs compared with Seattle or Sharma Refined. Three athletes were found to have a previously undetected cardiac abnormality (2 with hypertrophic cardiomyopathy and 1 with preexcitation).
Conclusions:
More recent ECG screening criteria substantially reduce the abnormal ECG rate and thus the number of athletes requiring additional testing. ECG screening criteria identified the predicted number (1/300) of young athletes with serious underlying cardiovascular disease. These criteria prompt not only additional cardiovascular testing but also a more thorough cardiovascular history.
Key Words: ECG screening, hypertrophic cardiomyopathy, sudden cardiac death
INTRODUCTION
The goal of cardiac screening of young athletes is to detect silent but potentially life-threatening cardiac conditions.1 The current standard of a preparticipation cardiac history and physical examination has a low sensitivity for discovering cardiac abnormalities.2 A screening 12-lead electrocardiogram (ECG) in the young athlete is reasonably sensitive for detection of these structural and electrical abnormalities.3,4 The Italian experience with screening ECG has led the European Society of Cardiology (ESC) to recommend a screening 12-lead ECG for young athletes.5 The International Olympic Committee and governing bodies of several US and international athletic leagues have endorsed or implemented screening ECGs.6 The American Heart Association (AHA) and American College of Cardiology (ACC), however, consider the use of screening 12-lead ECGs in relatively small cohorts of young healthy people, a class IIb indication.1 The AHA and ACC question the cost-effectiveness of screening ECGs because of a high false-positive rate, the lack of qualified interpreters of young athletes' ECGs, and whether the cardiovascular conditions responsible for sudden cardiac death can be detected effectively by screening ECGs.1
The goal of our study was to describe our experience with implementing an ECG screening program as part of the preparticipation physical in a university setting. We planned to measure the rate of screening ECG abnormalities using the 2005 European criteria, promulgated by the Italians. In 2011, however, modified interpretation criteria were published from Stanford University with reports of improved specificity without apparent decrease in sensitivity.7 After incorporating these newer criteria, we opted to measure the improvement in the rate of abnormal ECGs, comparing the 2005 European criteria with the 2011 Stanford criteria. At the conclusion of our study, two newer studies with more selective criteria were published (Seattle in 2013 and Sharma Refined in 2014), and we chose to apply these criteria retrospectively to our cohort.8,9
METHODS
Beginning in June 2008, all incoming varsity athletes at the University of Nevada, Reno underwent preparticipation screening by student health physicians, which included the 12-point cardiac examination recommended by the AHA. The University of Nevada, Reno is a National Collegiate Athletic Association (NCAA) Division I university. Division I includes the major collegiate athletic schools with larger budgets, more elaborate facilities, and more scholarships than Division II or III schools, and must have at least 14 sports programs with at least 7 sports for women. The athletic training staff, who had been trained by the student health physicians, administered a 12-lead ECG after a written protocol. ECG interpretation was performed by a dedicated group of four local board-certified adult cardiologists. Before beginning the study, the cardiologists reviewed the specific criteria for interpretation. ECGs were interpreted on-site by the cardiologist, faxed to the cardiologist within 24 hours or transmitted by a secure website for interpretation. From June 2008 to July 2011, the athlete's ECG was interpreted using the 2005 European criteria (Table 1).5 Starting in August 2011 through September 2013, the athlete's ECG was interpreted using the 2011 Stanford criteria (Table 2).7 If the ECG was interpreted as abnormal, the reading cardiologist recommended cardiac consultation for electrical abnormalities (long QTC, preexcitation, arrhythmias, etc.) or an echocardiogram for abnormal findings suggestive of possible cardiomyopathy (ST depression, T-wave inversion, etc.). Electrophysiologic testing and/or exercise stress testing were ordered on a subset of athletes based on individual symptoms and echocardiogram results. Athletes whose ECG was interpreted as abnormal were withheld from training and competition until further testing was completed.
TABLE 1.
