Abstract
Introduction
Highly Active Antiretroviral Therapy (HAART) has tremendously improved the life expectancy of the HIV-infected population over the past three decades. Protease inhibitors have been one of the major classes of drugs in HAART regimens that are effective in treating HIV. However, the emergence of resistance and cross-resistance against protease inhibitors encourages researchers to develop new PIs with broad-spectrum activity, as well as novel means of enhancing the efficacy of existing PIs.
Areas covered
In this article we discuss recent advances in HIV protease inhibitor (PI) development, focusing on both investigational and experimental agents. We also include a section on pharmacokinetic booster drugs for improved bioavailability of protease inhibitors. Further, we discuss novel drug delivery systems using a variety of nanocarriers for the delivery of PIs across the blood-brain barrier to treat the HIV in the brain.
Expert opinion
We discuss our opinion on the promises and challenges on the development of novel investigational and experimental PIs that are less toxic and more effective in combating drug-resistance. Further, we discuss the future of novel nanocarriers that have been developed to deliver PIs to the brain cells. Although these are promising findings, many challenges need to be overcome prior to making them a viable option.
Keywords: Protease inhibitors, HIV, pharmacoenhancers, antiretroviral therapy, drug delivery, nanocarriers
1. Introduction
HIV protease inhibitors (PIs) are one of the most important therapeutic agents for the treatment of HIV infection. These inhibitors block the crucial viral maturation stage and thereby reduce the spread of HIV [1]. PIs have played a key role in transforming HIV from an acute infection to a chronic disease since their introduction into the market in 1995. In recent times, in combination with other classes of HIV medication, mainly reverse transcriptase and integrase inhibitors, PIs have revolutionized HIV treatment paradigms and dramatically increased the life expectancy of the HIV-positive population [2]. As of now, darunavir (formerly known as TMC-114) is still the most recently (2006) Food and Drug Administration (FDA)-approved HIV PI on the market.
While recent guidelines recommend mostly integrase inhibitor-based regimens for initial treatment, PI-based regimens evidently have advantages over those without PIs for selected patient populations in which the use of integrase inhibitors are not appropriate [3]. Nonetheless, among different classes of anti-HIV medication, PIs are particularly associated with several adverse events, including dyslipidemia, insulin resistance, hyperglycemia, and lipodystrophy [4–6]. In addition, the PIs are involved in drug–drug interactions due to CYP3A4-mediated metabolism and may increase the risk of bleeding in HIV-positive hemophilic patients [7–9]. Considering the benefits of PIs in treating select patient populations as well as superior tolerability profiles, it is imperative to develop novel PIs or improve on the current PI structures to increase the arsenal for HIV treatment [10].
The goal of HIV therapy was always to achieve undetectable RNA in the body. Historically, HIV treatment started with nucleoside zidovudine (a reverse transcriptase inhibitor) monotherapy. But with the FDA approval of the first PI, saquinavir, the combinational therapy referred to highly active antiretroviral therapy (HAART) for HIV began. This HAART regimen greatly improved patient conditions by substantially suppressing viral load and improving the CD4+ T-cell count [11]. But saquinavir exhibited limited absorption and extensive first-pass metabolism that resulted in poor bioavailability [12]. To address this problem, patients had to take it three times daily. Soon after, the FDA approved two other PIs: ritonavir and indinavir. Ritonavir’s extended absorption rate and half-life (compared to saquinavir) mostly addressed the multiple dosing concerns encountered with saquinavir. Both monotherapy and combination therapy with ritonavir demonstrated impressive reduction in viral RNA levels [13–15]. Unfortunately, continuing ritonavir therapy was associated with increasingly resistant viral strains and extensive toxicity because of drug–drug interactions [16,17]. Similarly to ritonavir, indinavir displayed good suppression of viral load and increased CD4 T-cell count. Indinavir triple-drug combination therapy was found to be more effective than monotherapy and was implemented as the standard of care for HIV treatment in most parts of the world [18,19]. However, indinavir users suffered from strict dosing guidelines, renal toxicity, and gastrointestinal problems [20]. The fourth FDA-approved PI, nelfinavir, presented superior results to previous PIs in treatment-naïve HIV-infected individuals. The most common side effect with nelfinavir administration was diarrhea [21]. Some studies also observed PI-resistant viral strains in nelfinavir-treated patients, especially those who failed to adhere to the treatment paradigm which led to incomplete viral suppression [22]. Overall, the first-generation PI usage was limited by poor bioavailability and high pill burden, which led to treatment adherence problems and difficulty in maintaining low viral load in the blood. Failure of viral suppression caused the rise of multiple PI-resistant viral strains. One attempt to address this problem was to boost bioavailability by coadministration of a PI with low doses of ritonavir, which, in addition to its inhibitory effect on the viral protease, is also a potent inhibitor of the PI-metabolizing enzyme cytochrome P450 3A4. This boosting method enhanced the systemic exposure of saquinavir, but unfortunately had limitations such as nephrotoxicity and low bioavailability when used with other PIs [23].
This led to the development of the second generation of HIV PIs that have high potency against PI-resistant viral strains. Amprenavir/fosamprenavir, the next PI that was approved for twice-daily dosing, showed improved plasma concentration and high efficacy in combination therapy [24]. Treatment with the other approved PI, lopinavir, showed remarkably less phenotypic or genotypic resistance in HIV subjects who experienced virologic failure [25]. Further advances were made with the approval of atazanavir for once-daily dosing and tipranavir for patients who had a broad PI-resistance profile. Although treatment-experienced patients showed improved response to the tipranavir, its use in a clinical setting is limited because of its narrow indication, potential hepatotoxicity, and its twice-daily dosing when prescribed with ritonavir [26]. Darunavir was approved specifically for targeting drug-resistant viral strains [27]. But its great antiviral potency and low adverse reaction profile made darunavir the PI of choice in combination therapies [28]. Although it is less frequent than other PIs, resistance to darunavir was a significant concern in HIV treatment. Despite there being nine approved PIs on the market, increasing cross-resistance within the PI class, significant drug–drug interactions, and clinically relevant adverse drug reactions all challenge the research community to develop new PIs to effectively treat both treatment-naïve and treatment-experienced patients.
2. Investigational PIs
2.1. TMC310911
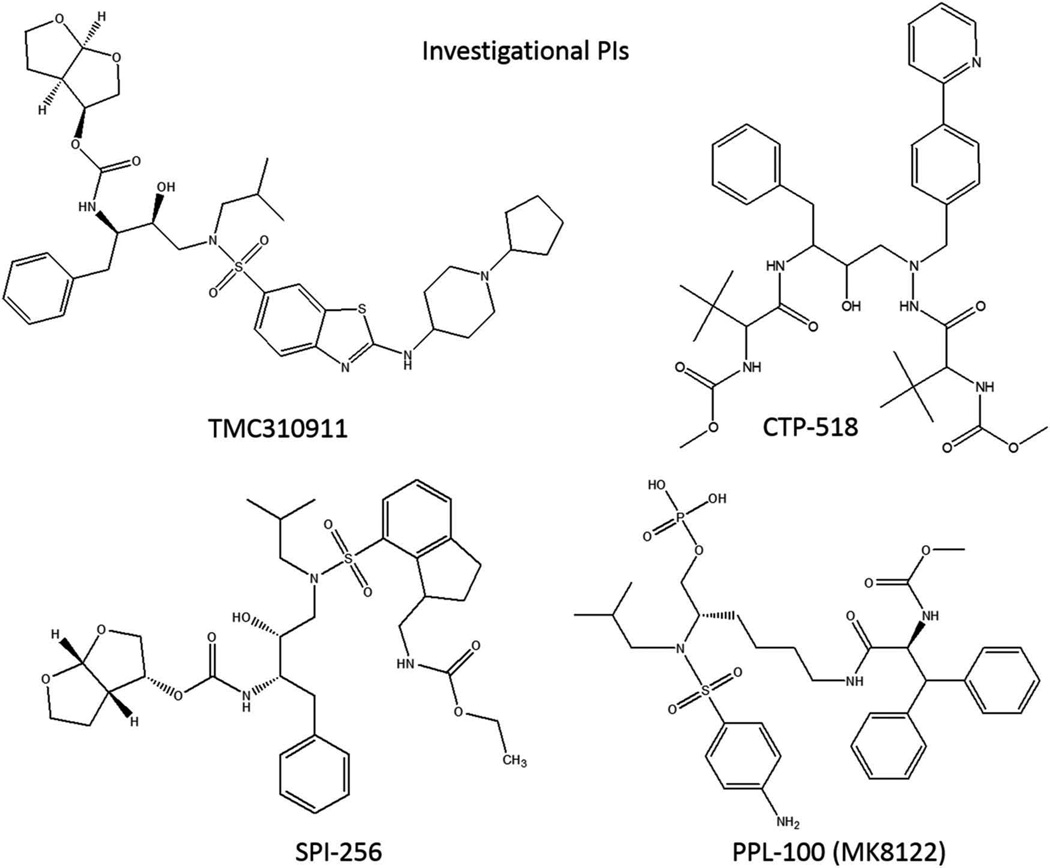
The emergence of viral resistance to existing anti-HIV drugs has led to renewed efforts aimed at the discovery and characterization of newer PIs, which are active against multidrug-resistant virus (Figure 1). One such study identified fused heteroaromatic sulfonamide to be effective in interacting with the aspartate-30 residue located in the P2′ pocket of the HIV-1 protease [29]. Virological characterization of the 2-(substituted-amino) benzothiazole sulfonamide, TMC310911, revealed in vitro effectiveness of this compound against several recombinant HIV-1 clinical isolates, including multiple PI-resistant strains [30]. In addition, compared to darunavir or lopinavir, this study also showed a reduced development of resistant strains and a lower incidence of viral mutations in the presence of TMC310911, warranting clinical evaluation of this novel PI. Subsequently, the safety and tolerability of TMC310911 was determined in two phase I clinical trials [30]. Apart from gastrointestinal side effects, no major complications were observed in healthy participants treated with TMC310911. A linear pharmacokinetic profile was observed for TMC310911 and coadministration of ritonavir boosted the bioavailability of this novel PI. An ensuing phase IIa trial evaluated the antiviral effectiveness of TMC310911, coadministered with ritonavir, in treatment-naïve HIV patients [31]. TMC310911 was found to possess potent antiviral activity, with a reduction of more than 1.5 log10 copies/ml of HIV RNA in plasma. Furthermore, the treatment was well-tolerated at all the evaluated doses. Based on the promising data from phase IIa trial, further clinical investigation of TMC310911 is currently underway (NCT00838162) [32].
