Abstract
Chronic infections with human immunodeficiency virus (HIV), hepatitis B virus (HBV) or hepatitis C virus (HCV) add to age-dependent bone loss and may contribute to lower bone strength in the elderly. In this review, we report recent highlights on the epidemiology of bone fragility in chronic viral infections with HIV, HCV and HBV, its physiopathology and discuss the interference of antiviral therapies with bone metabolism. Chronic infections influence bone through the interactions between risk factors for bone fragility and falls (which are highly prevalent in infected patients), virus activity and antiviral drugs. HIV-infected patients are at increased risk of fracture and the risk is higher in cases of co-infection with HIV and untreated chronic viral hepatitis. In HIV patients, the majority of bone loss occurs during virus activity and at initiation of antiretroviral therapy (ART). However, long-term elderly HIV-infected patients on successful ART display bone microstructure alterations only partially captured by dual energy X-ray absorptiometry (DXA). Bone loss is associated with an increase of bone resorption, reflecting the upregulation of the receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin (OPG) pathways via a crosstalk between virus activity, inflammation and the immune system. The use of some antiviral drugs, such as tenofovir (controlling both HBV and HIV infections) or protease inhibitors, may be associated with higher bone toxicity. The reduction of tenofovir plasma concentrations with the implementation of tenofovir alafenamide (TAF) attenuates bone mineral density (BMD) loss but it remains unknown whether it will contribute to reducing fracture risk in long-term HIV-treated patients. Moreover, to what extent the new direct-acting agents for treatment of HCV, including nucleotide inhibitors and protease inhibitors, may affect bone health similarly as ART in HIV should be investigated.
Keywords: bone mineral density, fracture, hepatitis B, hepatitis C, HIV, osteoporosis
Introduction
Age is a main determinant of bone fragility, and fracture risk exponentially increases after the age of 60 years in the general population [Kanis et al. 2005]. Major advances over the past 20 years in treatment of human immunodeficiency virus (HIV) and chronic viral liver diseases, hepatitis B (HBV) and hepatitis C (HCV), have contributed to a significant improvement of health status and an increase in the life expectancy of patients with these viral infections. As a result, a new population of elderly patients, with long-term infections of HIV, HCV, or HBV, is emerging. Usual risk factors for osteoporosis or fracture are frequently reported in these individuals and add to age-dependent bone loss [Compston, 2016]. It remains debated whether these individuals have lower bone strength and a higher risk of fracture than the general population, and whether viral infection itself or long-term exposure to drugs for controlling infection also contribute to bone fragility. These are key clinical issues for the management of bone fragility in these patients. If bone fragility is only associated with classical risk factors of fracture, standard clinical care should be considered as in the general population. On the other hand, if viral infection or its treatments have direct effects on bone health, this should be taken into account in the management of HIV and chronic viral hepatitis.
In this review, we report recent highlights on the epidemiology of bone fragility in patients presenting with HIV, HBV or untreated HCV infections, its pathophysiology and discuss the interference of antiviral therapies with bone metabolism.
Methods
A literature search of the Medline database was used to identify the publications on HIV, HBV or HCV infections and bone health up to March 2016. We searched using the keywords ‘HIV’ OR ‘hepatitis B’ OR ‘hepatitis C’ AND ‘osteoporosis’ OR ‘fracture’ OR ‘bone mineral density’. Many more data were published for HIV than for HBV or HCV.
Are patients presenting a chronic viral infection with HIV, HCV or HBV at higher risk of fracture?
It is quite challenging to assess whether HIV, HCV or HBV infections increase the risk of fragility fractures, because fracture risk may be associated both with a high prevalence of classical risk factors for fracture in infected individuals and also with virus and its treatments. No fracture data are available specifically in elderly populations with long-term treatment for HIV, chronic HBV or untreated HCV infections. In studies investigating fracture risk in chronic viral infections, control groups do not match for all the potent determinants of fractures linked to viral infection itself, patients’ characteristics and antiviral drugs. For instance, a study in male veterans showed a higher risk for fracture in HIV-infected compared with uninfected men. After adjusting for body mass index (BMI), which was lower in HIV-infected compared with uninfected men, HIV infection was no more associated with an increased fracture risk in this study [Womack et al. 2011]. Registry data with large sample size identified an increased fracture rate in HIV-infected populations compared with uninfected controls [Guerri-Fernandez et al. 2013; Hansen et al. 2012; Triant et al. 2008]. An almost three-fold increase in fracture risk has been shown in HIV-infected patients compared with that of age- and gender-matched uninfected patients in Danish registries [Prieto-Alhambra et al. 2014]. However, substantial heterogeneity is observed in baseline traditional risk factors for fracture and data are missing regarding the distinction of fragility fractures versus fractures associated with trauma, which may be highly prevalent in young infected populations. A number of cohort studies reported that HIV-infected people are at increased risk of fracture. A meta-analysis of these studies, including five controlled studies reporting incident fragility fractures, found a pooled incidence rate ratio of 1.35 [95% confidence interval (CI) 1.10–1.65] for fragility fracture in HIV-infected adults compared with controls [Shiau et al. 2013]. However, these cohort studies with more detailed data included a high proportion of young men (i.e individuals not at high risk of fragility fractures) in which few fracture events were recorded, in most cases self-reported [Collin et al. 2009; Young et al. 2011]. These studies also suffer from a lack of power, so that an increased risk of fracture was shown only in studies with the highest number of participants and longest follow up. For instance, while similar fracture incidence rates were initially reported among predominantly premenopausal participants of the Women’s Interagency HIV Study with or without HIV infection [Yin et al. 2010], follow-up with 5 additional observation years (median 10-year follow up) identified a higher adjusted fracture rate in middle-aged HIV-infected women compared with HIV-uninfected women [Sharma et al. 2015]. Taken together, it appears that HIV-infected individuals have an increased risk of fracture, but it remains unknown if it is associated with HIV infection itself or a pattern of classical risk factors of bone fragility shared by the HIV-infected population.