The 2005 European Criteria for Abnormal Preparticipation ECG in Young Athletes (Ref. 5)
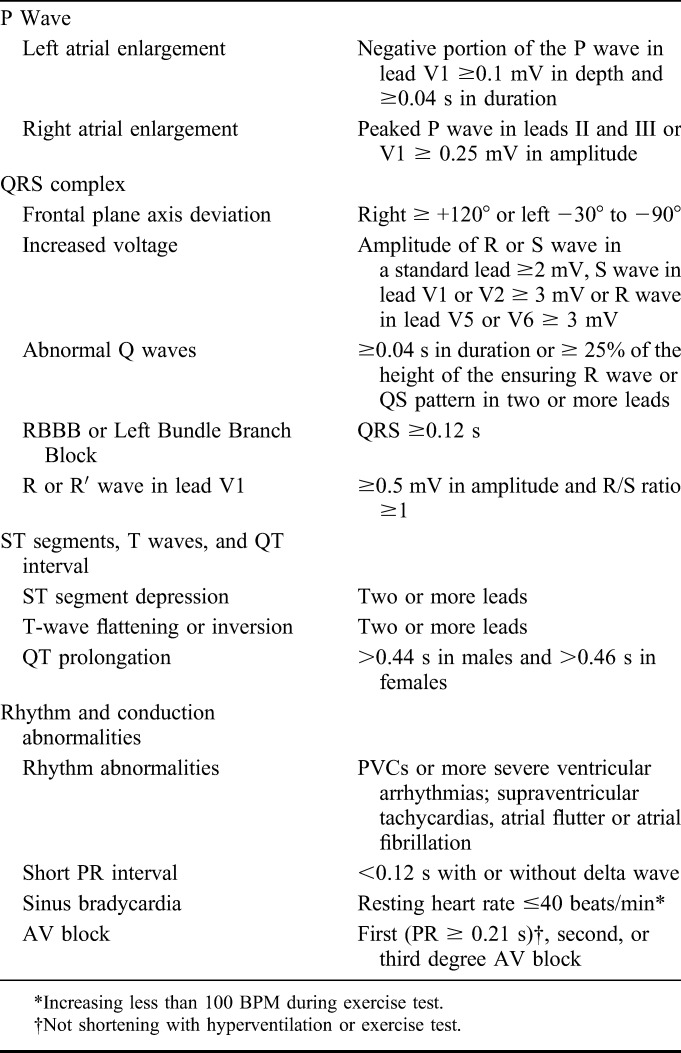
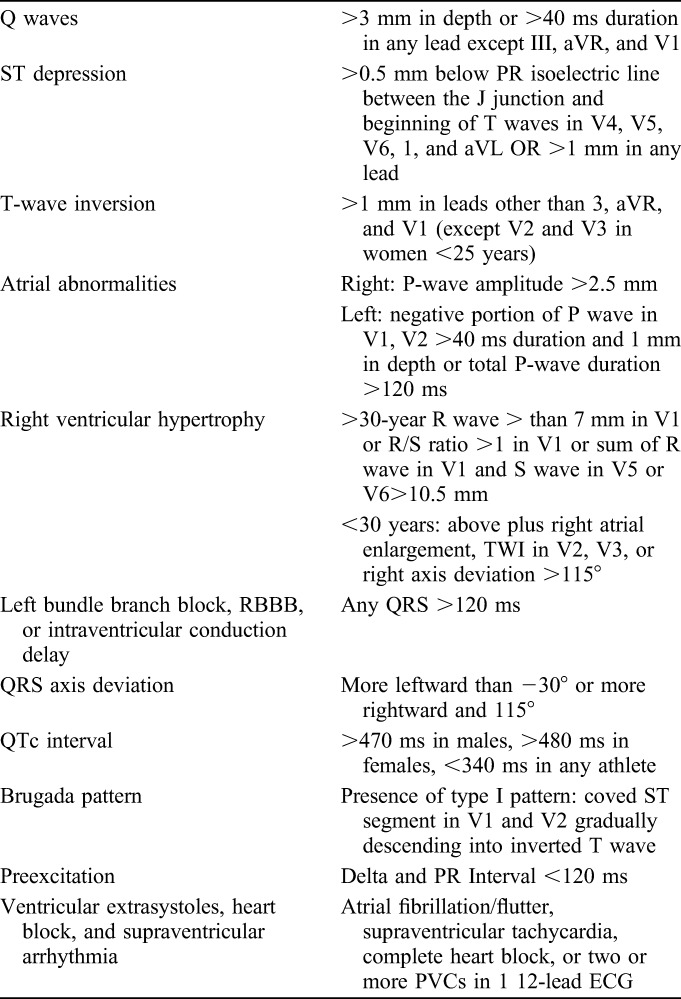
TABLE 2.