Figure 1.
Investigational protease inhibitors under different clinical developmental phases.
2.2. CTP-518
CTP-518 is a novel PI developed by Concert Pharmaceuticals. CTP-518 is a stable isotopolog of atazanavir, wherein certain key hydrogen atoms have been replaced with the nonradioactive hydrogen atom isotope deuterium. Following these substitutions, the hepatic metabolism of CTP-518 was drastically slowed and an improvement in half-life was reported. The phase I clinical trial assessing the pharmacokinetics, safety, and tolerability of CTP-518 in healthy volunteers concluded in 2011, but the results from this study have not been published (NCT01458769) [33].
2.3. SPI-256
Promising antiviral data for a novel PI, SPI-256, have been presented by researchers from Sequoia Pharmaceuticals at various national scientific conferences [34–36]. Following in vitro characterization, SPI-256 was found to be more potent against wild-type and mutant strains of HIV compared to commonly prescribed PIs [34]. Results from a subsequent study reported that the carbonyl oxygen of the P2′ urethane substituent of SPI-256 has a hydrogen-bonding interaction with the secondary amine of glycine-48 present in HIV protease [35]. In fact, all hydrogen bonds made by SPI-256 were found to be along the conserved regions of the HIV protease, such as the catalytic aspartates. These findings are thought to be responsible for the high barrier to resistance observed for SPI-256 in preclinical studies. Data from the clinical trial studies for SPI-256 have not been reported.
2.4. PPL-100
PPL-100 is a prodrug of a novel HIV-1 PI, PL-100, with a promising cross-resistance profile and a high genetic barrier for HIV mutation [37]. An in vitro screening study revealed that PL-100 has excellent antiviral activity against HIV isolates that are resistant to other PIs [38]. In a following study, when compared to amprenavir, presence of PL-100 was found to inhibit HIV replication for a considerably longer duration [39]. In fact, mild resistance toward PL-100 treatment was found to develop only in the presence of all four selected mutations in the HIV protease, suggesting a high barrier for viral resistance against PL-100. Furthermore, PL-100, a lysine sulfonamide peptidomimetic, was demonstrated to serve as both substrate and inhibitor for CYP3A4 in freshly isolated primary human hepatocytes, thereby predicting an un-boosted oral therapy for HIV patients [37]. Results from a phase I clinical trial demonstrated a good safety profile for PPL-100 with participants experiencing only mild side effects and no severe cardiovascular or hepatic adverse effects [40].
3. Experimental PIs
In addition to several investigational PIs, there are experimental PIs that are being developed as potentially novel PIs. Among these experimental PIs, many are darunavir analogs. Darunavir has several advantages over earlier PIs. It was designed specifically to bind heavily to the enzyme’s protein backbone, rather than the functional groups of the active site. This feature provides a significant genetic barrier to the development of resistance, and also circumvents many of the mutations that confer resistance to other inhibitors. In addition, darunavir also inhibits dimerization of the two HIV protease subunits, further decreasing its activity.
While darunavir is the most widely recommended antiretroviral PI and the only PI (excepting ritonavir, which is used as a boosting agent) to be recommended as a first line antiretroviral drug by the National Institute of Health, it is not without flaws. Although it is effective against many viral strains with resistance to older PIs, darunavir-resistant strains have begun to emerge. In addition, darunavir is a substrate of CYP3A4, one of the most promiscuous drug-metabolizing enzymes in humans, meaning that it is at significant risk of drug–drug interactions.
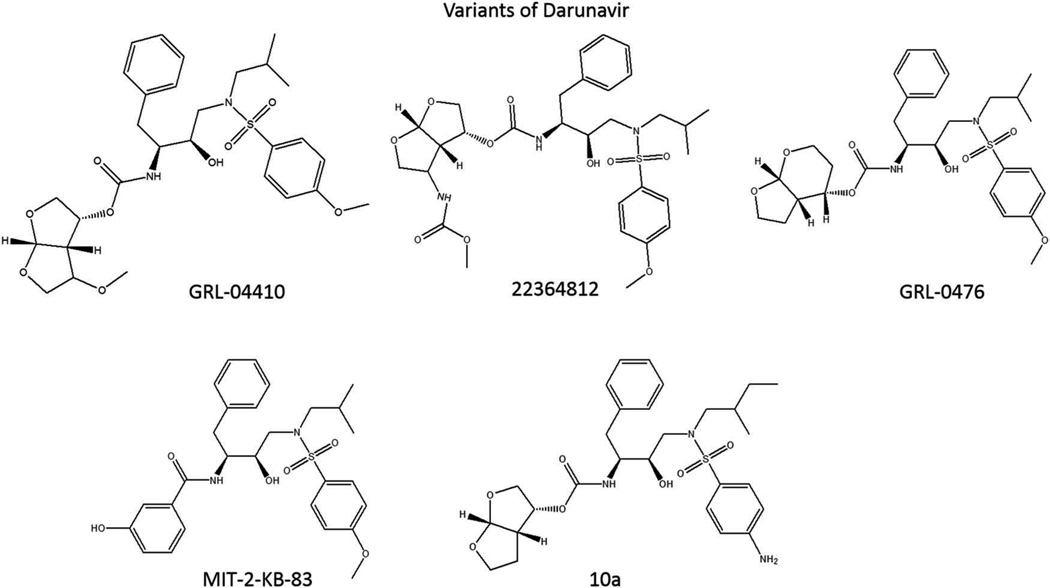
Earlier this year, Ghosh et al. published a comprehensive review of 145 experimental HIV PIs produced in the last two decades, a large portion of which are derivatives of darunavir [41]. It is beyond the scope of this review to discuss all the darunavir-derived PIs in detail, so instead we will focus on two alternative approaches to developing novel PIs from darunavir: the backbone-binding approach and the substrate envelope hypothesis (Figure 2).
Figure 2.
Experimental PIs that are developed based on the structure of darunavir.
3.1. Maximizing backbone binding
Darunavir was originally created by the Ghosh group with the specific intention of causing as much interaction as possible between the inhibitor and the backbone of the HIV protease active site [42]. By promoting hydrogen bonding with the backbone amino and carboxyl groups, the authors were able to induce tight binding to the active site while simultaneously avoiding the potential for resistance mutations by not having the inhibitor interact directly with the catalytic side chains. In the years since, the Ghosh group and others have worked to improve the ability of darunavir-based PIs to bind the protease backbone and produce even more potent inhibition.
For example, GRL-04410 is an experimental PI with the addition of a methoxyl group to darunavir’s bis-tetrahydrofuran P2 ligand. This structural change was based upon X-ray crystallography study of HIV-1 protease bound with darunavir, which suggested that the modification might form favorable bonds with the backbone amino group of glycine-48. GRL-04410 bound well with the protease, having a Ki of 2.9 pM compared to darunavir’s Ki of 16 pM. It also had a superior IC50 in MT-2 cells, at 2.4 nM, compared with 4.1 nM for darunavir [43].
Another experimental PI from the Ghosh group expanded on the aforementioned interactions of earlier inhibitors with glycine-48, this time exchanging the methoxyl group for a carbamate. This new functional group binds with the glycine-48 backbone carbonyl group, rather than the amino group. In addition, the carbamate methoxyl group fits into a hydrophobic pocket in a favorable manner. These and other combined elements produce an inhibitor that binds HIV protease with a Ki of 1.8 pM and has an IC50 of 1.6 nM in MT-2 cells, compared to 3 nM for darunavir in this publication. This inhibitor also showed effective inhibition of multiple drug-resistant strains of HIV, with overall higher EC50s than darunavir, but lower fold changes in efficacy between multidrug-resistant strains and the wild-type control [44].
Ghosh et al. also investigated changes to the tetrahydrofuran (THF) molecules that form the P2 ligands themselves, rather than simply adding different functional groups. The experimental inhibitor GRL-0476 replaced the bis-THF of darunavir with tetrahydropyranyl tetrahydrofuran (Tp-THF). This larger ring structure provides more flexibility to the inhibitor and allows closer interactions between the oxygen in the six-member ring of Tp-THF and the backbone amino group of aspartate-30. GRL-0476 exhibits strong binding, with a Ki of 2.7 pM and an IC50 of 0.5 nM in MT-2 cells, compared with IC50s of 30 nM and 15 nM, respectively, for amprenavir and saquinavir. It was also similarly effective in inhibiting multidrug-resistant strains of HIV, compared to darunavir, although its fold change in efficacy between various resistant strains and the wild-type control was notably different from darunavir’s [45].