Regarding chronic viral hepatitis, untreated HCV infection is associated with higher fracture risk [Hansen et al. 2014; Lo Re et al. 2012]. HIV/HCV co-infection is associated with a greater risk of fracture than HIV mono-infection, only partially explained by the severity of liver disease [Lo Re et al. 2012; Maalouf et al. 2013]. In chronic untreated HBV infection, no difference in hip fracture incidence was shown compared with uninfected patients, except in black populations. The risk of hip fracture was however increased in patients with hepatic decompensation (i.e. ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) [Byrne et al. 2014]. Similarly as for HCV, dually-treated HIV/HBV co-infection is associated with an increased risk of hip fracture compared with treated HIV-mono-infected and uninfected patients [Byrne et al. 2015].
To summarize, HIV and untreated HCV and HBV infections with severe liver disease are associated with an increased risk of fracture which is higher in case of co-infections.
Are bone mineral density or bone microstructure altered by HIV, HCV or HBV chronic infections?
Cross-sectional studies have shown that HIV-infected patients have low bone mineral density (BMD) and microstructural bone alterations associated with a higher bone resorption, independent of sex, age and gonadal status (Table 1) [Biver et al. 2014; Bolland et al. 2015; Calmy et al. 2013; Yin et al. 2013, 2014]. Although BMD is only 3–5% lower in patients infected with HIV than in uninfected patients in most cross-sectional studies, differences in bone cortical and trabecular microstructure parameters may approach 20% between long-term HIV-infected men and controls matched for age and BMI [Biver et al. 2014]. Concerning chronic viral hepatitis, untreated young patients (4–21 years old) with chronic HBV and HCV infection do not have a lower BMD than healthy patients [Mora et al. 2014]. A cross-sectional study showed that hepatitis B surface antigen (HBsAg) seropositivity in adult men was significantly associated with lower BMD [Baeg et al. 2016]. Regarding chronic hepatitis C, reduced BMD values were reported in untreated HCV-infected patients in some studies [Gaudio et al. 2012; Lin et al. 2012; Orsini et al. 2013], but inconsistently [Pelazas-Gonzalez et al. 2013].
Table 1.
Cross-sectional studies of BMD and bone microstructure in HIV-infected patients.
| Study reference | Population | HIV-infected cases | HIV-uninfected controls | Lower areal BMD | Cortical bone |
Trabecular bone |
||
|---|---|---|---|---|---|---|---|---|
| Lower volumetric BMD | Microstructure alteration | Lower volumetric BMD | Microstructure alteration | |||||
| Calmy et al. [2013] | White premenopausal women | n = 22 aged 39–46 years on successful ART | n = 44 age- and BMI-matched | Yes (spine) | Yes 3% | No | Yes 14–18% | Yes 9–16% |
| Yin et al. [2013] | Hispanic and African-American postmenopausal women | n = 46 aged 58 ± 18 years (mean ± SD) with menopause ⩾ 1 year | n = 60 | Yes (spine, hip, and radius) | No | Yes at the tibia 11–12% | No | No |
| Yin et al. [2014] | African-American or Hispanic Tanner stage 5 young men | n = 30 aged 20–25 years on ART (15 perinatally infected and 15 infected during adolescence) | n = 15 | Yes (spine, hip, and radius) | No | Yes 14–18% | Yes 19% | Yes 5–15% |
| Biver et al. [2014] | White elderly men | n = 28 aged 60–70 years on successful ART | n = 112 age- and BMI-matched | Yes (total hip and ultradistal radius) | Yes 3% | Yes 12–20% | Yes 12% | Yes 3–11% |
ART, antiretroviral therapy; BMD, bone mineral density; BMI, body mass index; HIV, human immunodeficiency virus; SD, standard deviation.
Pattern and determinants of bone loss in HIV, HCV and HBV-infected patients
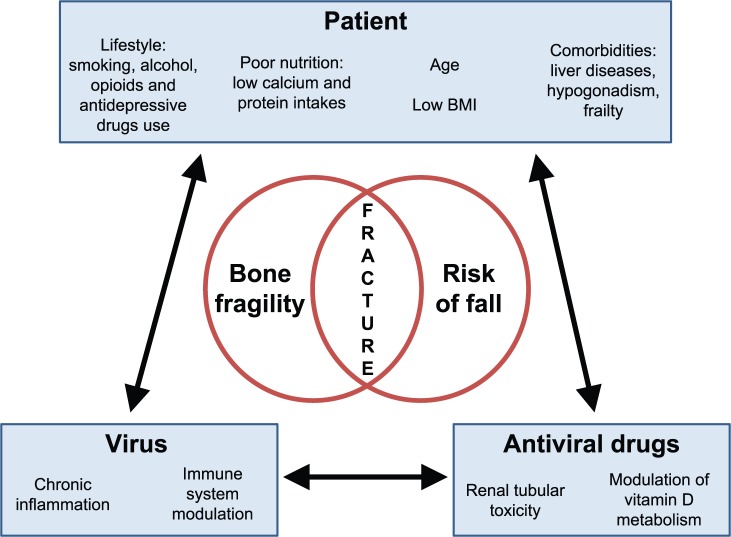
Bone loss in chronic viral infections is the result of the cumulative and time-dependent interactions between patient’s classical risk factors for osteoporosis, viral load and associated inflammation, and antiviral drug regimen (Figure 1). While cross-sectional studies do show lower BMD in HIV-infected patients compared with controls, longitudinal studies show a high proportion of virus-suppressed HIV-infected patients with increasing or stable BMD following 1–2 years after initiation of antiretroviral therapy (ART) [Bolland et al. 2015; Escota et al. 2016].
Figure 1.
Interactions between patient-related, virus-related and antiviral drug-related determinants of fracture risk in HIV and hepatitis B and C infections.
Patient-virus interaction: risk factors for fractures are highly prevalent in HIV/HCV/HBV-infected patients. Some patient’s lifestyle characteristics may be risk factors for virus infection (for instance, drug abuse and HCV). Virus infection may induce comorbidities which may have a direct negative effect on bone and fall risk, will further impair nutritional status and will induce loss of weight.