The 2011 Stanford Criteria for Abnormal Preparticipation ECG in Young Athletes (Ref. 7)
We projected that we would evaluate 900 NCAA athletes over 5 years. Based on a prevalence of 0.3% of serious cardiovascular abnormalities in young athletes, we expected detection by ECG of 3 athletes requiring disqualification from sports.10
Echocardiograms were performed in an Intersocietal Accreditation Commission certified laboratory and interpreted by board certified adult cardiologists. In the absence of echocardiographic abnormalities suggesting cardiomyopathy or significant valvular disease, athletes were cleared for sports. If echocardiographic findings were suggestive but not conclusive for cardiomyopathy, the athlete was withheld from sports and referred for cardiac consultation and additional testing.
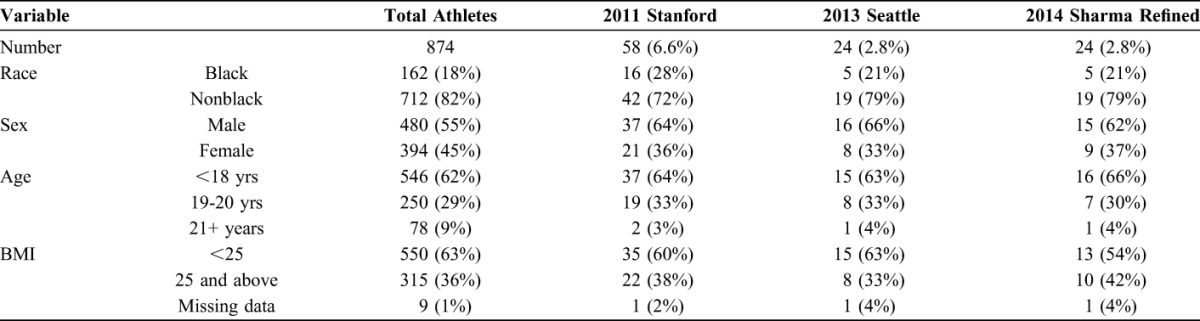
At the study conclusion, all ECGs were retrospectively reanalyzed using the 2011 Stanford criteria, 2013 Seattle criteria, and 2014 Sharma Refined criteria. We reviewed charts to obtain information on age, sex, ethnicity, sport, height, weight, systolic and diastolic blood pressure, cardiac history, and cardiovascular examination. The study protocol was approved by the University of Nevada, Reno Research Integrity Office.
Statistical Analysis
To ensure accuracy of the ECG interpretation, we used data from the retrospective analysis using the 2011 Stanford criteria, 2013 Seattle criteria, and 2014 Sharma Refined criteria as our outcome. Abnormal ECG rates for each of these three criteria were calculated and used as the outcome indicators. We grouped predictors into categorical variables, including sex, ethnicity (black vs nonblack athletes), sport, age (less than 18, 19–20 and 21 + years old), body mass index (BMI) (up to 24.9 vs 25 and above). Chi-square tests were performed to test whether there were statistically significant differences of abnormal rates between different predictor groups and between the screening criteria groups. Data analyses were conducted using Statistical Analysis Systems (9.4).
RESULTS
Prospective ECG Evaluation
Phase 1: From June 2008 until July 2011, 523 athletes were screened using the 2005 European criteria. Of these athletes, 46 (8.7%) had abnormal ECGs. One female athlete with marked ST depression was diagnosed with hypertrophic cardiomyopathy (HCM) and not cleared for sports. One asymptomatic male athlete with preexcitation was seen in consultation and cleared to play sports. The other 44 athletes with abnormal ECGs had no abnormalities on the subsequent testing and returned to their sport.
Phase 2: From August 2011 to September 2013, 351 athletes were screened using the 2011 Stanford ECG criteria. Of these athletes, 19 (5.4%) had abnormal ECGs. One male athlete with marked ST depression was diagnosed with HCM and not cleared for sports. The other 18 athletes with abnormal ECGs had no abnormalities on subsequent testing and returned to their sport.
