Abstract
Background:
Hemodynamic perturbations in spine surgeries are predominantly reported in cervical and thoracic level procedures. The literature related to negative cardiovascular changes (decrease of heart rate and blood pressure) in lumbar spine procedures is still scarce and only highlighted in few case reports/letters until now.
Methods:
With the help of a systematic literature review with predefined criteria, we, therefore, examined and synthesized here the probable underlying common cause of these hemodynamic disturbances in lumbar spine surgeries. Data aggregation to a model was done by a case survey method and established by a cause–effect relationship.
Results:
There are only 5 cases that met our strict predefined criteria and that were aggregated to an emergent model of an autonomous reflex arc.
Conclusion:
This review and consecutive data aggregation provides, for the first time, a concept of spinal cardiac reflex in lumbar spine surgeries.
Keywords: arterial hypotension, bradycardia, mechanism, spinal surgery, trigeminocardiac reflex
1. Introduction
Various types of cardiovascular changes are reported in neurosurgical patients, and these changes are the manifestations of different mechanisms including Cushing reflex, neurogenic reflex, brainstem manipulations, hypothalamic stimulation, cranial nerve stimulations, venous air embolism, and also the trigeminocardiac reflex (TCR) with its different subtypes.[1–10] On the other hand, hemodynamic perturbations in spine surgeries are limited to very few mechanisms including major bleeding, spinal shock, autonomic dysreflexia, parasympathetic nerve root activation, and venous air embolism.[1] Further to this, limited data exist related to hemodynamic changes during lumbar spine surgery[11–15] beyond the traumatic spinal cord injury. However, this gap in knowledge about hemodynamic perturbations during lumbar spine surgeries, and its clinical manifestation, similar to the neurosurgery well known TCR, suggests that there might also be a similar mechanism in the spinal as in the cerebral autonomous system.
Therefore, present article aims to provide the first detailed description of the possible mechanism underlying these adverse hemodynamic changes in lumbar spine surgeries. Here, we have presented a comprehensive review.
2. Method
The study presented here is a literature review, and it does not need any ethical approval.
2.1. Review of literature
We have searched terms including “Bradycardia,” “Hypotension,” “Asystole,” “lumbar spine surgery,” “lumbar laminectomy/discectomy,” “lumbar spine instrumentation,” “hemodynamic changes/perturbations/disturbances,” “cardiovascular changes/disturbances,” and “trans-foraminal lumbar interbody fusion” in various search engines including PubMed, Google Scholar, Science Direct, and Embase from January 1, 1970 to June 30, 2015.
2.2. Definition of hemodynamic changes: spinal cardiac reflex
For defining spinal cardiac reflex (SCR), a new entity for lumbar spinal cases, similar to TCR, we have used objective definitions of TCR.
Besides the decrease in heart rate (HR)/mean arterial blood pressure, we, therefore, define here based on our previous work[16] the following criteria: there should be present a unique cause–effect relationship–based criteria for SCR episode and highlight 4 major domains including plausibility, reversibility, repetition, and prevention. Further to this, we have divided these 4 major criteria to 2 major (plausibility and reversibility) and 2 minor criteria (repetition and prevention). For defining SCR, there should be at least 2 major criteria. Minor criteria may or may not be met in all cases.
2.3. Inclusion/exclusion criteria
The following inclusion criteria for the review were used: adult aged >18 years undergoing elective or emergent lumbar spinal surgery under general, regional, or local anesthesia, or a combination. Surgery for traumatic spinal cord injury is excluded. All papers, irrespective of the type and language, were included in this review (Table 1).