3.2. Substrate envelope hypothesis
It has recently been reported that part of the success of darunavir as a PI, aside from its affinity for the protease backbone, is its strong fit of the ‘substrate envelope’ [46]. The substrate envelope hypothesis states that the ability of HIV protease to bind to its substrate peptides depends not on the specific amino acid sequences of the substrates, but rather their overall three-dimensional conformation, which determines how well they fit into the protease active site. The conserved shape that protease substrates must retain to be cleaved is referred to as the ‘substrate envelope’, and inhibitors that can conform to the same shape, such as darunavir, can more effectively bind the active site and inhibit its activity. The hypothesis further proposes that because the shape of the substrate envelope cannot change without reducing the affinity of the protease for its substrates, resistance mutations within the envelope are disadvantageous for the virus. Furthermore, the hypothesis suggests that it is the functional groups of inhibitors that protrude beyond the substrate envelope which can be interfered with by resistance mutations without reducing the enzyme’s functionality. Therefore, novel inhibitors that conform to the substrate envelope as well as possible and protrude from that envelope as little as possible, would theoretically be as effective as darunavir without being affected by those mutations that confer resistance to it or other PIs.
As a demonstration of the relevance of the substrate envelope hypothesis, multiple variants of two darunavir-derived PIs with relatively flat resistance curves were created with a series of increasingly large structural modifications. These modifications protruded progressively further out of the substrate envelope and correlated with a loss of efficacy against mutant strains of HIV, but not the wild-type virus. The fact that those mutants with low affinity for inhibitors that protruded from the envelope had mutations specifically at locations where different amino acids could potentially interact directly with the protruding functional groups was a strong piece of evidence in support of the hypothesis. The variant PIs that most closely fit within the substrate envelope were also assessed for inhibitory capacity in vitro, and while all the novel inhibitors tested proved to have higher absolute EC50s than darunavir, each also showed similar fold changes in their EC50s against drug-resistant strains to darunavir, indicating that fitting well within the substrate envelope could be an effective constraint in designing PIs to inhibit drug-resistant strains of HIV [47].
The substrate envelope hypothesis can be used to predict potential inhibitors by computational modeling of the target enzyme’s active site. For example, a computational analysis produced a list of hundreds of potential darunavir-like inhibitors, of which those that had the highest affinities for the protease in silico were synthesized and tested in vitro. Most had experimental Kis on par with older, FDA-approved inhibitors such as ritonavir and saquinavir, but not as low as darunavir (8 pM in this case). Two inhibitors in particular, MIT-2-KB-83 and MIT-2-KB-93, had notably low-fold changes in inhibition of drug-resistant strains, with worst fold losses being 14-fold and 16-fold, respectively. Both of those were significantly better than darunavir, which had a worst fold loss of 41, indicating that both of the novel inhibitors better retained efficacy when used against drug-resistant viruses than darunavir did [48].
Having established that darunavir-derived inhibitors designed to conform to the substrate envelope are less susceptible to resistance mutations than PIs that do not conform to it, the focus was moved to improving the inhibitor’s binding ability while staying within that constraint. Increasing the hydrophobicity of darunavir’s isobutyl P1′ ligand was hypothesized to increase van der Waal’s interactions with isoleucine-50 that are lost in the darunavir-resistance-defining mutation I50V. Multiple inhibitors were designed with small variations in the P1′ and P2′ ligands of darunavir. Remarkably, all 10 of these inhibitors bound to wild-type HIV protease with similar or superior Kis to darunavir, and several of them also maintained superior binding profiles and antiviral activity against multidrug-resistant strains of HIV, when compared to darunavir. To discuss but one of the novel PIs, given the temporary moniker of Inhibitor 10a, a Ki of 15 pM was observed for wild-type protease, compared to 5 pM for darunavir. However, where darunavir had an average Ki of 98 pM amongst the wild-type protease and three mutant strains, inhibitor 10a retained very similar binding affinity between strains, with an average Ki of 12 pM. In terms of antiviral activity, experimental inhibitor 10a had sub-nanomolar EC50 values against a panel of different wild-type and drug-resistant HIV strains, which were consistently lower than darunavir [49].
3.3. Non-darunavir-based experimental PIs
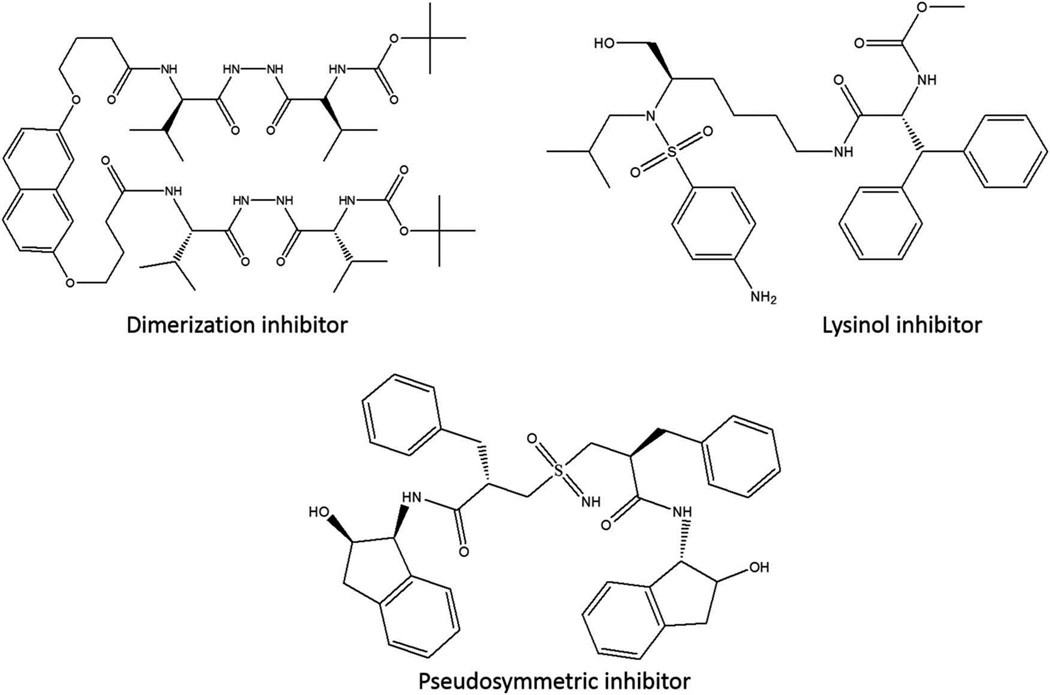
Not all recent novel experimental PIs have been derived from darunavir; other structures are being investigated (Figure 3). Lysinol-derived inhibitors, which bear some resemblance to the scaffold of darunavir but have vastly different P1′ and P2 ligand structures, have been studied. Two lysinol-based inhibitors sharing an isopentyl P1′ ligand and biphenyl P2 ligand with the addition of a methyl or ethyl group to the lysine backbone had noteworthy IC50s of 7 pM and 16 pM, respectively, in an inhibition assay [50].
Figure 3.
Non-darunavir-based experimental protease inhibitors.
Another group of experimental inhibitors recently tested was pseudo-symmetric sulfoximine inhibitors. The dimeric nature of HIV protease implied that it could respond well to inhibition by identical P2 and P2′ ligands. A sulfoximine moiety was hypothesized to play the role of a transition state mimetic and act as a hydrogen bond donor and acceptor with the two catalytic aspartic acid residues present at the active site. However, the inhibitory capabilities of these pseudo-symmetric inhibitors were lackluster. The most potent of the tested compounds had an IC50 of 2.5 nM against purified protease, and an IC50 of 410 nM against whole virus [51].
3.4. Dimerization inhibitors
As mentioned previously, aside from its inhibitory activity at the active site, darunavir also inhibits dimerization of HIV protease. As the protease is only active while dimerized, inhibition of the dimerization process is an attractive alternative approach to PI design. A number a dimerization inhibitors have been developed, although they differ significantly from darunavir. One of the most potent recently designed dimerization inhibitors is composed of two carbonyl hydrazide ‘tongs’ attached by a naphthalene scaffold. This inhibitor has a Ki of 50 nM for wild-type protease and an average Ki of 120 nM for proteases from two drug-resistant strains [52].
While inhibition of protease dimerization has potential as a novel avenue for antiretroviral design, it has some notable drawbacks. Dimerization inhibitors are typically peptidomimetic, which makes them substrates for degradation by peptidases, reducing their efficacy. They are also typically highly hydrophobic, and this hydrophobicity, as measured by their calculated partition coefficient between aqueous and lipophilic phases, or clogP, which is an indicator of potential bioavailability. A higher clogP value is indicative of greater hydrophobicity, and most drug-like molecules with clogP values greater than 5 are considered too hydrophobic for use as orally administered therapeutics, in accordance with Lipinski’s rule of five [53]. The inhibitor mentioned above has a clogP of 9.3, compared to darunavir’s clogP of 2.23 [54]. Inhibitory concentrations of even the most potent dimerization inhibitors are also generally much higher than traditional HIV PIs. There are currently no FDA-approved commercially available antiretroviral drugs that specifically target protease dimerization. Despite these limitations, dimerization inhibitors may still have value as a potential new class of antiretroviral. In fact, it was recently reported that HIV protease has a previously unrecognized binding pocket during dimerization that can be bound by darunavir and tipranavir, which could serve as a new target for inhibitor design [55].