Retrospective ECG Evaluation
Phase 1: The 2011 Stanford ECG criteria were retrospectively applied to the 523 ECGs prospectively analyzed using the 2005 European criteria (Figure 1). Of the 46 athletes with ECGs considered abnormal by the 2005 European criteria, only 22 athletes (40%) were considered abnormal by the 2011 Stanford criteria. The other 24 (60%) had isolated QRS voltage criteria for left ventricular hypertrophy (LVH) (n = 14), flat T waves (n = 7), short PR interval (n = 1), first-degree atrioventricular (AV) block (n = 1), and a large R in V1 (n = 1). These ECG findings are now considered to be common and training-related findings.11 Of the remaining 477 athletes, whose ECGs were read as normal by the 2005 European criteria, 10 were found on retrospective analysis to meet the 2011 Stanford (and the 2005 European) criteria with specific findings, including: left atrial enlargement (n = 3), Q wave (n = 2), right bundle branch block (RBBB) (n = 2), ST depression (n = 1), right axis deviation (n = 1), and left axis deviation (n = 1). Overall in the phase 1 ECGs, using the 2005 European criteria, there were a total of 56 abnormal ECGs (46 prospective and 10 retrospective) of 523 total resulting in an abnormal ECG a rate of 10.7%.
FIGURE 1.
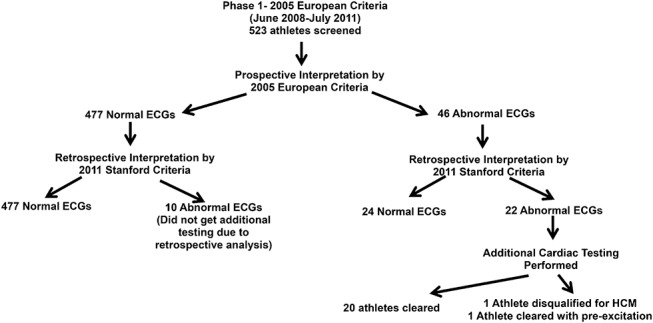
Flowsheet of ECG analysis during phase 1 of the study.
Phase 2: The 2011 Stanford ECG criteria were reapplied retrospectively to the 351 athletes' ECGs, and 7 athletes' ECGs were found to have abnormalities missed during initial prospective analysis (Figure 2). In the 7 athletes, the abnormalities found included: left atrial enlargement (n = 7), ST segment depression (n = 2), and premature ventricular contractions (n = 2).
FIGURE 2.
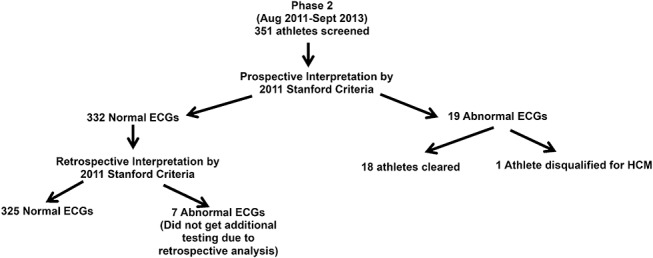
Flowsheet of ECG analysis during phase 2 of the study.
For the entire cohort of 874 athletes, 58 (6.6%) met the 2011 Stanford criteria, 24 (2.8%) met the Seattle criteria and 24 (2.8%) met the Sharma Refined criteria for an abnormal ECG (Table 3). All athletes with abnormal ECGs identified by the Seattle criteria and the Sharma Refined criteria had been identified using the Stanford criteria.
TABLE 3.