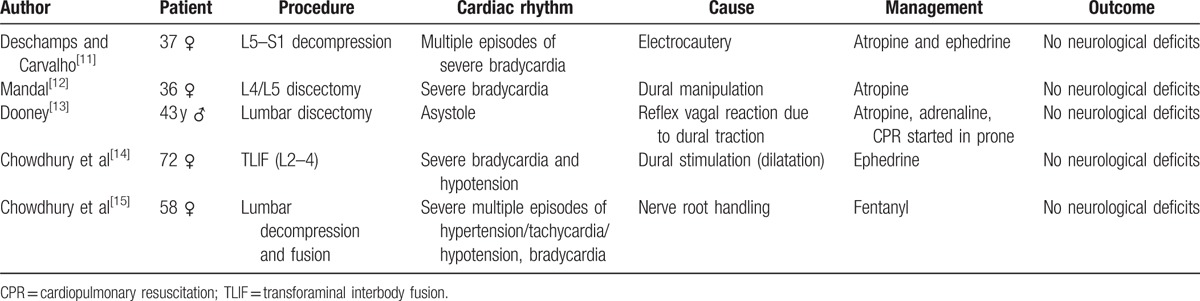
Table 1.
Negative chronotropic changes in lumbar spine surgery.
2.4. Data extraction and quality assessment and bias
For data extraction, 1 reviewer (TC) selected all titles/abstract. Articles that could not be excluded by title and/or abstracts were assessed for defined eligibility criteria in full text. If there was no agreement, the articles were read and checked for inclusion by a second reviewer (BS) independently, and the decision was made after thorough discussion according to PRISMA guidelines (Fig. 1). The selection of the included literature was performed according to the data contained in summary. If we were not able to confirm all including criteria through the summary, the whole publication was evaluated. However, the chances of publication bias cannot be eliminated.
Figure 1.
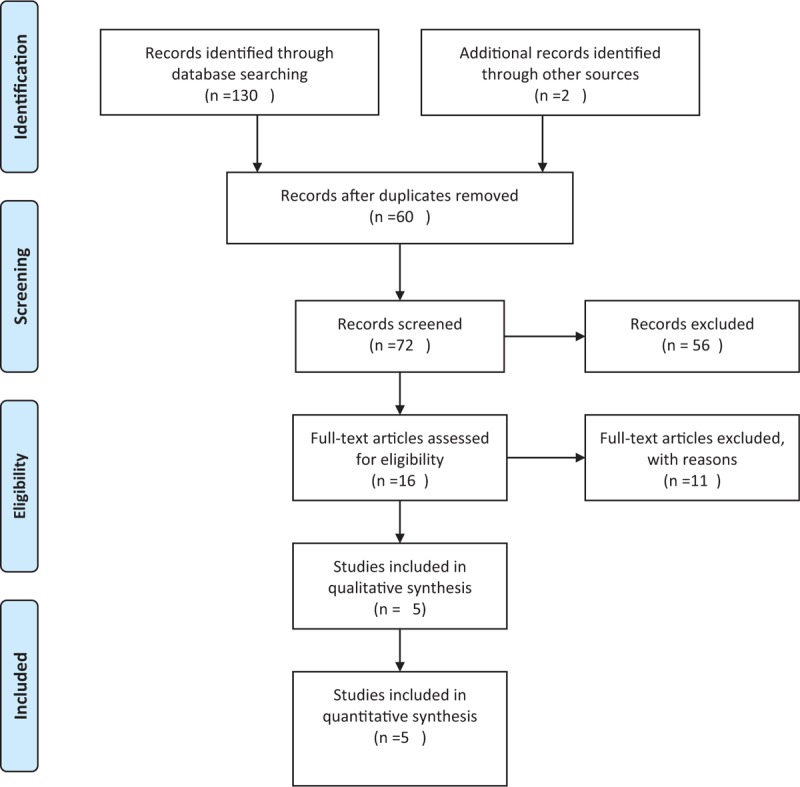
PRISMA flow diagram.
2.5. Statistical analysis
All the statistical analyses were performed using statistical software (JMP, SAS Institute Inc.; Cary, NC) on a commercially available computer. Data were tested for normality using the D’Agostino and Person omnibus normality test. Data normally distributed are represented by mean (standard deviation [SD]).