4. Novel pharmacokinetic enhancers for PIs
With one exception, all currently approved PIs are extensively metabolized by CYP3A, and thus require ‘pharmacoenhancement’ via the addition of a CYP3A inhibitor. Historically, this has been done with ritonavir, a PI with low antiretroviral activity but potent anti-CYP3A activity. While coadministration of ritonavir, either in a single tablet co-formulated with a PI or in an individual pill alongside a PI, results in therapeutic concentrations of the PI, the medication is not without complications. First, ritonavir has activity as a PI, and thus there is a concern that resistance may develop to a subtherapeutic concentration of the drug. Second, ritonavir has been shown to exert effects on lipid levels and can cause gastrointestinal intolerance [56].
More recently, cobicistat has been approved, and is being co-formulated with many antiretrovirals. Cobicistat is a potent CYP3A inhibitor, but has no antiviral activity, unlike ritonavir. Cobicistat has a similarly favorable side effect profile to ritonavir, although a decrease in creatinine clearance has been commonly reported with the drug. Cobicistat-including regimens are thus not recommended for individuals who have a beginning creatinine clearance of <70 ml/min [57]. Cobicistat, known as GS-9350 throughout its development, has been compared with ritonavir as a pharmacoenhancer through a variety of clinical trials. In general, these studies showed that cobicistat is non-inferior to ritonavir over treatment durations up to 144 weeks [58]. The success of cobicistat-containing regimens in these trials has resulted in its co-formulation with a number of PIs, including atazanavir and darunavir [59]. The studies comparing darunavir and ritonavir pairings to darunavir and cobicistat treatments have shown a similar pharmacokinetic profile for the two pharmacoenhancers, and a co-formulation of darunavir and cobicistat (prescobix) has since been approved [60]. It is likely that as new PIs are developed, they will either be co-formulated or coadministered with cobicistat.
Other CYP3A inhibitors have been under development in the past as well. Notably, Sequoia Pharmaceuticals was developing SPI-452, although there have been few developments with that drug in the last few years [61]. Furthermore, Pfizer is developing a booster drug, PF-03716539. A phase I study to evaluate the safety, tolerability, and pharmacokinetics of a single oral dose has been completed, although the results have yet to be posted (NCT00783484) [62]. TMC558445, a Tibotec Pharmaceuticals drug, is being developed as a stand-alone pharmacokinetic booster and for use in fixed-dose combinations with the novel PI TMC310911 and also with darunavir (NCT00838760) [63]. Jonckers et al. are developing benzoxazole and benzothiazole amide modifications of ritonavir that would provide the potent CYP3A-inhibitory effects of ritonavir while removing any antiretroviral effects [64]. The investigators designed three novel compounds and tested their CYP3A inhibition in animals, showing significant increases in AUC and Cmax of darunavir, a CYP3A substrate. The further development of these molecules may provide valuable new pharmacoenhancers to improve the pharmacokinetic profiles of PIs.
5. Novel drug-delivery systems for PIs to combat PI-induced toxicity, and effective treatment of HIV in sanctuary sites
Most PIs are rapidly metabolized by the liver CYP3A4 [65]. Through this metabolic process, PIs produce reactive oxygen species and reactive metabolites that are toxic to cells [66]. Recent reports also suggest that PIs are also metabolized by CYP3A4 in non-hepatic cells such as monocytes, astrocytes, and neurons [65]. This is likely to cause cellular toxicity and suboptimal therapeutic concentration of PIs at the target cells such as HIV-infected lymphocytes and monocytes. Furthermore, drug efflux transporters, which are predominantly present in gut, liver, monocytes, and blood-brain barrier (BBB), are known to efflux PIs and reduce their concentrations to below their therapeutic ranges [67]. Thus, it is important to design PI delivery systems so that PIs retain their activity and optimal concentrations reach the target cells. Furthermore, most PIs do not effectively cross the BBB, and therefore very low level of PIs are found in the brain tissue to combat HIV-infected microglia and perivascular macrophages [68,69]. Although, among all the PIs, darunavir and lopinavir have shown some ability to cross the BBB, the concentration is too low to be effective for reducing actively replicating HIV in these cells [68,70]. Therefore, there is a further need to design a targeted drug-delivery system to deliver PIs effectively to these infected cells in the CNS. There are many varieties of nanocarriers (nanomaterial-based transport packages for enhancing drug delivery) to deliver antiretrovirals to the brain and a more comprehensive review of those nanocarriers has been published recently [71].
In principle, a nanoparticle delivery mechanism can increase bioavailability of a given drug and increase its half-life through sustained release. Nanocarriers constructed of polymers of polycaprolactone and l-lactide/Ɛ-caprolactone have been shown to increase the blood concentration of darunavir and atazanavir ~2–2.5-fold compared to free drugs, when orally administered to rats [72]. Similarly, polyvinyl alcohol-stabilized nanoparticles containing lopinavir have been found to have a ~3-fold higher bioavailability than ritonavir-boosted free lopinavir, when orally administered to rats [73]. Nanocarriers constructed of polylactic-co-glycolic acid (PLGA) used to administer lopinavir and ritonavir to primary human peripheral blood mononuclear cells (PBMCs). Those drugs remained detectable within the cells for 28 days due to slow release from the nanocarriers, compared to 2 days for cells treated with free drugs [74]. This has obvious implications for potentially reducing pill burdens for patients undergoing antiretroviral therapy (ART), although further investigation is necessary. PLGA nanoparticles have additionally been shown to increase delivery of saquinavir to cancer cells, an alternative usage of the drug [75].
One means of increasing drug penetrance into the CNS is through inhibition of export proteins in the BBB. P85 is a lipid polymer that forms micelles in solution and is known to have an inhibitory effect on P-glycoprotein, a major drug efflux protein [76]. In vitro experiments have shown that P85 can inhibit HIV+ macrophages, and in vivo experiments in mice have shown that a commonly used ART cocktail containing nelfinavir, when formulated with P85, results in higher drug concentrations and lower viral replication in the CNS without damaging the integrity of the BBB [77]. An inorganic type of nanocarrier called a quantum rod has been conjugated to transferrin, allowing for transferrin receptor-mediated translocation of the carrier across an in vitro model of the BBB using brain microvascular endothelial cells and normal human astrocytes. This has allowed in vitro delivery of saquinavir across the model BBB (without any significant cytotoxicity) to HIV-infected PBMCs, where it inhibits viral replication by 91%, compared to control [78]. A similar technique using quantum dot nanocarriers has also been performed by the same group with amprenavir, to similar effect [79].
As macrophages are capable of traversing the BBB, nanoparticles that specifically target macrophages or monocytes can be carried into the CNS where they can control viral replication, in a manner very similar to the ‘Trojan Horse hypothesis’ of HIV CNS infection. One manner of macrophage-targeting that has been tested is the use of folic acid-conjugated nanoparticles that are recognized by macrophages expressing the folate receptor. Folic acid-conjugated nanoparticles carrying atazanavir were taken up by primary monocyte-derived macrophages in significantly greater quantities than non-targeted nanocarriers and released at similar rates to free atazanavir. In addition, the targeted nanocarriers inhibited viral replication by 81% compared to control, better than the non-targeted nanocarrier [80]. These nanoparticles were also shown to have antiretroviral efficacy in mice when delivered intramuscularly [81].
Targeted delivery of ARTs, especially PIs, to HIV-1-infected T cells and macrophages would improve the efficacy of antiviral drugs, reduce toxicity, reduce resistant viral mutants, and decrease viral production. A biocompatible nano-formulation has been engineered to deliver ART drugs such as ritonavir, indinavir, and lopinavir [82]. This formulation significantly increases the therapeutic concentration of these drugs at the target sites. Similarly, macrophages have been used as cellular transporters for PI-containing nanoparticles, which are expected to increase the efficacy of antiretroviral medications significantly [80]. A recent study has shown that a single intravenous dose of nano-ART can elicit high sustained tissue and plasma drug levels in the reticuloendothelial system and brain [83]. It can be taken up within minutes by circulating monocytes and released in tissues over a period of 2 weeks [84]. Such a drug delivery system is expected to decrease drug toxicity and increase efficacy. In another example, Tat-peptide-conjugated ritonavir-loaded nanoparticles have shown to be an effective treatment strategy in controlling viral replication in HIV-infected brain cells such as monocyte-derived macrophages [82]. Similarly, a nanoparticle-conjugated delivery of ritonavir and lopinavir has shown sustained release (up to 28 days) of these drugs in vivo, and their antiviral activity was comparable to that of free drugs in vitro [85].
6. Strategies for better adverse event profiles of novel PIs
In general, the chemical structure of the drug molecule can contribute to the adverse effects that are observed with the use of a particular PI. For instance, ritonavir, lopinavir, and amprenavir decrease glucose uptake, while atazanavir does not [86]. Similarly, unlike other PIs, atazanavir does not cause dyslipidemia [87,88]. Additionally, adverse reactions occur due to nonspecific binding of PIs to various intracellular molecules that are necessary for metabolic regulation. For example, most PIs modulate the function of sterol regulatory element-binding protein 1, which is critical for lipid metabolism [89,90]. Similarly, PI-induced insulin resistance is mainly due to inhibition of glucose transporter-4 by most PIs [91,92]. Therefore, the strategies that use approved PIs’ scaffolds would exploit structural nuances of the PIs to find solutions to mitigate or eliminate drug side effects.