Patient-antiviral drug interaction: antiviral drugs may improve patient’s health status, but some of them may have toxicity which affects bone metabolism and the risk of fall.
Virus-antiviral drug interaction: antiviral drugs decrease viral load and restore the consequences of viral infection on inflammation, immunity and liver metabolism. Efficacy of antiviral drugs may vary according to virus resistance and patient adherence to treatment.
BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Patients’ risk factors for osteoporosis
In wealthy countries, classical risk factors for osteoporosis and fragility fractures may be prevalent in patients infected with HIV, HCV and HBV (depending on the transmission route): low body weight, poor nutrition, smoking habits, alcohol consumption, opioids or antidepressive drug use, liver diseases, hypogonadism and frailty [Compston, 2016]. This suggests that HIV, HCV and HBV infections may be markers of lifestyle and health associated with risk factors for low BMD. This hypothesis is well illustrated in studies showing low BMD in homosexual men before or soon after the diagnosis of HIV and in studies on pre-exposure prophylaxis with ART [Grijsen et al. 2010, 2013; Liu et al. 2011]. In addition, cross-sectional studies showing lower BMD in HIV-infected patients compared with age- and sex-matched noninfected controls showed a parallel lower body weight. Adjustment for between-group differences in bodyweight results in a remaining BMD difference of only 2% between groups [Bolland et al. 2015]. Some of these risk factors are influenced by the virus and its treatment. In particular, body weight loss, which is common in untreated HIV infection, is usually reversed over subsequent years after ART initiation (Table 2).
Table 2.
Pathophysiology of bone loss in HIV infection.
| Stages of HIV infection | Classical risk factor for osteoporosis | CD4+ T-cells | B-cells | RANKL/OPG ratio | Pro-inflammatory cytokines | Result | BMD changes | |
|---|---|---|---|---|---|---|---|---|
| Pre-HIV infection | ++ | ↔ | ↔ | ↔ | ↔ | High prevalence of risk factors for osteoporosis Low BMD |
↔ | |
| HIV-infection | ART naïve | ++ | ↓ | ↓ co-stimulation T and B-cells ↓ OPG ↑ RANKL |
↑ | ↑ IL6 (viral chronic inflammation) | Stimulation of osteoclasts ↑ bone resorption |
↓ |
| ART initiation | ++ | CD4+ T-cell repopulation ↑ RANKL ↑ TNF-α |
↑ co-stimulation T and B- cells ↑ RANKL ↑ TNF-α |
↑↑ | ↑ TNF-α (immune reconstitution) | Stimulation of osteoclasts ↑↑ bone resorption |
↓↓ | |
| ART continuation | + * | ↔ | ↔ | ↔ | ↔ | No stimulation of osteoclasts No excess of bone resorption |
↑ then ↔ | |
ART, antiretroviral therapy; BMD, bone mineral density; HIV, human immunodeficiency virus; IL6, interleukin 6; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor-kappaB ligand; TNF-α, tumor necrosis factor α.
may improve with intervention.
Chronic inflammation
Whether bone loss may also be linked with virus-associated chronic inflammation has been investigated in ART-naïve HIV-infected adults. High-sensitivity C-reactive protein and interleukin-6 levels are elevated in HIV-infected patients [Neuhaus et al. 2010]. BMD loss associated with higher interleukin-6 levels has been observed in ART-naïve HIV-infected adults compared with noninfected patients [Hileman et al. 2014]. A positive association between low BMD and time since HIV diagnosis has been reported in ART-naïve HIV-infected adults, but without association with CD4 cell count or viral load [Carr et al. 2015], highlighting the importance of traditional risk factors for bone loss in these populations. Low BMD values have also been associated with longer time since HIV diagnosis in HIV-infected treated individuals, without association with treatment variables and cumulative exposure to ART [Cotter et al. 2014].
Antiviral drugs
It might be expected that viral load suppression with antiviral drugs decreases bone loss associated with chronic viral inflammation. All HIV-infected patients initiating ART present an accelerated BMD loss of about 2% at the spine and the hip, in comparison with patients to whom ART is deferred [Bolland et al. 2015]. Bone loss is associated with an increase of bone resorption markers. In addition, a biphasic pattern of BMD change is observed after ART initiation in HIV infection: a short-term decrease of 2–4% in BMD at the hip and spine that occurs mainly within the first year of treatment, followed by a stability of BMD thereafter [Bolland et al. 2015]. Thus, the magnitude of bone loss after ART initiation is quite small (for comparison, post-menopausal bone loss is about 1% per year). Moreover, the degree of BMD loss varies according to the ART regimens.
The decrease in BMD is greater when the initiating regimen containing tenofovir is compared with a regimen with nucleoside reverse transcriptase inhibitors (NRTIs) [McComsey et al. 2011; Stellbrink et al. 2010] or when switching to tenofovir rather than to NRTI [Cotter et al. 2013; Haskelberg et al. 2012; Rasmussen et al. 2012]. BMD loss is also observed when tenofovir is used in the absence of HIV infection [Mulligan et al. 2015]. After the initial BMD decline, BMD is however also stabilized in long-term regimens containing tenofovir, and limited to a 2–4% decrease from baseline after 3 years [Cassetti et al. 2007; Gallant et al. 2004]. In the setting of hepatitis B, the effect of tenofovir on BMD remains unclear and poorly investigated [Maggi et al. 2015; Tien et al. 2015]. Conversely, tenofovir-sparing regimens with integrase inhibitor in ART-naïve HIV-infected adults are associated with lower BMD loss than with tenofovir-containing regimens [Bernardino et al. 2015]. In treated virologically-suppressed HIV-infected patients, switching from tenofovir- to abacavir/lamivudine-containing regimens is associated with a decrease in bone turnover markers [Wohl et al. 2016]. In addition to the increase in bone resorption, the use of nucleotide analogues such as tenofovir and adefovir has been associated with rare cases of proximal renal tubular toxicity and hypophosphatemic osteomalacia, which may contribute to bone alterations seen with HIV and hepatitis B treatments [Hall et al. 2011]. Considering the risk of tenofovir-related renal or bone toxicity, a novel tenofovir prodrug, tenofovir alafenamide (TAF), has been developed to reduce tenofovir plasma concentrations by 90% compared with the classical prodrug tenofovir disoproxil fumarate (TDF). Similar virologic efficacy has been demonstrated with these two tenofovir prodrugs. In treatment-naïve HIV-infected patients, BMD loss is attenuated in patients receiving a TAF-containing regimen compared with a TDF-containing regimen [Gallant et al. 2016; Sax et al. 2015]. Switching from a TDF-containing to a TAF-containing regimen has been shown to be noninferior for maintenance of viral suppression and leads to preserved BMD and renal function [Mills et al. 2016].