2.6. Case survey method to model aggregation
We have used the case survey method that accounts for a well established method to aggregate existing research.[16,17] With the help of a set of structured criteria about the outcomes of interest are ascertained. The answers are determined in the same manner for each of the selected cases, and the resulting data are put in a machine-readable form. Thus, qualitative and descriptive information found in case studies are set in a form susceptible to quantitative analysis.
Our criteria were used as previously defined[18] to demonstrate a cause–effect relationship in TCR.
2.6.1. Plausibility
The appearance of the reflex answer must be explainable by an adequate stimulation of the trigeminal nerve.
The reflex appears promptly after the stimulus is applied.
2.6.2. Reversibility
Stimulus cessation abolished the reflex and cardiopulmonary parameters return to baseline.
2.6.3. Repetition
Reapplication of the stimulus on cranial nerve V will result in similar hemodynamic changes.
2.6.4. Prevention
-
1.
A lighter stimulus of the same type does not lead to the same severe reflex.
-
2.
Trigeminal nerve block abolished the TCR.
-
3.
Application of anticholinergic drugs blocks the occurrence of the reflex.
3. Results
3.1. Summary of literature review
Hitherto, there are only 5 cases that met our strict predefined criteria (Table 1). The mean age was 49.2 years with SD 15.5 years. Female patients comprised 80% of the total cases. In majority of the cases (75%), the level of spine operation was mainly limited to lower lumbar and sacral regions. In all cases, a severe bradycardia/asystole occurred that was treated in 3 out of 5 cases (60%) with atropine.
3.2. Detailed case review
In the first case,[11] a young women (37 years, 95 kg) undergoing lumbosacral (L5–S1) spine decompression developed several episodes of bradycardia during the use of electrocautery.[11] The first 2 episodes of bradycardia were mild (<45 bpm) in nature; however, the other episodes were very severe, and patient's HR dropped down to 10 bpm that was further managed with intravenous anticholinergics/ephedrine. The dural handling during electrocautery was noted during these changes. Similarly, in the second case,[12] a young male (36 years) patient developed 2 episodes of severe bradycardia (<30 bpm) during L4/L5 discectomy under general anesthesia.[12] Due to the persistence and severity of bradycardia, atropine was given intravenously. These hemodynamic changes were also coincided with dural manipulation. Further to this, the exaggerated manifestation of dural manipulation was reported in the third case,[13] in which a 43-year-old male patient who was undergoing L4 to L5 microscopic discectomy developed severe bradycardia followed with asystole. Anticholinergic medication was administered, and cardiopulmonary resuscitation was started even in the prone position, and eventually, the patient was successfully resuscitated.[13] Similar to these reports, the fourth case,[14] a 72-year-old female patient undergoing lower lumbar transforaminal interbody fusion (TLIF) developed severe bradycardia (<38 bpm) and hypotension (60/40 mm Hg) during dilatation near L2 level.[14] Interestingly, after the cessation of stimuli, the HR reverted to normal, but blood pressure remained low for more than a minute. Here also, the author could not exclude the dural manipulation. On the contrary to these 4 cases, fifth case[15] reported a fluctuating type of severe hemodynamic changes during lumbar spine instrumentation in a 58-year-old female patient.[15] In this, during microscopic decompression phase, the patient developed severe hypotensive and hypertensive episodes and abolished after the conclusion of the surgery.
3.3. Aggregation to an emerging model
Each of the 5 cases was included and assessed in the predefined criteria. According to those data aggregation, the cause relationship criteria (temporal precedence, covariation of the cause and effect, and no plausible alternative explanations) were proofed, and the model of SCR established.