ECG Abnormalities in 874 NCAA Division I Athletes Using the 2011 Stanford, 2013 Seattle, and 2014 Sharma Refined Criteria
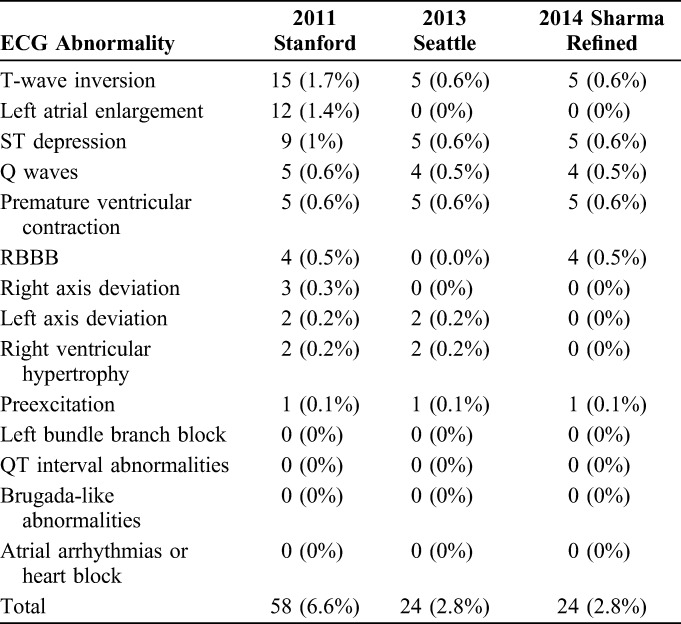
Black athletes comprised 18% of the cohort, but were 28% of the population with the 2011 Stanford abnormal ECGs. This percentage of black athletes with abnormal EGGs was lowered to 22% using either the Seattle criteria or the Sharma Refined criteria (Table 4). There were no statistically significant differences in abnormal ECGs by sex, BMI, or age between the overall cohort and the group of athletes with abnormal ECGs using the Stanford, Seattle, or the Sharma Refined criteria.
TABLE 4.
Percentage of Abnormal ECGS by Modern Criteria by Race, Sex, Age, and BMI
Three of the 874 athletes (0.3%) were found to have a serious underlying cardiovascular abnormality potentially placing them at risk for sudden cardiac death. One asymptomatic athlete was found to have electrocardiographic findings of preexcitation and was cleared for sports after electrophysiologic consultation and has remained asymptomatic. Two athletes were found to have HCM. For the two athletes with HCM, their ECGs were interpreted as abnormal using any of the four of the criteria used in the study.
One female white soccer player showed marked ST depression and deep lateral T-wave inversion (Figure 3). Echocardiography revealed left ventricular end-diastolic dimension 4.1 cm with 1.2 cm concentric LVH. After echocardiography, the patient then admitted to episodes of syncope seconds after running, which she had not reported during the initial cardiac history. Her magnetic resonance imaging revealed moderate concentric LVH (14 mm septum) without any late gadolinium enhancement. Stress echocardiography provoked left ventricular outflow gradient of 40 mm Hg and mild systolic anterior motion. Her father then was found to have an abnormal ECG and LVH. The diagnosis was HCM and an automatic implantable cardioverter-defibrillator was placed. She returned to intramural sports.
FIGURE 3.
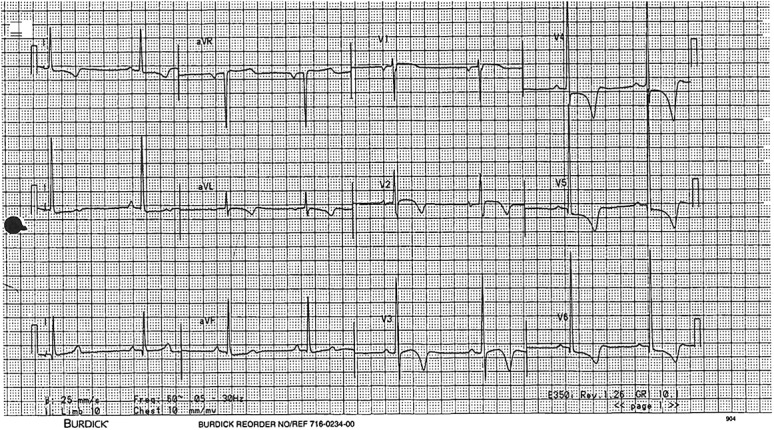
ECG of female athlete disqualified because of hypertrophic cardiomyopathy.
One black male basketball player showed marked ST depression and deep lateral T-wave inversion (Figure 4). Echocardiography revealed left ventricular end-diastolic dimension of 3.7 cm and concentric LVH at 1.9 cm. There was no history of hypertension. He initially denied any cardiac symptoms related to this finding but after being diagnosed, his family acknowledged that he had been previously diagnosed with HCM. He was medically disqualified from participating in sports at our institution.
FIGURE 4.