In addition to tenofovir-containing regimens, BMD loss seems to be greater in protease inhibitor-containing regimens and correlates with the duration of treatment with this ART class [Brown et al. 2015b; Duvivier et al. 2009]. Discontinuation of protease inhibitors allows recovery of BMD, especially in the lumbar spine [Kinai et al. 2014]. Efavirenz, a popular first-line drug from the nonnucleoside retrotranscriptase inhibitor, is associated with a decrease in vitamin D levels and an increase in the risk of vitamin D deficiency [Havers et al. 2014; Wohl et al. 2014]. The mechanism of efavirenz’s effect on vitamin D has been linked to modulation of various cytochromes and enzymes involved in the inactivation or activation of vitamin D, or possibly of vitamin D binding protein [Havers et al. 2014].
Oral nucleoside or nucleotide analogs are also widely used long-term to treat chronic hepatitis B. Few data are available on the bone safety profile of these treatments in a non-HIV related context. TDF-exposed patients with chronic hepatitis B have reduced BMD at total hip compared with non-TDF-exposed patients with chronic hepatitis B [Gill et al. 2015]. In studies of cohorts with chronic hepatitis B, the incidence of hip fracture is low and does not differ between treated and untreated patients [Porcelli et al. 2014; Wong et al. 2015]. However, exposure to nucleotide analogues increased the risk of hip fracture compared with nucleoside analogs [hazard ratio (HR) = 5.69; 95% CI: 1.98–16.39; p = 0.001], suggesting that tenofovir-containing treatments may also slightly increase the risk of hip fracture in the context of chronic hepatitis B [Wong et al. 2015].
Concerning HCV treatment, no significant bone loss was observed with treatment with ribavirin in patients who underwent an orthotopic liver transplantation for HCV-related cirrhosis [Trombetti et al. 2002]. A recent study reported that bone turnover markers in HIV/HCV-co-infected patients controlled for HIV, and who received pegylated interferon-α and ribavirin, were markedly reduced [Bedimo et al. 2016]. However, it is unknown whether this is a direct effect of interferon or a result of HCV viral clearance, and whether the reduction of bone turnover will result in improved BMD. Treatment of chronic hepatitis C has recently been enriched with oral interferon-free therapies including the hepatitis C nucleotide inhibitor, sofosbuvir, in a combination regimen with ribavirin; nonstructural protein 5A inhibitors, ledipasvir, daclatasvir and ombitasvir; the protease inhibitor simeprevir and the ritonavir-boosted protease inhibitor paritaprevir; and the nonnucleoside inhibitor, dasabuvir. The impact of these direct-acting agents on bone health has not been evaluated up to now. It is unknown if an increase of bone turnover and bone loss at initiation will be observed similarly as for ART initiation in HIV-infected patients, and if the potential bone toxicity would be reversible after treatment discontinuation. In this context, studies investigating the effect of new interferon-free regimens on bone are highly warranted.
Pathophysiology of increased bone resorption and bone loss in the course of HIV infection
The mechanisms of the increase of bone resorption during HIV infection have recently been elucidated (Table 2). HIV-transgenic rats reproduce the pattern of bone and fat metabolism observed in HIV-infected patients. These rats are characterized by a decrease in BMD, an increase in bone resorption with normal bone formation, and a decrease in BMI (weighing 21% lower than controls, without a difference in food and water consumption). In this animal model, an up-regulation of RANKL expression by B-cells has been observed, compounded by a simultaneous decline in expression of its physiological moderator, OPG [Vikulina et al. 2010]. Similar observations were found in ART-naïve HIV-infected patients in which B-cell RANKL/OPG ratios correlated with total hip and femoral neck BMD, suggesting that B-cell dysregulation promotes HIV-induced bone loss through an imbalance in the RANKL/OPG ratio [Titanji et al. 2014]. In addition, initiation of ART (lopinavir/ritonavir plus TDF/emtricitabine) in these patients induces a rapid increase in bone resorption markers carboxy-terminal collagen crosslinks (CTX) followed by an increase in RANKL and tumor necrosis factor (TNF)-α levels which positively correlates with the magnitude of CD4R T-cell repopulation [Ofotokun et al. 2016b]. Based on these data, it has been hypothesized that ART-induced T-cells restoration and immune reactivation leads to the production of the osteoclastogenic cytokines RANKL and TNF-α by T-cells and B-cells. These cytokines would contribute to osteoclast formation and activation, to the increase of bone resorption and consequently to bone loss [Ofotokun et al. 2015].