3.4. Physioanatomical parts of the model
The efferent part of the model‘s reflex arc is originating from the medulla oblongata and similar that TCR model that is previously described in detail.[2,3,25] The afferent part, however, is as follows:
The nerve supply of the spinal dura mater is via direct branches from the sympathetic chain and via the sinuvertebral nerves that originate from the rami communicantes[32] forming the afferent reflex arc that is carrying the sensation of the action potential to the spinal cord and via not yet described connections to the medulla oblongata.
4. Discussion
In all 5 cases having intraoperative hemodynamic disturbances during spine surgery, no postoperative neurological deficits were noted. In the abovepresented situations, we could rule out other associated factors including anesthetics, bleeding, drug error, venous air embolism, positioning related, and so on and present in every case a clear cause–effect relationship; however, the possibility of direct surgical dural manipulation cannot be ignored. The appearance of the negative chronotropic effect of all these cases was noted during surgical manipulation of dura only (directly and indirectly), which therefore, strongly suggests that neurogenic control does have some link with these observed changes. The most common postulation is the activation of parasympathetic nerves that causes these adverse changes in HR similar to vasovagal reflex. The mechanical stretch of the spinal dura with its intrinsic and extrinsic innervation[19] is the most potent provoking factor in inciting this reflex. As in every reflex, also this spinal reflex has a certain threshold from which the reflex will be triggered, explaining that not every dural manipulation will lead to a reflex response. However, such vasovagal reaction is also and in a substantial part thought to be the manifestation of either pain/fear/other emotional factors and/or decrease in the venous return due to any reason.[20] The usual manifestation of vasovagal includes bradycardia (parasympathetic activation) and/or hypotension (sympathetic inhibition). For the latter mechanism, sudden activation of left ventricle receptors incites a potent depressor response known as Bezold–Jarisch reflex.[21] However, it is also evident that before syncope the heart emptiness or powerful contractions are not required, therefore, postulates some alternative mechanism to explain this abrupt autonomic switch.[22]
In above cases, the trigger remains the dural stretch that may provoke a stretch response similar to TCR reported in various neurosurgical procedures during direct or indirect (e.g., thermic) dural manipulation.[23] According to our definition, the cessation of hemodynamic response after every stop of the spinal dural manipulation (2 major criteria of plausibility and reversibility),[16] the repetitive provocation of the answer (1 minor criteria of repetition), and the prevention by anticholinergic drugs (1 minor criteria of prevention) implicit a full cause–effect relationship as necessary to define a TCR subgroup[2,24–28] that is different from the abovedescribed differential diagnostic nociceptive background. However, the best known “classical” TCR is a brainstem reflex that mediates through trigeminal nerve (afferent) and vagus nerve (efferent), and manifests as bradycardia, hypotension, asystole, gastric hypermotility, and apnea having different subgroups of which the SCR is one of it. However, TCR mechanism is also validated for the non-nociceptive stimulation[2,24–28] and thus gets differentiated from vasovagal reflex in general. Similarly, the SCR should be a separate entity that cannot be the mere reflection of pure vasovagal response. The dural manipulation during surgical decompression/dilatation phase of TLIF provokes parasympathetic nerves stimulation and gives emergent evidence to a different afferent but the similar efferent pathway to the TCR. However, there should be some central connection to the brainstem, which requires explaining various manifestations including bradycardia, hypotension, and respiratory changes noted in these reports.[14,15]
The question remains whether only a few subjects manifested this SCR until now. Answering this must be a goal of future research. Probably, there was not much attention on this entity as it is usually a transient phenomenon or invasive hemodynamic monitoring is rarely used in such cases. The majority of the reported cases were young patients without preexisting cardiac disease, suggesting that younger age may be a risk factor as noted in vasovagal reactions.[2] However, on the contrary, younger patients are not the principal patient population for spinal surgery.[29,30] Interestingly, we also noted a female preponderance in this study. Whether or not female gender could be a risk factor for inciting SCR episodes (as indicated in 80% of the cases) is need to be further elaborated. However, human and animal data show a substantial gender differences in basal function of the autonomic nervous system. It highlights females having higher parasympathetic, cardiac autonomic activity.[31]
5. Limitation
The present review is based on the guidelines for conducting a systemic examination; however, only a few reports could be retrieved for the final inclusion, therefore, should not be considered as noble systemic review or meta-analysis. Therefore, it represents more hypothetical yet logical knowledge about the negative chronotropic effects of lumbar spine surgeries. However, by using this mixed methodological approach, we could aggregate the available data to a model. Second, the presence of this phenomenon highlighted in above 5 reports further questions about the importance of SCR in the lumbar spine surgeries. However, it should also be noted that in most of the lumbar laminectomies, discectomies, and minimally invasive spine surgeries, the invasive arterial line monitoring is rarely used, and NIBP is usually set up 3- to 5-minute interval that can easily miss the relevant blood pressure variations.