Other important factors that can cause severe deleterious reactions while treating HIV patients are the physiological concentration of the drug and PIs’ interactions with other therapeutic agents [93]. Comorbidities such as tuberculosis and hepatitis C are very common with the HIV infection. As PIs are predominantly metabolized by CYP3A4, and many other medications are also either substrates or inducers for CYP enzymes, it is highly possible that concomitant usage of these medications worsens their adverse events profiles and increases drug-induced toxicity [94,95]. Hence, preclinical characterization of drug interaction profiles of novel PIs with the other common medications, especially antituberculosis medications such as rifampin, is necessary to understand and predict detrimental effects prior to further drug development. As metabolic abnormalities and drug–drug interactions are the critical side effects of PI-based regimens, it is essential that investigational PIs be screened for the known adverse effects prior to proceeding to clinical trials. Alternatively, the newly designed PIs can be developed as prodrugs [96,97] or nanoformulations [78,98] that can give better bioavailability profile with limited adverse effects.
7. Conclusions
In conclusion, design and development of novel HIV PIs, as well as novel means of delivering and boosting the effectiveness of existing PIs, are expected to play a critical role in the advancement of ART. These are important to combat drug resistance and reduce/abolish PI-induced toxicity. In the past few years, new investigational drugs have been designed and are at various stages of clinical trials. In addition, several darunavir-based novel experimental drugs have been designed that have potential to be a viable drugs in the future. Further, the development of novel pharmacoenhancers, as well as the use of existing pharmacoenhancers in different regimens, is important for the success of HIV therapy. In this context, cobicistat, which was originally developed as a pharmacoenhancer for the integrase inhibitor elvitegravir, also shows promising results with darunavir. Finally, novel advancements in drug-delivery systems using nanocarriers have the potential to reduce pill burdens and facilitate drug transport across the BBB, which is critical to treat neuroAIDS. In the next few years, we expect to see relatively enhanced progress in this area of basic science research as well as clinical trials.
8. Expert opinion
As discussed above, although the application of ART regimens, especially PIs, has been impressive in controlling HIV replication, they also pose significant challenges in the forms of drug resistance and PI-induced drug toxicity [8,99–101]. The effective treatment of HIV relies upon the development of new PIs that are less toxic and more effective in combating drug resistance. In the recent past, new integrase inhibitors became available and have replaced ART regimens that include PIs, at least to some extent in the United States [102]. However, PIs (e.g. darunavir/ritonavir and lopinavir/ritonavir) are still the most widely used ART regimens in the world, especially in African and Asian countries [UNAIDS, 2015]. In the United States, ART regimens containing PIs are the alternative choice if the first line of treatment fails, or in special populations in which the first line of treatment is not recommended [103–105]. Therefore, it is still important to consistently find better PIs in the case of emerging viral resistance. Second-generation PIs, particularly darunavir, were developed to address these concerns. However, it is equally important to design novel delivery systems for the existing PIs to overcome PI-induced toxicity not only to liver and blood cells, but also in the brain. In fact, designing a successful delivery system for old PIs is more pragmatic than developing novel drugs because the drug design process is very slow and expensive.
8.1. Darunavir-based novel inhibitors
Since darunavir is the most recently developed second-generation PI and an important part of ART regimens, design and development of novel darunavir-based PIs with improved pharmacological properties and better drug-resistance profiles are of great importance. As described in Section 3, several darunavir-based novel PIs have been designed and synthesized. Since the backbone conformations of the wild-type and mutant protease enzymes show minimal conformational change, PIs with enhanced binding to the backbone should be effective against typically PI-resistant strains. Using this strategy, the Ghosh group has developed a new generation of PIs, which are exceedingly potent and demonstrate very high efficacy against multidrug-resistant HIV-1 variants [106,107]. A number of PIs have novel structures and show clinical potential. For example, TMC310911, structurally similar to darunavir, is in advanced phase of clinical development. In addition, a few other darunavir analogs are in preclinical development. Thus, unlike other enzymes such as HIV reverse transcriptase or integrase, HIV-1 protease is a biochemical target that allows us to design transition-state binding inhibitors that have excellent antiviral activity against multidrug-resistant HIV-1 variants. Further design of the PIs that target the transition state of the protease will continue to evolve and could yield more effective novel PIs. Similarly, design of novel PIs that target the protein backbone to combat drug resistance will continue to occur.
8.2. Novel delivery system for PIs
As described in the previous section, the advancement in drug delivery of ART, especially PIs, is important because: (1) there is an increased prevalence of neuroAIDS, especially among drug abusers and aging populations [108], (2) the majority of PIs do not cross the BBB [68,69], (3) brain macrophages and microglia are major viral sanctuary sites from which HIV is not eliminated by current ART drug treatments [109,110], and (4) PIs are toxic to astrocytes and neurons [111–113]. Several groups have developed and utilized a number of nanocarriers such as liposomes, nanoemulsions, polymeric micelles, and solid-lipid nanoparticles for PI drug delivery across BBB [71,83,114]. Most notably, the Nair group has utilized a magnetic nanoparticle-based drug-delivery system through direct transport of PIs in magnetic nanocarriers, as well as through macrophage-packaged magnetic nanocarriers [115]. The PIs from these magnetic nanocarriers can be released to the HIV-infected microglia and astrocytes in a controlled manner with respect to time and concentration of the drugs released. Interestingly, a controlled magnetic/electrical field can be applied to remove these drugs and drug metabolites upon their action. Although the advancement in research for the delivery of PIs in the brain is very promising, further research is required with respect to different navigation and drug release strategies, as well as regarding their biocompatibility and efficacy. Thus, getting ART drugs (especially PIs), into the brain seems possible via exploring and optimizing compartmentalization-based nanomedicine for the management of neuroAIDS. Once well-characterized and validated, an optimized nano-formulation strategy can be developed, which may be explored to treat neuroAIDS. Further, this technology is likely to help treat other CNS diseases and neurological disorders such as Huntington’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, and Alzheimer’s disease. In these diseases, site-specific drug delivery is the key to managing symptoms and improving treatment.
In conclusion, as a result of the development of novel investigational and experimental PIs, novel uses of known pharmacoenhancers for PIs, and development of novel nanocarriers for PI drugs, the use of PIs in HIV therapy could continue to grow. In particular, the development of novel means of delivering PI drugs across the BBB to treat neuroAIDS and eliminate hiding virus from brain macrophages and microglia appears possible. However, these novel PIs and PI-loaded nanocarriers must go through a number of further studies and clinical trials prior to their use in humans. Though the future of novel PI-based treatment of HIV and neuroAIDS is bright, these drugs/nanocarriers may pose new challenges that need to be overcome. These challenges may include clinical testing to ensure a better absorption, distribution, metabolism, and excretion profile of novel investigational and experimental drugs. In addition, the controlled release of PIs from nanocarriers and the biocompatibility of those nanocarriers need to be further investigated.
Article highlights.
Novel investigational PIs are under development that have better side effect profiles, and may be able to treat resistant strains effectively.
Novel experimental darunavir-based PIs are under development, and have potential to treat resistant virus strains.
Known and new pharmacoenhancers are being investigated for the development of new regimens that are relatively more effective than the current regimens.
Several nanocarriers are being developed to deliver PIs into the CNS to treat infected brain macrophages and microglia.
These novel drugs and drug delivery systems are likely to help treat neuroAIDS.
This box summarizes key points contained in the article.
Acknowledgments
Funding
The authors acknowledge financial support from the National Institute of Health grant 1R01AA022063-01A1.