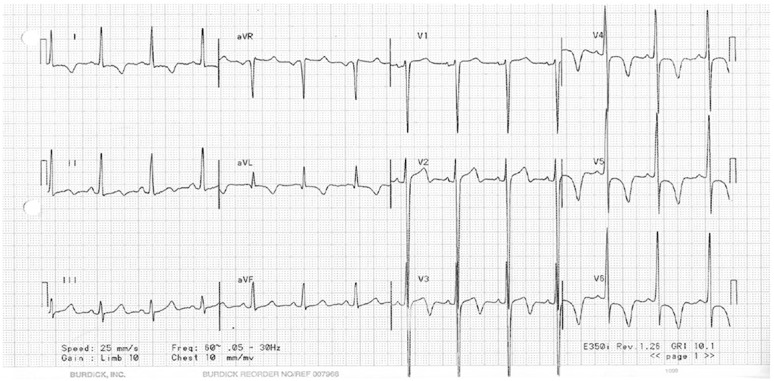
ECG of male athlete disqualified because of hypertropic cardiomyopathy.
Personal cardiac history alone led to 1 student, with exercise-induced chest pain, having treadmill testing, which was normal. No other athletes were referred for further testing based on personal cardiac history alone. The physicians, on learning of an historical abnormality in an athlete, queried about the status of the ECG: if ECG was normal, the historical abnormalities were considered insignificant. The physician staff estimated the number of athletes in this category was less than 1%. Neither cardiac family history nor cardiac physical examination alone led to athletes requiring additional cardiovascular testing. No athletes sustained cardiac death during the study.
DISCUSSION
Using the 2005 European criteria for interpretation of young athletes' ECGs in phase 1 of our study revealed 10.7% athletes with abnormal ECG findings. Those criteria, based on adult ECG interpretation, were used in the landmark prospective Italian screening evaluation of 42 386 young athletes.12,13 In retrospect, the percentage of abnormal ECGs in the Italian study and older studies was high, secondary to including common and training related ECG findings as abnormal.11 There are structural and electrical alterations in the young athlete's heart that are physiologic rather than pathologic and result in ECG changes such as isolated voltage criteria for LVH, early repolarization, incomplete RBBB, sinus bradycardia, and first-degree AV block. These changes are seldom associated with underlying cardiac pathology.4,11
In 2010, the ESC published updated consensus recommendations for interpretation of athlete's ECGs, emphasizing the distinction between group 1 (common and training related) and group 2 (uncommon and training unrelated) ECG changes.11 Shortly thereafter the 2011 Stanford criteria, reflecting minor differences from the 2010 European ECG criteria, were published and then applied retrospectively to the ECGs from phase 1 of our study. Sixty percent of the abnormal ECGs identified using the 2005 European criteria were considered common and training-related findings by the Stanford criteria and not related to any underlying cardiac pathology. The 2011 Stanford criteria, when applied to all our 874 athletes, revealed 6.6% rate of abnormal ECGs. This represents an improvement from the 10.7% rate using the 2005 European criteria. After the close of our prospective study in 2013, a group from Seattle published more selective criteria for the interpretation of young athlete's ECGs. When applied retrospectively to all 874 athletes in our study, the number of abnormal ECGs was reduced by more than 50%–2.8%. Drezner applied the 2013 Seattle criteria retrospectively to 790 NCAA athletes with 2.8% of their athletes having abnormal ECGs.14
The number of abnormal ECGs was almost double in black versus nonblack athletes by the 2011 Stanford, a trend seen in other studies as well.9 Black athletes, compared with white athletes, develop greater amounts of physiologic hypertrophy, which might explain a higher rate of ST and T-wave abnormalities, thereby reducing the specificity of ECGs in black athletes.9 The reason for greater physiologic hypertrophy in black athletes is not known. In our study, however, applying either the Seattle or the Sharma Refined criteria reduced the percentage of abnormal ECGs in black athletes.
It is reasonable to ask whether the 2010 European and 2011 Stanford criteria, documented to improve specificity and reduce false-positive rates, could reduce sensitivity to detect serious cardiovascular disease. The most significant change is that isolated voltage criteria for LVH by ECG do not require further evaluation.7,11 The improvement in specificity is approximately 40% with an estimated reduction sensitivity of only 1% to 2%.15 Recently, the Sharma proposed revised criteria for which left atrial abnormality, right atrial abnormality, left axis deviation, right axis deviation, right ventricular hypertrophy, and T-wave inversion V1-V4 proceeded by convex ST segment elevation in black athletes (found in 2.5% of our black athletes), if found in isolation, should be considered group 1 findings.9 These refinements markedly improve specificity in black and white athletes without compromising sensitivity and would further reduce the number of athletes requiring additional cardiovascular testing.9 When the Sharma Refined criteria were applied to the ECGs of our cohort of Division I athletes, only 2.8% were abnormal.