We have relative strict inclusion criteria (including definition of the reflex) so that our review certainly has an under-representation of this spinal reflex. However, we have also certainly no false positive cases included, which was the principal goal of this study.
6. Conclusion
The presented review and consecutive data aggregation (based on accepted cause–effect relationship criteria) to a model of a reflex arc show, for the first time, the existence of a SCR during direct or indirect manipulation of the spinal dura when performing spinal surgery. The exact incidence and predisposing factors of this reflex should not be related to this review and requires further well designed prospective studies.
Footnotes
Abbreviations: HR = heart rate, SCR = spinal cardiac reflex, SD = standard deviation, TCR = trigeminocardiac reflex, TLIF = transforaminal interbody fusion.
TC and BS are the academic editors of medicine.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Chowdhury T, Petropolis A, Cappellani RB. Cardiac emergencies in neurosurgical patients. Biomed Res Int 2015;2015:751320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chowdhury T, Mendelowitz D, Govlanov E, et al. Trigeminocardiac reflex: an update of the current clinical and physiological knowledge. J Neurosurg Anesthesiol 2015;27:136–47. [DOI] [PubMed] [Google Scholar]
- [3].Schaller B, Cornelius JF, Prabhakar H, et al. The trigemino-cardiac reflex: an update of the current knowledge. J Neurosurg Anesthesiol 2009;21:187–95. [DOI] [PubMed] [Google Scholar]
- [4].Arasho B, Sandu N, Spiriev T, et al. Management of the trigeminocardiac reflex: facts and own experience. Neurol India 2009;57:375–80. [DOI] [PubMed] [Google Scholar]
- [5].Schaller BJ, Filis A, Buchfelder M. Trigemino-cardiac reflex in humans initiated by peripheral stimulation during neurosurgical skull-base operations. Its first descriötion. Acta Neurochir (Wien) 2008;150:715–8. [DOI] [PubMed] [Google Scholar]
- [6].Schaller BJ. Trigeminocardiac reflex. J Neurosurg 2007;107:243. [DOI] [PubMed] [Google Scholar]
- [7].Sandu N, Sadr-Eshkevari P, Schaller BJ. Usefulness of case reports to improve medical knowledge regarding trigemino-cardiac reflex in skull base surgery. J Med Case Report 2011;5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chowdhury T, Sandu N, Meuwly C, et al. Trigeminocardiac reflex: differential behavior and risk factor in the course of the trigeminal nerve. Future Neurol 2014;9:41–7. [Google Scholar]
- [9].Schaller BJ, Filis A, Buchfelder M. Cardiac autonomic control in neurosurgery: the example of trigemino-cardiac reflex. Arch Med Sci 2007;3:287–92. [Google Scholar]
- [10].Spiriev T, Sandu N, Kondoff S, et al. Tic and autonomic symptoms. J Neurosurg 2012;116:1397–8. [DOI] [PubMed] [Google Scholar]
- [11].Deschamps A, Carvalho G. Lumbo-sacral spine surgery and severe bradycardia (Letter). Can J Anesth 2004;51:277.Erratum: Can J Anesth 2004; 51: 643. [DOI] [PubMed] [Google Scholar]
- [12].Mandal N. More on lumbo-sacral spine surgery and bradycardia (Letter). Can J Anaesth 2004;51:942. [DOI] [PubMed] [Google Scholar]
- [13].Dooney N. Prone CPR for transient asystole during lumbosacral spinal surgery. Anaesth Intensive Care 2010;38:212–3. [PubMed] [Google Scholar]