This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Konvalinka J, Krausslich HG, Muller B. Retroviral proteases and their roles in virion maturation. Virology. 2015;479–480:403–417. doi: 10.1016/j.virol.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 2.De Clercq E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int J Antimicrob Agents. 2009;33(4):307–320. doi: 10.1016/j.ijantimicag.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Geneva: World Health Organization; 2015. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. [PubMed] [Google Scholar]
- 4.Holstein A, Plaschke A, Egberts EH. Lipodystrophy and metabolic disorders as complication of antiretroviral therapy of HIV infection. Exp Clin Endocrinol Diabetes. 2001;109(8):389–392. doi: 10.1055/s-2001-18990. [DOI] [PubMed] [Google Scholar]
- 5.Calza L, Manfredi R, Chiodo F. Insulin resistance and diabetes mellitus in HIV-infected patients receiving antiretroviral therapy. Metab Syndr Relat Disord. 2004;2(4):241–250. doi: 10.1089/met.2004.2.241. [DOI] [PubMed] [Google Scholar]
- 6.Calvo M, Martinez E. Update on metabolic issues in HIV patients. Curr Opin HIV AIDS. 2014;9(4):332–339. doi: 10.1097/COH.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Kumar A. Differential effects of ethanol on spectral binding and inhibition of cytochrome P450 3A4 with eight protease inhibitors antiretroviral drugs. Alcohol Clin Exp Res. 2011;35(12):2121–2127. doi: 10.1111/j.1530-0277.2011.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolbach A, Paziana K, Heverling H, et al. A review of the toxicity of HIV medications II: interactions with drugs and complementary and alternative medicine products. J Med Toxicol. 2015;11(3):326–341. doi: 10.1007/s13181-015-0465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbuthnot C, Wilde JT. Increased risk of bleeding with the use of tipranavir boosted with ritonavir in haemophilic patients. Haemophilia. 2008;14(1):140–141. doi: 10.1111/j.1365-2516.2007.01447.x. [DOI] [PubMed] [Google Scholar]
- 10.Lv Z, Chu Y, Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV AIDS (Auckl) 2015;7(1):95–104. doi: 10.2147/HIV.S79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collier AC, Coombs RW, Schoenfeld DA, et al. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS clinical trials group. N Engl J Med. 1996;334(16):1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 12.Vella S. HIV therapy advances. Update on a proteinase inhibitor. AIDS. 1994;8:S25–S30. doi: 10.1097/00002030-199409001-00006. [DOI] [PubMed] [Google Scholar]
- 13.Danner SA, Carr A, Leonard JM, et al. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. European-Australian collaborative ritonavir study group. N Engl J Med. 1995;333(23):1528–1533. doi: 10.1056/NEJM199512073332303. [DOI] [PubMed] [Google Scholar]
- 14.Markowitz M, Saag M, Powderly WG, et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333(23):1534–1539. doi: 10.1056/NEJM199512073332204. [DOI] [PubMed] [Google Scholar]
- 15.Mathez D, Bagnarelli P, Gorin I, et al. Reductions in viral load and increases in T lymphocyte numbers in treatment-naive patients with advanced HIV-1 infection treated with ritonavir, zidovudine and zalcitabine triple therapy. Antivir Ther. 1997;2(3):175–183. [PubMed] [Google Scholar]
- 16.Schmit JC, Ruiz L, Clotet B, et al. Resistance-related mutations in the HIV-1 protease gene of patients treated for 1 year with the protease inhibitor ritonavir (APT-538) AIDS. 1996;10(9):995–999. doi: 10.1097/00002030-199610090-00010. [DOI] [PubMed] [Google Scholar]
- 17.Piroth L, Grappin M, Waldner A, et al. Discontinuing protease inhibitor treatment of HIV-1 patients for intolerance. Longitudinal study of 309 patients. Presse Med. 1999;28(8):381–387. French. [PubMed] [Google Scholar]
- 18.Gulick RM, Mellors JW, Havlir D, et al. Simultaneous vs sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection: 100-week follow-up. JAMA. 1998;280(1):35–41. doi: 10.1001/jama.280.1.35. [DOI] [PubMed] [Google Scholar]
- 19.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337(11):734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 20.Boyle BA. Recent advances in the management and treatment of GI and hepatic diseases associated with HIV: part I. AIDS Read. 2001;11(7):354–355. 359–361, 363. [PubMed] [Google Scholar]
- 21.Moyle GJ, Youle M, Higgs C, et al. Safety, pharmacokinetics, and antiretroviral activity of the potent, specific human immunodeficiency virus protease inhibitor nelfinavir: results of a phase I/II trial and extended follow-up in patients infected with human immunodeficiency virus. J Clin Pharmacol. 1998;38(8):736–743. doi: 10.1002/j.1552-4604.1998.tb04814.x. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiya K, Matsuoka-Aizawa S, Yasuoka A, et al. Primary nelfinavir (NFV)-associated resistance mutations during a follow-up period of 108 weeks in protease inhibitor naive patients treated with NFV-containing regimens in an HIV clinic cohort. J Clin Virol. 2003;27(3):252–262. doi: 10.1016/s1386-6532(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 23.Becker SL. The role of pharmacological enhancement in protease inhibitor-based highly active antiretroviral therapy. Expert Opin Investig Drugs. 2003;12(3):401–412. doi: 10.1517/13543784.12.3.401. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-French A, Boghossian J, Gray GE, et al. The NEAT study: a 48-week open-label study to compare the antiviral efficacy and safety of GW433908 versus nelfinavir in antiretroviral therapy-naive HIV-1-infected patients. J Acquir Immune Defic Syndr. 2004;35(1):22–32. doi: 10.1097/00126334-200401010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Masquelier B, Breilh D, Neau D, et al. Human immunodeficiency virus type 1 genotypic and pharmacokinetic determinants of the virological response to lopinavir-ritonavir-containing therapy in protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2002;46(9):2926–2932. doi: 10.1128/AAC.46.9.2926-2932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wensing AM, Van Maarseveen NM, Nijhuis M. Fifteen years of HIV protease inhibitors: raising the barrier to resistance. Antiviral Res. 2010;85(1):59–74. doi: 10.1016/j.antiviral.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 27.De Meyer S, Azijn H, Surleraux D, et al. Tmc114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother. 2005;49(6):2314–2321. doi: 10.1128/AAC.49.6.2314-2321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKeage K, Perry CM, Keam SJ. Darunavir: a review of its use in the management of HIV infection in adults. Drugs. 2009;69(4):477–503. doi: 10.2165/00003495-200969040-00007. [DOI] [PubMed] [Google Scholar]
- 29.Surleraux DL, de Kock HA, Verschueren WG, et al. Design of HIV-1 protease inhibitors active on multidrug-resistant virus. J Med Chem. 2005;48(6):1965–1973. doi: 10.1021/jm049454n. [DOI] [PubMed] [Google Scholar]
- 30.Dierynck I, Van Marck H, Van Ginderen M, et al. TMC310911, a novel human immunodeficiency virus type 1 protease inhibitor, shows in vitro an improved resistance profile and higher genetic barrier to resistance compared with current protease inhibitors. Antimicrob Agents Chemother. 2011;55(12):5723–5731. doi: 10.1128/AAC.00748-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stellbrink HJ, Arasteh K, Schurmann D, et al. Antiviral activity, pharmacokinetics, and safety of the HIV-1 protease inhibitor TMC310911, coadministered with ritonavir, in treatment-naive HIV-1-infected patients. J Acquir Immune Defic Syndr. 2014;65(3):283–289. doi: 10.1097/QAI.0000000000000003. • A clinical study that reported the effectiveness of TMC310911 in HIV-infected patients.
- 32.A study to determine the antiviral activity of TMC310911 when administered with ritonavir in treatment-naive human immunodeficiency virus - type 1 (HIV-1) infected patients, NCT00838162. 2013 [cited 2016 Jun 13] Available from: https://clinicaltrials.Gov/ct2/show/study/nct00838162.
- 33.Pharmacokinetics, safety & tolerability of isotopologs of atazanavir (ATV), with pharmacokinetic comparison to reyataz, NCT01458769. 2013 [cited 2016 Jun 13] Available from: https://clinicaltrials.Gov/ct2/show/nct01458769?Term=ctp-518&rank=1.
- 34.Gulnik EASV, Eissenstat M, Parkin N, et al. Spi-256, a highly potent HIV protease inhibitor with broad activity against mdr. 13th CROI conference on retroviruses and opportunistic infections; 2006 Feb 5–8; Denver (CO). [Google Scholar]
- 35.Abelardo Silva EA, Erickson J, Eissenstat M, et al. Design and crystal structure of SPI-256: an experimental HIV protease inhibitor with a high genetic barrier to resistance. Infect Dis Soc America. 2008 Oct 26;:H-1266. [cited 2016 Jul 7]. Available from: https://idsa.confex.com/idsa/2008/webprogram/Paper27769.html.
- 36.SPI-256. SPI-256: a novel investigational protease inhibitor. 2016 [cited 2016 Jun 13]. Available from: http://wwweatgorg/news/162302/SPI-256%3A_a_novel_investigational_protease_inhibitor. [Google Scholar]
- 37.Wu JJ, Stranix BR, Milot G, et al. Pl-100, a next generation protease inhibitor against drug-resistant HIV: in vitro & in vivo metabolism. 46th annual ICAAC interscience conference on antimicrobial agents and chemotherapy; 2006 Sep 27–30; San Francisco (CA). [Google Scholar]
- 38. Dandache S, Sevigny G, Yelle J, et al. In vitro antiviral activity and cross-resistance profile of PL-100, a novel protease inhibitor of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2007;51(11):4036–4043. doi: 10.1128/AAC.00149-07. • In vitro study that showed effectiveness of PL-100 against various protease inhibitor-resistant HIV isolates.
- 39.Dandache S, Coburn CA, Oliveira M, et al. PL-100, a novel HIV-1 protease inhibitor displaying a high genetic barrier to resistance: an in vitro selection study. J Med Virol. 2008;80(12):2053–2063. doi: 10.1002/jmv.21329. [DOI] [PubMed] [Google Scholar]
- 40.PPL-100. PPL-100 phase I report: new PI from Merck. 2016 [cited 2016 Jun 13]. Available from: www.natap.org/2006/HIV/121206_05.htm. [Google Scholar]
- 41. Ghosh AK, Osswald HL, Prato G. Recent progress in the development of HIV-1 protease inhibitors for the treatment of HIV/AIDS. J Med Chem. 2016;59:5172–5208. doi: 10.1021/acs.jmedchem.5b01697. •• A comprehensive review of recent experimental protease inhibitor structures and their inhibitory capabilities.