Three athletes, 0.3% of our cohort, which was the expected prevalence, had potentially serious previously undetected cardiovascular abnormalities detected by ECG.10 The success of the more recent screening criteria in identifying athletes with serious cardiovascular pathology answers a question about successful detection expressed in the AHA/ACC Scientific Statement.1 Both athletes diagnosed with HCM had an initial negative screening cardiovascular history. These 2 athletes admitted to having an abnormal history and cardiac symptoms only after discussing the results of their abnormal echocardiogram. Others have found similar difficulties when using the preparticipation cardiac history and physical examination.16 For college athletes, the desire to participate in sport and the fear that any abnormal cardiovascular history may jeopardize their ability to play (and potentially jeopardize their academic scholarship) makes the cardiac history difficult to rely on as a sensitive screening tool for life-threatening cardiac pathology. The ECG is reliable and reproducible. It is abnormal in 95% of cases of sudden cardiac death caused by HCM and abnormal in the 70% to 80% of cases of sudden cardiac death caused by arrhythmogenic right ventricular cardiomyopathy.17,18 An ECG will identify most of the cases of preexcitation, Brugada syndrome, and long and short QT interval syndromes.19 The sensitivity of the preparticipation ECG to detect potentially lethal cardiovascular disease in young athletes exceeds 70%.4 Group 2 findings on the screening ECG should prompt a more probing cardiac personal and family history.
Our study did have some limitations. The goal of our study was to provide a “real-world” experience of implementing an ECG screening program in a university setting. Because we were limited to the number of athletes at our university, this limited power for statistical analysis, especially regarding differences by ethnicity. Additionally, we may have underestimated the true abnormal rate for European criteria because we did not retrospectively reassess phase 1 ECGs using the 2005 European criteria.
We also chose to report the abnormal ECG rate instead of the false-positive rate. During retrospective analysis, we found 17 athletes with abnormal ECGs initially misclassified as normal and thus did not receive an echocardiogram. Although none of these athletes developed cardiac issues during their university athletic careers, because of the lack an echocardiogram, which is considered the confirmatory test diagnosing HCM, we could not confirm them as having a false-positive ECG. This also points out some of the practical difficulties with ECG screening; whereas our cardiologists had reviewed the diagnostic criteria as a group and were dedicated to reading these ECGs by the defined criteria, 17 abnormal ECGs were only found on retrospective analysis.
Whether ECG interpretation of a young athlete is part of an evaluation of symptoms or used for screening, training a core of physicians (sports medicine physicians, sports cardiologists, etc.) to accurately interpret ECGs is essential. Using technology to specifically alert providers about potential abnormal findings when they are interpreting the ECG or creating a web-based centralized site, staffed by qualified ECG readers, to interpret young athletes' ECGs may prove helpful.
CONCLUSIONS
Modern criteria for interpretation of young athletes' ECGs have led to lower percentages of athletes requiring additional cardiovascular studies during preparticipation evaluation. In Division I NCAA athletes, using these more recent selective criteria, we found screening ECG abnormality rates of: the 2011 Stanford 6.6%, the 2013 Seattle 2.8%, and the 2014 Sharma Refined 2.8%. Other cohorts may be similar to the extent that demographic characteristics, including race, ethnicity, and gender, are also similar. In our 5-year experience, screening ECG detected two athletes with HCM, whereas the cardiac history and examination did not. An abnormal screening ECG should prompt a more probing cardiovascular history. Of every 1000 young athletes, 3 will have previously undetected serious cardiovascular disease, potentially placing them at risk of sudden cardiac death on playing fields. The annual risk of sudden cardiac death in Division 1 NCAA athletes with serious cardiovascular disease may be as high as 1%.20 It seems prudent to train sports medicine physicians and cardiologists in the correct interpretation of young athletes' ECGs.