Management of bone health in patients infected with HIV, HBV or HCV
Assessment of fracture risk
Fracture risk increases with age. Similarly as in the general population, fracture risk should be assessed in the context of HIV or viral hepatitis in individuals who have clinical risk factors for fracture, particularly in men aged ⩾50 years and postmenopausal women or patients with a history of fragility fracture [Brown et al. 2015a]. Beyond DXA, FRAX is currently the main tool used to assess fracture risk in the general population [Kanis et al. 2008]. However, it has not been validated for patients under 40 years-old and in the context of HIV or viral hepatitis infection. It has been proposed to consider HIV as a cause of secondary osteoporosis when calculating FRAX in HIV-infected individuals. Accuracy of FRAX to predict fracture improves when HIV is included as a cause of secondary osteoporosis, but only a small proportion of HIV-infected individuals who sustain incident fractures reach a suggested FRAX threshold for pharmacological intervention with anti-osteoporotic drugs [Yin et al. 2016]. This can be explained by the fact that FRAX does not incorporate some risks that are specific to viral infection (co-infection HIV/HCV, duration of antiviral exposure) or risks that are predominant in patients with chronic viral infections (such as poor nutrition, frailty or risk of fall). Thus, clinicians should take into account the complex interactions between patient, virus and antiviral drug-related determinants of fracture risk for the management of bone health in HIV, HBV and HCV infections for each patient individually. The longitudinal pattern of bone loss, especially the transient accelerated bone loss after ART initiation in HIV infection, should also be kept in mind. A high prevalence of subclinical vertebral fractures has been reported in HIV-infected patients (12–46%), independently of BMD status [Gazzola et al. 2015; Porcelli et al. 2014; Stephens et al. 2016]. As prior vertebral fracture is a risk factor for subsequent fracture, screening of vertebral fractures with vertebral fracture assessment (VFA) concomitantly with DXA may help to assess fracture risk, especially in osteopenic patients [Damiano et al. 2006].
Prevention and management of fracture risk
Management of patients should focus on modifiable risk factors, especially nutritional status, toxic habits, promotion of physical activity and prevention of falls. It has been recommended to avoid TDF or boosted protease inhibitors in patients at high risk of fracture [Brown et al. 2015a]. An alternative may be considering TAF-containing regimens. BMD loss in the setting of HIV infection on ART is preventable [Overton et al. 2015; Pinzone et al. 2014]. Optimization of calcium, protein and vitamin D intake is recommended. This is supported by a recent randomized controlled study in ART-naïve HIV-infected adults showing that supplementation with high-dose vitamin D and calcium (4000 IU of cholecalciferol daily plus 500 mg of calcium carbonate twice daily) attenuates bone loss at initiation of efavirenz/emtricitabine/TDF [Overton et al. 2015]. Alendronate and zoledronate increase BMD and are well tolerated in HIV-infected patients, but no study in the context of HIV or viral hepatitis infection was designed to demonstrate a reduction of fracture with bisphosphonates [Pinzone et al. 2014]. A single dose of 5 mg zoledronic acid administered at ART initiation prevents ART-induced bone loss through the first 48 weeks of ART [Ofotokun et al. 2016a]. Interestingly, it has been shown that BMD response to alendronate in HIV-infected patients is greater in those with higher baseline CTX and TNF-α levels, although the levels of OPG, RANKL or inflammatory markers do not change with alendronate treatment [Natsag et al. 2016]. There are no data on the efficacy and safety of raloxifene, denosumab or teriparatide in HIV and chronic viral hepatitis infections. It has been shown that raloxifene improves the efficacy of antiviral treatment of postmenopausal women with chronic hepatitis C, suggesting that it may be of potential interest as an adjuvant to the antiviral treatment in osteoporotic women with chronic hepatitis C. This rationale is based on a potential positive interaction between estrogen levels and the virologic response of hepatitis C to antiviral treatment [Furusyo et al. 2012]. Lastly, considering the role of the upregulation of the RANKL pathway in HIV- and ART-associated bone loss, denosumab may be an interesting treatment in this context; data on its efficacy and safety in this context are highly warranted.
Conclusion
The impact of long-term infection with HIV, HBV and untreated HCV on bone results in a complex interaction between highly prevalent risk factors for bone fragility and fall in infected patients, virus activity and antiviral drugs. The majority of bone loss occurs in patients with virus activity and at early stage on antiviral therapy, illustrating the crosstalk between the immune system, inflammation and RANKL/OPG regulation of osteoclast activity and bone resorption. However, long-term HIV-infected elderly patients on successful ART display a certain degree of bone microstructure alterations only partially captured by DXA. In addition to registries studies, increased fracture rate is now depicted in observational studies with long-term follow up of HIV-infected patients. Co-infection of HIV/HCV increases the risk of fracture and the risk of fracture in chronic hepatitis B seems mainly associated with the degree of hepatopathy. Whether the implementation of TAF to reduce tenofovir plasma concentrations and toxicity will contribute to reduced fracture risk in long-term HIV-treated patients remains to be investigated, in addition to the interest of denosumab to target the RANKL pathways which seem upregulated in the pathophysiology of HIV-related bone loss. Moreover, it remains unknown to what extent the new oral interferon-free therapies of chronic hepatitis C, including nucleotide inhibitors and protease inhibitors, may affect bone health similarly as with ART in HIV.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest. AC has received unrestricted educational and research grant from GILEAD Switzerland, and unrestricted educational grants from AbbVie, ViiV Healthcare, MSD, BMS, and Janssen-Cilag Switzerland (HIV Unit).
Contributor Information
Emmanuel Biver, Division of Bone Diseases, Department of Internal Medicine Specialties, Geneva University Hospitals and Faculty of Medicine, Rue Gabrielle-Perret-Gentil 4, 1211 Geneva 14, Switzerland.