- [14].Chowdhury T, Sapra H, Dubey S. Severe hypotension in transforaminal lumbar interbody fusion surgery: Is it vasovagal or? Asian J Neurosurg. [Publish ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chowdhury T, Narayanasamy S, Dube SK, et al. Acute hemodynamic disturbances during lumbar spine surgery. J Neurosurg Anesthesiol 2012;24:80–1. [DOI] [PubMed] [Google Scholar]
- [16].Lucas WA. The case survey method: aggregating case experience. San Diego:Rand Corp; 1974. [Google Scholar]
- [17].Sandu N, Chowdhury T, Schaller BJ. How to apply case reports in clinical practice using surrogate models via example of the trigeminocardiac reflex. J Med Case Rep 2016;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Meuwly C, Golanov E, Chowdhury T, et al. Trigeminal cardiac reflex: new thinking model about the definition based on a literature review. Medicine (Baltimore) 2015;94:e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chowdhury T, Meuwly C, Sandu N, et al. Coronary spasm in neurosurgical patients and role of trigeminocardiac reflex. Neurol Res Int 2014;2014:974930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kinsella SM, Tuckey JP. Perioperative bradycardia and asystole: relationship to vasovagal syncope and the Bezold-Jarisch reflex. Br J Anaesth 2001;86:859–68. [DOI] [PubMed] [Google Scholar]
- [21].Hainsworth R. Reflexes from the heart. Physiol Rev 1991;71:617–58. [DOI] [PubMed] [Google Scholar]
- [22].Hainsworth R. Syncope: what is the trigger? Heart 2003;89:123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med 2002;347:878–85. [DOI] [PubMed] [Google Scholar]
- [24].Groen GJ, Baljet B, Drukker J. The innervation of the spinal dura mater: anatomy and clinical implications. Acta Neurochir 1988;92:39–46. [DOI] [PubMed] [Google Scholar]
- [25].Schaller B, Probst R, Strebel S, et al. Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg 1999;90:215–20. [DOI] [PubMed] [Google Scholar]
- [26].Schaller B. Trigeminocardiac reflex. J Neurol 2004;251:658–65. [DOI] [PubMed] [Google Scholar]
- [27].Meuwly C, Chowdhury T, Sandu N, et al. Anesthetic influence on occurrence and treatment of the trigemino-cardiac reflex: a systematic literature review. Medicine (Baltimore) 2015;94:e807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chowdhury T, Ahuja N, Schaller B. Severe bradycardia during neurosurgical procedure: depth of anesthesia matters and leads to a new surrogate model of the trigeminocardiac reflex: a case report. Medicine (Baltimore) 2015;94:e2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sandu N, Pöpperl G, Toubert ME, et al. Current molecular imaging of spinal tumors in clinical practice. Mol Med 2011;17:308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sandu N, Schaller B, Arasho B, et al. Wallis interspinous implantation to treat degenerative spinal disease: description of the method and case series. Expert Rev Neurother 2011;11:799–807. [DOI] [PubMed] [Google Scholar]
- [31].Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc Res 2002;53:678–87. [DOI] [PubMed] [Google Scholar]
- [32].Groen GJ, Baljet B, Drukker J. The innvervation of the spinal dura mater: anatomy and clinical implications. Acta Neurochir (Wien) 1988;92:39. [DOI] [PubMed] [Google Scholar]