ACKNOWLEDGMENTS
We would like to thank Dr. Alejandro De La Parra Landa for his assistance with the ECG review.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Maron BJ, Friedman RA, Kligfield P, et al. Assessment of the 12-lead ECG as a screening test for detection of cardiovascular disease in healthy general populations of young people (12-25 Years of Age): a scientific statement from the American Heart Association and the American College of Cardiolog. Circulation. 2014;130:1303–1334. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Shirani J, Poliac LC, et al. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. Web site. http://www.ncbi.nlm.nih.gov/pubmed/8667563. Accessed June 16, 2014. [PubMed] [Google Scholar]
- 3.Borjesson M, Dellborg M. Is there evidence for mandating electrocardiogram as part of the pre-participation examination? Clin J Sport Med. 2011;21:13–17. [DOI] [PubMed] [Google Scholar]
- 4.Drezner J, Corrado D. Is there evidence for recommending electrocardiogram as part of the pre-participation examination? Clin J Sport Med. 2011;21:18–24. [DOI] [PubMed] [Google Scholar]
- 5.Corrado D, Pelliccia A, Bjørnstad HH, et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and. Eur Heart J. 2005;26:516–524. [DOI] [PubMed] [Google Scholar]
- 6.Ljungqvist A, Jenoure P, Engebretsen L, et al. The International Olympic Committee (IOC) Consensus Statement on periodic health evaluation of elite athletes March 2009. Br J Sports Med. 2009;43:631–643. [DOI] [PubMed] [Google Scholar]
- 7.Uberoi A, Stein R, Perez MV, et al. Interpretation of the electrocardiogram of young athletes. Circulation. 2011;124:746–757. [DOI] [PubMed] [Google Scholar]
- 8.Drezner JA, Ackerman MJ, Anderson J, et al. Electrocardiographic interpretation in athletes: the “Seattle Criteria.” Br J Sports Med. 2013;47:122–124. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh N, Papadakis M, Ghani S, et al. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation. 2014;129:1637–1649. [DOI] [PubMed] [Google Scholar]
- 10.Maron BJ, Thompson PD, Ackerman MJ, et al. Recommendations and Considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;115:1643–1645. [DOI] [PubMed] [Google Scholar]
- 11.Corrado D, Pelliccia A, Heidbuchel H, et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J. 2010;31:243–259. [DOI] [PubMed] [Google Scholar]
- 12.Corrado D, Basso C, Schiavon M, et al. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med. 1998;339:364–369. [DOI] [PubMed] [Google Scholar]
- 13.Corrado D, Basso C, Pavei A, et al. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296:1593–1601. [DOI] [PubMed] [Google Scholar]
- 14.Drezner JA, Prutkin JM, Harmon KG, et al. Cardiovascular screening in college athletes. J Am Coll Cardiol. 2015;65:2353–2355. [DOI] [PubMed] [Google Scholar]
- 15.Corrado D, Calore C, Zorzi A, et al. Improving the interpretation of the athlete's electrocardiogram. Eur Heart J. 2013;34:3606–3609. [DOI] [PubMed] [Google Scholar]
- 16.Wilson MG, Basavarajaiah S, Whyte GP, et al. Efficacy of personal symptom and family history questionnaires when screening for inherited cardiac pathologies: the role of electrocardiography. Br J Sports Med. 2008;42:207–211. [DOI] [PubMed] [Google Scholar]
- 17.Maron BJ, Roberts WC, Epstein SE. Sudden death in hypertrophic cardiomyopathy: a profile of 78 patients. Circulation. 1982;65:1388–1394. Web site. http://www.ncbi.nlm.nih.gov/pubmed/7200406. Accessed June 16, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Corrado D, Basso C, Thiene G, et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512–1520. Web site. http://www.ncbi.nlm.nih.gov/pubmed/9362410. Accessed June 16, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Marcus FI. Electrocardiographic features of inherited diseases that predispose to the development of cardiac arrhythmias, long QT syndrome, arrhythmogenic right ventricular cardiomyopathy/dysplasia, and Brugada syndrome. J Electrocardiol. 2000;33(Suppl 1–10). Web site. http://www.ncbi.nlm.nih.gov/pubmed/11265707. Accessed June 16, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Harmon KG, Asif IM, Klossner D, et al. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123:1594–1600. [DOI] [PubMed] [Google Scholar]