Alexandra Calmy, Division of Infectious Diseases, HIV Unit, Department of Internal Medicine Specialties, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
René Rizzoli, Division of Bone Diseases, Department of Internal Medicine Specialties, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
References
- Baeg M., Yoon S., Ko S., Han K., Choi H., Bae S., et al. (2016) Males seropositive for hepatitis B surface antigen are at risk of lower bone mineral density: the 2008–2010 Korea National Health and Nutrition Examination Surveys. Hepatol Int 10: 470–477. [DOI] [PubMed] [Google Scholar]
- Bedimo R., Kang M., Tebas P., Overton E., Hollabaugh K., McComsey G., et al. (2016) Effects of pegylated interferon/ribavirin on bone turnover markers in HIV/Hepatitis C virus-coinfected patients. AIDS Res Hum Retroviruses 32: 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardino J., Mocroft A., Mallon P., Wallet C., Gerstoft J., Russell C., et al. (2015) Bone mineral density and inflammatory and bone biomarkers after darunavir-ritonavir combined with either raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults with HIV-1: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV 2: e464–e473. [DOI] [PubMed] [Google Scholar]
- Biver E., Calmy A., Delhumeau C., Durosier C., Zawadynski S., Rizzoli R. (2014) Microstructural alterations of trabecular and cortical bone in long-term HIV-infected elderly men on successful antiretroviral therapy. AIDS 28: 2417–2427. [DOI] [PubMed] [Google Scholar]
- Bolland M., Grey A., Reid I. (2015) Skeletal health in adults with HIV infection. Lancet Diabetes Endocrinol 3: 63–74. [DOI] [PubMed] [Google Scholar]
- Brown T., Hoy J., Borderi M., Guaraldi G., Renjifo B., Vescini F., et al. (2015a) Recommendations for evaluation and management of bone disease in HIV. Clin Infect Dis 60: 1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Moser C., Currier J., Ribaudo H., Rothenberg J., Kelesidis T., et al. (2015b) Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis 212: 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne D., Newcomb C., Carbonari D., Nezamzadeh M., Leidl K., Herlim M., et al. (2014) Risk of hip fracture associated with untreated and treated chronic hepatitis B virus infection. J Hepatol 61: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne D., Newcomb C., Carbonari D., Nezamzadeh M., Leidl K., Herlim M., et al. (2015) Increased risk of hip fracture associated with dually treated HIV/hepatitis B virus coinfection. J Viral Hepat 22: 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmy A., Chevalley T., Delhumeau C., Toutous-Trellu L., Spycher-Elbes R., Ratib O., et al. (2013) Long-term HIV infection and antiretroviral therapy are associated with bone microstructure alterations in premenopausal women. Osteoporos Int 24: 1843–1852. [DOI] [PubMed] [Google Scholar]
- Carr A., Grund B., Neuhaus J., Schwartz A., Bernardino J., White D., et al. (2015) Prevalence of and risk factors for low bone mineral density in untreated HIV infection: a substudy of the INSIGHT Strategic Timing of Antiretroviral Treatment (START) trial. HIV Med 16(Suppl. 1): 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassetti I., Madruga J., Suleiman J., Etzel A., Zhong L., Cheng A., et al. (2007) The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1-infected patients. HIV Clin Trials 8: 164–172. [DOI] [PubMed] [Google Scholar]
- Collin F., Duval X., Le Moing V., Piroth L., Al Kaied F., Massip P., et al. (2009) Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS 23: 1021–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston J. (2016) HIV infection and bone disease. J Intern Med 280: 350–358. [DOI] [PubMed] [Google Scholar]
- Cotter A., Sabin C., Simelane S., Macken A., Kavanagh E., Brady J., et al. (2014) Relative contribution of HIV infection, demographics and body mass index to bone mineral density. AIDS 28: 2051–2060. [DOI] [PubMed] [Google Scholar]
- Cotter A., Vrouenraets S., Brady J., Wit F., Fux C., Furrer H., et al. (2013) Impact of switching from zidovudine to tenofovir disoproxil fumarate on bone mineral density and markers of bone metabolism in virologically suppressed HIV-1 infected patients; a substudy of the prepare study. J Clin Endocrinol Metab 98: 1659–1666. [DOI] [PubMed] [Google Scholar]
- Damiano J., Kolta S., Porcher R., Tournoux C., Dougados M., Roux C. (2006) Diagnosis of vertebral fractures by vertebral fracture assessment. J Clin Densitom 9: 66–71. [DOI] [PubMed] [Google Scholar]
- Duvivier C., Kolta S., Assoumou L., Ghosn J., Rozenberg S., Murphy R., et al. (2009) Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 23: 817–824. [DOI] [PubMed] [Google Scholar]
- Escota G., Mondy K., Bush T., Conley L., Brooks J., Onen N., et al. (2016) High prevalence of low bone mineral density and substantial bone loss over 4 years among HIV-infected persons in the era of modern antiretroviral therapy. AIDS Res Hum Retroviruses 32: 59–67. [DOI] [PubMed] [Google Scholar]
- Furusyo N., Ogawa E., Sudoh M., Murata M., Ihara T., Hayashi T., et al. (2012) Raloxifene hydrochloride is an adjuvant antiviral treatment of postmenopausal women with chronic hepatitis C: a randomized trial. J Hepatol 57: 1186–1192. [DOI] [PubMed] [Google Scholar]
- Gallant J., Brunetta J., Crofoot G., Benson P., Mills A., Brinson C., et al. (2016) Efficacy and safety of switching to a single-tablet regimen of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) in HIV-1/hepatitis B coinfected adults. J Acquir Immune Defic Syndr. Epub ahead of print 11 May 2016. DOI: 10.1097/QAI.0000000000001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Staszewski S., Pozniak A., Dejesus E., Suleiman J., Miller M., et al. (2004) Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 292: 191–201. [DOI] [PubMed] [Google Scholar]
- Gaudio A., Pennisi P., Muratore F., Bertino G., Ardiri A., Pulvirenti I., et al. (2012) Reduction of volumetric bone mineral density in postmenopausal women with hepatitis C virus-correlated chronic liver disease: a peripheral quantitative computed tomography (pQCT) study. Eur J Intern Med 23: 656–660. [DOI] [PubMed] [Google Scholar]
- Gazzola L., Savoldi A., Bai F., Magenta A., Dziubak M., Pietrogrande L., et al. (2015) Assessment of radiological vertebral fractures in HIV-infected patients: clinical implications and predictive factors. HIV Med 16: 563–571. [DOI] [PubMed] [Google Scholar]