- 42.Tie Y, Boross PI, Wang YF, et al. High resolution crystal structures of HIV-1 protease with a potent non-peptide inhibitor (UIC-94017) active against multi-drug-resistant clinical strains. J Mol Biol. 2004;338(2):341–352. doi: 10.1016/j.jmb.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh AK, Martyr CD, Steffey M, et al. Design of substituted bistetrahydrofuran (bis-THF)-derived potent HIV-1 protease inhibitors, protein-ligand X-ray structure, and convenient syntheses of bis-THF and substituted bis-THF ligands. ACS Med Chem Lett. 2011;2(4):298–302. doi: 10.1021/ml100289m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghosh AK, Chapsal BD, Steffey M, et al. Substituent effects on P2-cyclopentyltetrahydrofuranyl urethanes: design, synthesis, and X-ray studies of potent HIV-1 protease inhibitors. Bioorg Med Chem Lett. 2012;22(6):2308–2311. doi: 10.1016/j.bmcl.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh AK, Chapsal BD, Baldridge A, et al. Design and synthesis of potent HIV-1 protease inhibitors incorporating hexahydrofuropyranol-derived high affinity P(2) ligands: structure-activity studies and biological evaluation. J Med Chem. 2011;54(2):622–634. doi: 10.1021/jm1012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lefebvre E, Schiffer CA. Resilience to resistance of HIV-1 protease inhibitors: profile of darunavir. AIDS Rev. 2008;10(3):131–142. [PMC free article] [PubMed] [Google Scholar]
- 47.Shen Y, Altman MD, Ali A, et al. Testing the substrate-envelope hypothesis with designed pairs of compounds. ACS Chem Biol. 2013;8(11):2433–2441. doi: 10.1021/cb400468c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altman MD, Ali A, Reddy GS, et al. HIV-1 protease inhibitors from inverse design in the substrate envelope exhibit subnanomolar binding to drug-resistant variants. J Am Chem Soc. 2008;130(19):6099–6113. doi: 10.1021/ja076558p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nalam MN, Ali A, Reddy GS, et al. Substrate envelope-designed potent HIV-1 protease inhibitors to avoid drug resistance. Chem Biol. 2013;20(9):1116–1124. doi: 10.1016/j.chembiol.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones KL, Holloway MK, Su HP, et al. Epsilon substituted lysinol derivatives as HIV-1 protease inhibitors. Bioorg Med Chem Lett. 2010;20(14):4065–4068. doi: 10.1016/j.bmcl.2010.05.082. [DOI] [PubMed] [Google Scholar]
- 51.Lu D, Sham YY, Vince R. Design, asymmetric synthesis, and evaluation of pseudosymmetric sulfoximine inhibitors against HIV-1 protease. Bioorg Med Chem. 2010;18(5):2037–2048. doi: 10.1016/j.bmc.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Dufau L, Marques Ressurreicao AS, Fanelli R, et al. Carbonylhydrazide-based molecular tongs inhibit wild-type and mutated HIV-1 protease dimerization. J Med Chem. 2012;55(15):6762–6775. doi: 10.1021/jm300181j. [DOI] [PubMed] [Google Scholar]
- 53.Lipinski CA, Lombardo F, Dominy BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 54.Kruse CG, Timmerman H. Towards drugs of the future: key issues in lead finding and lead optimization. Amsterdam: IOS Press; 2008. [Google Scholar]
- 55.Pietrucci F, Vargiu AV, Kranjc A. HIV-1 protease dimerization dynamics reveals a transient druggable binding pocket at the interface. Sci Rep. 2015;5:18555. doi: 10.1038/srep18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arya V, Robertson SM, Struble KA, et al. Scientific considerations for pharmacoenhancers in antiretroviral therapy. J Clin Pharmacol. 2012;52(8):1128–1133. doi: 10.1177/0091270011410569. [DOI] [PubMed] [Google Scholar]
- 57.Nathan B, Bayley J, Waters L, et al. Cobicistat: a novel pharmacoenhancer for co-formulation with HIV protease and integrase inhibitors. Infect Dis Ther. 2013;2(2):111–122. doi: 10.1007/s40121-013-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallant JE, Koenig E, Andrade-Villanueva JF, et al. Brief report: cobicistat compared with ritonavir as a pharmacoenhancer for atazanavir in combination with emtricitabine/tenofovir disoproxil fumarate: week 144 results. J Acquir Immune Defic Syndr. 2015;69(3):338–340. doi: 10.1097/QAI.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 59.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2016 [cited 2016 Jun 13]. Available from: https://aidsinfo.Nih.Gov/contentfiles/lvguidelines/adultandadolescentgl.Pdf.
- 60.Putcharoen O, Do T, Avihingsanon A, et al. Rationale and clinical utility of the darunavir-cobicistat combination in the treatment of HIV/AIDS. Drug Des Devel Ther. 2015;9:5763–5769. doi: 10.2147/DDDT.S63989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilkin TJ, Taylor B, Olender S, et al. Advances in antiretroviral therapy. Top HIV Med. 2009;17(2):68–88. [PubMed] [Google Scholar]
- 62.A study to evaluate the safety, tolerability and pharmacokinetics of single oral doses of PF-03716539 in healthy adult subjects, NCT00783484. 2009 [cited 2016 Jun 13]. Available from: https://clinicaltrials.Gov/ct2/show/nct00783484.
- 63.PEPI-TiDP23-C103: first-in-human study to examine the safety, tolerability, and plasma pharmacokinetics of increasing single and repeated oral doses of TMC558445 and of a combined single day dosing of oral TMC558445 and oral TMC310911 and also oral darunavir, NCT00838760. 2010 [cited 2016 Jun 13]. Available from: https://clinicaltrials.Gov/ct2/show/nct00838760. [Google Scholar]
- 64.Jonckers TH, Rouan MC, Hache G, et al. Benzoxazole and benzothiazole amides as novel pharmacokinetic enhancers of HIV protease inhibitors. Bioorg Med Chem Lett. 2012;22(15):4998–5002. doi: 10.1016/j.bmcl.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 65.Kumar S, Jin M, Ande A, et al. Alcohol consumption effect on antiretroviral therapy and HIV-1 pathogenesis: role of cytochrome P450 isozymes. Expert Opin Drug Metab Toxicol. 2012;8(11):1363–1375. doi: 10.1517/17425255.2012.714366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reyskens KM, Essop MF. HIV protease inhibitors and onset of cardiovascular diseases: a central role for oxidative stress and dysregulation of the ubiquitin-proteasome system. Biochim Biophys Acta. 2014;1842(2):256–268. doi: 10.1016/j.bbadis.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 67.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6(8):591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 68.Ene L, Duiculescu D, Ruta SM. How much do antiretroviral drugs penetrate into the central nervous system? J Med Life. 2011;4(4):432–439. [PMC free article] [PubMed] [Google Scholar]
- 69.Nau R, Sorgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calcagno A, Yilmaz A, Cusato J, et al. Determinants of darunavir cerebrospinal fluid concentrations: impact of once-daily dosing and pharmacogenetics. AIDS. 2012;26(12):1529–1533. doi: 10.1097/QAD.0b013e3283553619. [DOI] [PubMed] [Google Scholar]
- 71. Nair M, Jayant RD, Kaushik A, et al. Getting into the brain: potential of nanotechnology in the management of NeuroAIDS. Adv Drug Deliv Rev. 2016;103:202–217. doi: 10.1016/j.addr.2016.02.008. •• A review of the progress in development of various nanocarriers to deliver antiretrovirals across the BBB.
- 72.Meshram SM, Kumar YV, Dodoala S, et al. Biodegradable polymeric nanoparticles for delivery of combination of antiretroviral drugs. Int J ChemTech Res. 2015;7(2):716–724. [Google Scholar]
- 73.Jain S, Sharma JM, Jain AK, et al. Surface-stabilized lopinavir nanoparticles enhance oral bioavailability without coadministration of ritonavir. Nanomedicine (Lond) 2013;8(10):1639–1655. doi: 10.2217/nnm.12.181. [DOI] [PubMed] [Google Scholar]
- 74.Destache CJ, Belgum T, Christensen K, et al. Combination antiretroviral drugs in PLGA nanoparticle for HIV-1. BMC Infect Dis. 2009;9:198. doi: 10.1186/1471-2334-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh R, Kesharwani P, Mehra NK, et al. Development and characterization of folate anchored saquinavir entrapped PLGA nanoparticles for anti-tumor activity. Drug Dev Ind Pharm. 2015;41(11):1888–1901. doi: 10.3109/03639045.2015.1019355. [DOI] [PubMed] [Google Scholar]
- 76.Pedersen JS, Gerstenberg MC. The structure of P85 pluronic block copolymer micelles determined by small-angle neutron scattering. Colloids Surf Physicochem Eng Aspects. 2003;213(2–3):175–187. [Google Scholar]
- 77.Spitzenberger TJ, Heilman D, Diekmann C, et al. Novel delivery system enhances efficacy of antiretroviral therapy in animal model for HIV-1 encephalitis. J Cereb Blood Flow Metab. 2007;27(5):1033–1042. doi: 10.1038/sj.jcbfm.9600414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahajan SD, Roy I, Xu G, et al. Enhancing the delivery of anti retroviral drug “saquinavir” across the blood brain barrier using nanoparticles. Curr HIV Res. 2010;8(5):396–404. doi: 10.2174/157016210791330356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahajan SD, Aalinkeel R, Law WC, et al. Anti-HIV-1 nanotherapeutics: promises and challenges for the future. Int J Nanomedicine. 2012;7:5301–5314. doi: 10.2147/IJN.S25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Puligujja P, McMillan J, Kendrick L, et al. Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections. Nanomedicine. 2013;9(8):1263–1273. doi: 10.1016/j.nano.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puligujja P, Araínga M, Dash P, et al. Pharmacodynamics of folic acid receptor targeted antiretroviral nanotherapy in HIV-1-infected humanized mice. Antiviral Res. 2015;120:85–88. doi: 10.1016/j.antiviral.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borgmann K, Rao KS, Labhasetwar V, et al. Efficacy of Tat-conjugated ritonavir-loaded nanoparticles in reducing HIV-1 replication in monocyte-derived macrophages and cytocompatibility with macrophages and human neurons. AIDS Res Hum Retroviruses. 2011;27(8):853–862. doi: 10.1089/aid.2010.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nowacek A, Gendelman HE. NanoART, neuroAIDS and CNS drug delivery. Nanomedicine (Lond) 2009;4(5):557–574. doi: 10.2217/nnm.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta U, Jain NK. Non-polymeric nano-carriers in HIV/AIDS drug delivery and targeting. Adv Drug Deliv Rev. 2010;62(4–5):478–490. doi: 10.1016/j.addr.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 85.Shibata A, McMullen E, Pham A, et al. Polymeric nanoparticles containing combination antiretroviral drugs for HIV type 1 treatment. AIDS Res Hum Retroviruses. 2013;29(5):746–754. doi: 10.1089/aid.2012.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noor MA, Flint OP, Maa JF, et al. Effects of atazanavir/ritonavir and lopinavir/ritonavir on glucose uptake and insulin sensitivity: demonstrable differences in vitro and clinically. AIDS. 2006;20(14):1813–1821. doi: 10.1097/01.aids.0000244200.11006.55. [DOI] [PubMed] [Google Scholar]
- 87.Carey D, Amin J, Boyd M, et al. Lipid profiles in HIV-infected adults receiving atazanavir and atazanavir/ritonavir: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2010;65(9):1878–1888. doi: 10.1093/jac/dkq231. [DOI] [PubMed] [Google Scholar]
- 88.Cahn PE, Gatell JM, Squires K, et al. Atazanavir–a once-daily HIV protease inhibitor that does not cause dyslipidemia in newly treated patients: results from two randomized clinical trials. J Int Assoc Physicians AIDS Care (Chic) 2004;3(3):92–98. doi: 10.1177/154510970400300304. [DOI] [PubMed] [Google Scholar]
- 89.Caron M, Auclair M, Sterlingot H, et al. Some HIV protease inhibitors alter lamin A/C maturation and stability, SREBP-1 nuclear localization and adipocyte differentiation. AIDS. 2003;17(17):2437–2444. doi: 10.1097/00002030-200311210-00005. [DOI] [PubMed] [Google Scholar]
- 90.Hui DY. Effects of HIV protease inhibitor therapy on lipid metabolism. Prog Lipid Res. 2003;42(2):81–92. doi: 10.1016/s0163-7827(02)00046-2. [DOI] [PubMed] [Google Scholar]
- 91.Hresko RC, Hruz PW. HIV protease inhibitors act as competitive inhibitors of the cytoplasmic glucose binding site of gluts with differing affinities for GLUT1 and GLUT4. PLoS One. 2011;6(9):e25237. doi: 10.1371/journal.pone.0025237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hruz PW. HIV protease inhibitors and insulin resistance: lessons from in-vitro, rodent and healthy human volunteer models. Curr Opin HIV AIDS. 2008;3(6):660–665. doi: 10.1097/COH.0b013e3283139134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Josephson F. Drug-drug interactions in the treatment of HIV infection: focus on pharmacokinetic enhancement through CYP3A inhibition. J Intern Med. 2010;268(6):530–539. doi: 10.1111/j.1365-2796.2010.02301.x. [DOI] [PubMed] [Google Scholar]
- 94.Mallolas J, Sarasa M, Nomdedeu M, et al. Pharmacokinetic interaction between rifampicin and ritonavir-boosted atazanavir in HIV-infected patients. HIV Med. 2007;8(2):131–134. doi: 10.1111/j.1468-1293.2007.00442.x. [DOI] [PubMed] [Google Scholar]
- 95.Karageorgopoulos DE, El-Sherif O, Bhagani S, et al. Drug interactions between antiretrovirals and new or emerging direct-acting antivirals in HIV/hepatitis C virus coinfection. Curr Opin Infect Dis. 2014;27(1):36–45. doi: 10.1097/QCO.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 96.Birkus G, Bam RA, Willkom M, et al. Intracellular activation of tenofovir alafenamide and the effect of viral and host protease inhibitors. Antimicrob Agents Chemother. 2016;60(1):316–322. doi: 10.1128/AAC.01834-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu L, Hruska M, Hwang C, et al. Pharmacokinetic interactions between BMS-626529, the active moiety of the HIV-1 attachment inhibitor prodrug bms-663068, and ritonavir or ritonavir-boosted atazanavir in healthy subjects. Antimicrob Agents Chemother. 2015;59(7):3816–3822. doi: 10.1128/AAC.04914-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gautam N, Puligujja P, Balkundi S, et al. Pharmacokinetics, biodistribution, and toxicity of folic acid-coated antiretroviral nanoformulations. Antimicrob Agents Chemother. 2014;58(12):7510–7519. doi: 10.1128/AAC.04108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seden K, Back D, Khoo S. Antiretroviral drug interactions: often unrecognized, frequently unavoidable, sometimes unmanageable. J Antimicrob Chemother. 2009;64(1):5–8. doi: 10.1093/jac/dkp152. [DOI] [PubMed] [Google Scholar]
- 100.Weber IT, Kneller DW, Wong-Sam A. Highly resistant HIV-1 proteases and strategies for their inhibition. Future Med Chem. 2015;7(8):1023–1038. doi: 10.4155/fmc.15.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kurt Yilmaz N, Swanstrom R, Schiffer CA. Improving viral protease inhibitors to counter drug resistance. Trends Microbiol. 2016;24:547–557. doi: 10.1016/j.tim.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong E, Trustman N, Yalong A. HIV pharmacotherapy: a review of integrase inhibitors. JAAPA. 2016;29(2):36–40. doi: 10.1097/01.JAA.0000475465.07971.19. [DOI] [PubMed] [Google Scholar]
- 103.Robertson J, Feinberg J, Darunavir A. nonpeptidic protease inhibitor for antiretroviral-naive and treatment-experienced adults with HIV infection. Expert Opin Pharmacother. 2012;13(9):1363–1375. doi: 10.1517/14656566.2012.681776. [DOI] [PubMed] [Google Scholar]
- 104.Geretti AM, Moeketsi M, Demasi R, et al. Efficacy of once daily darunavir/ritonavir in PI-Naïve, NNRTI-experienced patients in the ODIN trial. AIDS Res Treat. 2015;2015:1–6. doi: 10.1155/2015/962574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brochot A, Kakuda TN, Van De Casteele T, et al. Model-based once-daily darunavir/ritonavir dosing recommendations in pediatric HIV-1-infected patients aged ≥3 to <12 years. CPT Pharmacometrics Syst Pharmacol. 2015;4(7):406–414. doi: 10.1002/psp4.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghosh RK, Ghosh SM, Chawla S. Recent advances in antiretroviral drugs. Expert Opin Pharmacother. 2011;12(1):31–46. doi: 10.1517/14656566.2010.509345. [DOI] [PubMed] [Google Scholar]
- 107.Ghosh AK, Dawson ZL, Mitsuya H. Darunavir, a conceptually new HIV-1 protease inhibitor for the treatment of drug-resistant HIV. Bioorg Med Chem. 2007;15(24):7576–7580. doi: 10.1016/j.bmc.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burdo TH, Katner SN, Taffe MA, et al. Neuroimmunity, drugs of abuse, and neuroaids. J Neuroimmune Pharmacol. 2006;1(1):41–49. doi: 10.1007/s11481-005-9001-3. [DOI] [PubMed] [Google Scholar]
- 109.Kolson DL, Lavi E, Gonzalez-Scarano F. The effects of human immunodeficiency virus in the central nervous system. Adv Virus Res. 1998;50:1–47. doi: 10.1016/s0065-3527(08)60804-0. [DOI] [PubMed] [Google Scholar]
- 110.Gotfredsen A, Podenphant J, Nilas L, et al. Discriminative ability of total body bone-mineral measured by dual photon absorptiometry. Scand J Clin Lab Invest. 1989;49(2):125–134. doi: 10.3109/00365518909105410. [DOI] [PubMed] [Google Scholar]
- 111.Akay C, Cooper M, Odeleye A, et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014;20(1):39–53. doi: 10.1007/s13365-013-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Romao PR, Lemos JC, Moreira J, et al. Anti-HIV drugs nevirapine and efavirenz affect anxiety-related behavior and cognitive performance in mice. Neurotox Res. 2011;19(1):73–80. doi: 10.1007/s12640-009-9141-y. [DOI] [PubMed] [Google Scholar]
- 113.Gill AJ, Kolson DL. Chronic inflammation and the role for cofactors (hepatitis C, drug abuse, antiretroviral drug toxicity, aging) in hand persistence. Curr HIV/AIDS Rep. 2014;11(3):325–335. doi: 10.1007/s11904-014-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Edagwa BJ, Zhou T, McMillan JM, et al. Development of HIV reservoir targeted long acting nanoformulated antiretroviral therapies. Curr Med Chem. 2014;21(36):4186–4198. doi: 10.2174/0929867321666140826114135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kaushik A, Jayant RD, Sagar V, et al. The potential of magnetoelectric nanocarriers for drug delivery. Expert Opin Drug Deliv. 2014;11(10):1635–1646. doi: 10.1517/17425247.2014.933803. • Magnetically inducible drug release through nanocarriers has potential for tissue- and time-specific antiretroviral treatment.