- Gill U., Zissimopoulos A., Al-Shamma S., Burke K., Mcphail M., Barr D., et al. (2015) Assessment of bone mineral density in tenofovir-treated patients with chronic hepatitis B: can the fracture risk assessment tool identify those at greatest risk? J Infect Dis 211: 374–382. [DOI] [PubMed] [Google Scholar]
- Grijsen M., Vrouenraets S., Steingrover R., Lips P., Reiss P., Wit F., et al. (2010) High prevalence of reduced bone mineral density in primary HIV-1-infected men. AIDS 24: 2233–2238. [DOI] [PubMed] [Google Scholar]
- Grijsen M., Vrouenraets S., Wit F., Stolte I., Prins M., Lips P., et al. (2013) Low bone mineral density, regardless of HIV status, in men who have sex with men. J Infect Dis 207: 386–391. [DOI] [PubMed] [Google Scholar]
- Guerri-Fernandez R., Vestergaard P., Carbonell C., Knobel H., Aviles F., Castro A., et al. (2013) HIV infection is strongly associated with hip fracture risk, independently of age, gender, and comorbidities: a population-based cohort study. J Bone Miner Res 28: 1259–1263. [DOI] [PubMed] [Google Scholar]
- Hall A., Hendry B., Nitsch D., Connolly J. (2011) Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis 57: 773–780. [DOI] [PubMed] [Google Scholar]
- Hansen A., Gerstoft J., Kronborg G., Larsen C., Pedersen C., Pedersen G., et al. (2012) Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study. AIDS 26: 285–293. [DOI] [PubMed] [Google Scholar]
- Hansen A., Omland L., Krarup H., Obel N. (2014) Fracture risk in hepatitis C virus infected persons: results from the DANVIR cohort study. J Hepatol 61: 15–21. [DOI] [PubMed] [Google Scholar]
- Haskelberg H., Hoy J., Amin J., Ebeling P., Emery S., Carr A., et al. (2012) Changes in bone turnover and bone loss in HIV-infected patients changing treatment to tenofovir-emtricitabine or abacavir-lamivudine. PLoS ONE 7: e38377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havers F., Detrick B., Cardoso S., Berendes S., Lama J., Sugandhavesa P., et al. (2014) Change in vitamin D levels occurs early after antiretroviral therapy initiation and depends on treatment regimen in resource-limited settings. PLoS ONE 9: e95164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hileman C., Labbato D., Storer N., Tangpricha V., McComsey G. (2014) Is bone loss linked to chronic inflammation in antiretroviral-naive HIV-infected adults? A 48-week matched cohort study. AIDS 28: 1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis J., Borgstrom F., De Laet C., Johansson H., Johnell O., Jonsson B., et al. (2005) Assessment of fracture risk. Osteoporos Int 16: 581–589. [DOI] [PubMed] [Google Scholar]
- Kanis J., Johnell O., Oden A., Johansson H., Mccloskey E. (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinai E., Nishijima T., Mizushima D., Watanabe K., Aoki T., Honda H., et al. (2014) Long-term use of protease inhibitors is associated with bone mineral density loss. AIDS Res Hum Retroviruses 30: 553–559. [DOI] [PubMed] [Google Scholar]
- Lin J., Hsieh T., Wu C., Chen P., Chueh T., Chang W., et al. (2012) Association between chronic hepatitis C virus infection and bone mineral density. Calcif Tissue Int 91: 423–429. [DOI] [PubMed] [Google Scholar]
- Liu A., Vittinghoff E., Sellmeyer D., Irvin R., Mulligan K., Mayer K., et al. (2011) Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS ONE 6: e23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Re V., 3rd, Volk J., Newcomb C., Yang Y., Freeman C., Hennessy S., et al. (2012) Risk of hip fracture associated with hepatitis C virus infection and hepatitis C/human immunodeficiency virus coinfection. Hepatology 56: 1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf N., Zhang S., Drechsler H., Brown G., Tebas P., Bedimo R. (2013) Hepatitis C co-infection and severity of liver disease as risk factors for osteoporotic fractures among HIV-infected patients. J Bone Miner Res 28: 2577–2583. [DOI] [PubMed] [Google Scholar]
- McComsey G., Kitch D., Daar E., Tierney C., Jahed N., Tebas P., et al. (2011) Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids clinical trials group A5224s, a substudy of ACTG A5202. J Infect Dis 203: 1791–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi P., Montinaro V., Leone A., Fasano M., Volpe A., Bellacosa C., et al. (2015) Bone and kidney toxicity induced by nucleotide analogues in patients affected by HBV-related chronic hepatitis: a longitudinal study. J Antimicrob Chemother 70: 1150–1154. [DOI] [PubMed] [Google Scholar]
- Mills A., Arribas J., Andrade-Villanueva J., Diperri G., Van Lunzen J., Koenig E., et al. (2016) Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis 16: 43–52. [DOI] [PubMed] [Google Scholar]
- Mora S., Giacomet V., Vigano A., Maruca K., Capelli S., Nannini P., et al. (2014) Areal bone mineral density in pediatric patients with chronic hepatitis B or chronic hepatitis C. Calcif Tissue Int 95: 218–221. [DOI] [PubMed] [Google Scholar]
- Mulligan K., Glidden D., Anderson P., Liu A., McMahan V., Gonzales P., et al. (2015) Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 61: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsag J., Kendall M., Sellmeyer D., McComsey G., Brown T. (2016) Vitamin D, osteoprotegerin/receptor activator of nuclear factor-kappaB ligand (OPG/RANKL) and inflammation with alendronate treatment in HIV-infected patients with reduced bone mineral density. HIV Med 17: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus J., Jacobs D., Jr., Baker J., Calmy A., Duprez D., La Rosa A., et al. (2010) Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 201: 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofotokun I., Titanji K., Lahiri C., Vunnava A., Foster A., Sanford S., et al. (2016a) A single-dose zoledronic acid infusion prevents antiretroviral therapy-induced bone loss in treatment-naive HIV-infected patients: a phase IIb trial. Clin Infect Dis 63: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofotokun I., Titanji K., Vikulina T., Roser-Page S., Yamaguchi M., Zayzafoon M., et al. (2015) Role of T-cell reconstitution in HIV-1 antiretroviral therapy-induced bone loss. Nat Commun 6: 8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofotokun I., Titanji K., Vunnava A., Roser-Page S., Vikulina T., Villinger F., et al. (2016b) Antiretroviral therapy induces a rapid increase in bone resorption that is positively associated with the magnitude of immune reconstitution in HIV infection. AIDS 30: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini L., Pinheiro M., Castro C., Silva A., Szejnfeld V. (2013) Bone mineral density measurements, bone markers and serum vitamin D concentrations in men with chronic non-cirrhotic untreated hepatitis C. PLoS ONE 8: e81652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton E., Chan E., Brown T., Tebas P., McComsey G., Melbourne K., et al. (2015) Vitamin D and calcium attenuate bone loss with antiretroviral therapy initiation: a randomized trial. Ann Intern Med 162: 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelazas-Gonzalez R., Gonzalez-Reimers E., Aleman-Valls M., Santolaria-Fernandez F., Lopez-Prieto J., Gonzalez-Diaz A., et al. (2013) Bone alterations in hepatitis C virus infected patients. Eur J Intern Med 24: 92–96. [DOI] [PubMed] [Google Scholar]
- Pinzone M., Moreno S., Cacopardo B., Nunnari G. (2014) Is there enough evidence to use bisphosphonates in HIV-infected patients? A systematic review and meta-analysis. AIDS Rev 16: 213–222. [PubMed] [Google Scholar]
- Porcelli T., Gotti D., Cristiano A., Maffezzoni F., Mazziotti G., Foca E., et al. (2014) Role of bone mineral density in predicting morphometric vertebral fractures in patients with HIV infection. Osteoporos Int 25: 2263–2269. [DOI] [PubMed] [Google Scholar]
- Prieto-Alhambra D., Guerri-Fernandez R., De Vries F., Lalmohamed A., Bazelier M., Starup-Linde J., et al. (2014) HIV infection and its association with an excess risk of clinical fractures: a nationwide case-control study. J Acquir Immune Defic Syndr 66: 90–95. [DOI] [PubMed] [Google Scholar]
- Rasmussen T., Jensen D., Tolstrup M., Nielsen U., Erlandsen E., Birn H., et al. (2012) Comparison of bone and renal effects in HIV-infected adults switching to abacavir or tenofovir based therapy in a randomized trial. PLoS ONE 7: e32445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax P., Wohl D., Yin M., Post F., Dejesus E., Saag M., et al. (2015) Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 385: 2606–2615. [DOI] [PubMed] [Google Scholar]
- Sharma A., Shi Q., Hoover D., Anastos K., Tien P., Young M., et al. (2015) Increased fracture incidence in middle-aged HIV-infected and HIV-uninfected women: updated results from the women’s interagency HIV study. J Acquir Immune Defic Syndr 70: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau S., Broun E., Arpadi S., Yin M. (2013) Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. AIDS 27: 1949–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellbrink H., Orkin C., Arribas J., Compston J., Gerstoft J., Van Wijngaerden E., et al. (2010) Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 51: 963–972. [DOI] [PubMed] [Google Scholar]
- Stephens K., Rubinsztain L., Payan J., Rentsch C., Rimland D., Tangpricha V. (2016) Dual-energy X-ray absorptiometry and calculated FRAX risk scores May underestimate osteoporotic fracture risk in vitamin D-deficient veterans with HIV infection. Endocr Pract 22: 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien C., Xu J., Chan L., Chang M., Lim C., Lee S., et al. (2015) Long-term treatment with tenofovir in Asian-American chronic hepatitis B patients is associated with abnormal renal phosphate handling. Dig Dis Sci 60: 566–572. [DOI] [PubMed] [Google Scholar]
- Titanji K., Vunnava A., Sheth A., Delille C., Lennox J., Sanford S., et al. (2014) Dysregulated B cell expression of RANKL and OPG correlates with loss of bone mineral density in HIV infection. PLoS Pathog 10: e1004497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant V., Brown T., Lee H., Grinspoon S. (2008) Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large US healthcare system. J Clin Endocrinol Metab 93: 3499–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetti A., Giostra E., Mentha G., Negro F., Rizzoli R. (2002) Lack of evidence for ribavirin-induced bone loss. Hepatology 36: 255–257. [DOI] [PubMed] [Google Scholar]
- Vikulina T., Fan X., Yamaguchi M., Roser-Page S., Zayzafoon M., Guidot D., et al. (2010) Alterations in the immuno-skeletal interface drive bone destruction in HIV-1 transgenic rats. Proc Natl Acad Sci U S A 107: 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl D., Bhatti L., Small C., Edelstein H., Zhao H., Margolis D., et al. (2016) The ASSURE study: HIV-1 suppression is maintained with bone and renal biomarker improvement 48 weeks after ritonavir discontinuation and randomized switch to abacavir/lamivudine + atazanavir. HIV Med 17: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl D., Orkin C., Doroana M., Pilotto J., Sungkanuparph S., Yeni P., et al. (2014) Change in vitamin D levels and risk of severe vitamin D deficiency over 48 weeks among HIV-1-infected, treatment-naive adults receiving rilpivirine or efavirenz in a Phase III trial (ECHO). Antivir Ther 19: 191–200. [DOI] [PubMed] [Google Scholar]
- Womack J., Goulet J., Gibert C., Brandt C., Chang C., Gulanski B., et al. (2011) Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS ONE 6: e17217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Tse Y., Wong V., Yip T., Tsoi K., Chan H. (2015) Long-term safety of oral nucleos(t)ide analogs for patients with chronic hepatitis B: a cohort study of 53,500 subjects. Hepatology 62: 684–693. [DOI] [PubMed] [Google Scholar]
- Yin M., Lund E., Shah J., Zhang C., Foca M., Neu N., et al. (2014) Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS 28: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M., Shi Q., Hoover D., Anastos K., Sharma A., Young M., et al. (2010) Fracture incidence in HIV-infected women: results from the women’s interagency HIV study. AIDS 24: 2679–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M., Shiau S., Rimland D., Gibert C., Bedimo R., Rodriguez-Barradas M., et al. (2016) Fracture prediction with modified-FRAX in older HIV-infected and uninfected men. J Acquir Immune Defic Syndr 72: 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M., Shu A., Zhang C., Boutroy S., Mcmahon D., Ferris D., et al. (2013) Trabecular and cortical microarchitecture in postmenopausal HIV-infected women. Calcif Tissue Int 92: 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B., Dao C., Buchacz K., Baker R., Brooks J. (2011) Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis 52: 1061–1068. [DOI] [PubMed] [Google Scholar]