Abstract
Reducing low-density lipoprotein cholesterol (LDL-C) to target ≤1.81 mmol/L is a common therapeutic goal after acute coronary syndrome (ACS). This study aimed to examine the factors associated with reaching or not this LDL-C target after 1 year of optimal statin therapy postpercutaneous coronary intervention (PCI). This was a retrospective study of 633 consecutive prospectively enrolled patients with ACS treated between January 2011 and December 2012 at the Beijing Hospital (China). All patients were treated with PCI and statins for 1 year. A multivariate analysis was carried out to identify the factors associated with reaching the LDL-C target of ≤1.81 mmol/L. The rate of unreached LDL-C goal after 1 year was 48%. Compared with those who achieved their LDL-C goal, patients not achieving their LDL-C goal showed a higher proportion of females (37.9% vs 28.7%, P < 0.001), higher LDL-C levels at admission (2.82 ± 0.75 vs 2.08 ± 0.70 mmol/L, P < 0.001), lower proportion of patients with a history of PCI (17.6% vs 24.8%, P = 0.03), and younger age (66.7 ± 10.6 vs 68.9 ± 10.1 years, P = 0.009). A multivariate analysis showed that lower LDL-C levels on admission were predictive of LDL-C goal achievement (odds ratio [OR] = 4.81; 95% confidence interval [CI]: 3.46–6.70; P < 0.001), together with older age (OR: 0.98; 95% CI: 0.96–0.997; P = 0.026), and male gender (OR: 0.64; 95% CI: 0.42–0.98; P = 0.040). Higher LDL-C levels at admission, younger age, and female gender were independently associated with not reaching the LDL-C target after 1 year of optimal statin therapy after PCI.
Keywords: acute coronary syndrome, coronary artery disease, low-density lipoprotein cholesterol, percutaneous coronary intervention, statin, treatment target
1. Introduction
Acute coronary syndrome (ACS) refers to a wide spectrum of diseases caused by acute myocardial ischemia and/or necrosis secondary to reduced coronary blood flow caused by unstable angina, non-ST-elevation myocardial infarction, or ST-elevation myocardial infarction.[1–4] There are about 230 million patients with cardiovascular diseases in China alone.[5] Age, male gender, dyslipidemia, obesity, tobacco exposure, diabetes, hypertension, and a previous history of cardiovascular diseases are major risk factors for ACS.[3,4] Elevated low-density lipoprotein cholesterol (LDL-C) is an independent risk factor for coronary heart disease (CHD).[6] Epidemiological studies and clinical trials have shown that plasma cholesterol levels are linearly correlated with the prognosis of CHD.[7–9]
Lipid-lowering therapy is used to reduce the risk of recurrent cardiovascular events among patients with ACS. Nevertheless, since the primary aim of LDL-C-lowering therapy is the prevention of a novel coronary event, risk stratification is needed to be determined before treatment.[10] The latest guidelines and studies suggest that LDL-C target for patients with ACS should be ≤1.81 mmol/L after percutaneous coronary intervention (PCI).[10–12] Supporting this target, previous studies suggested that cardiovascular events could be reduced by 20% to 50% when LDL-C levels are decreased to ≤1.81 mmol/L.[13,14] Statins are presently the main drugs used to decrease LDL-C levels; their efficacy in reducing secondary coronary events is well-established.[8,13,14]
PCI is presently the gold standard treatment for ACS, especially for patients with significant left main coronary artery disease, patients with 3-vessel disease, or patients with suboptimal revascularization and ongoing symptoms despite maximal nonsurgical therapy.[1,2] These patients are considered at high recurrence risk and their LDL-C levels should be ≤1.81 mmol/L.[1,2] The DYSIS-China study showed that the global proportion of patients reaching this LDL-C target was only 61.5%, and even reaching proportions as low as 39.7% and 54.8% in very high-risk and high-risk patients, respectively.[15] A previous study from Hong Kong showed a low rate of reaching LDL-C goals at discharge and during follow-up.[16] In addition, most studies assessing LDL-C goal attainment were carried out in Caucasians, and few results are available for Chinese.
Therefore, the aim of the present study was to examine the rate of patients reaching the LDL-C target of <1.81 mmol/L after 1 year of post-PCI statin therapy and the factors associated with goal fulfillment. These results could allow the early identification of patients needing a closer follow-up, leading to better treatment outcomes after PCI.
2. Methods
2.1. Study design
This was a retrospective study of consecutive, prospectively enrolled patients with ACS treated between January 1st, 2011 and December 31st, 2012 at the Department of Cardiology of Beijing Hospital (China). Four cardiologists participated in the original study. The study was approved by the ethics committee of the Beijing Hospital. All patients provided a written informed consent.
2.2. Patients
The inclusion criteria were: available coronary angiography showing stenosis ≥75% (or ≥50% for the left main coronary artery) in at least 1 main coronary artery (left main coronary artery, left anterior descending artery, circumflex branch of left coronary artery, or right coronary artery); underwent PCI; and had been treated with optimal statin regimen for 1 year after PCI.
The exclusion criteria were: allergy or intolerance to statins; stroke or history of visceral bleeding disorder within 6 months; severe liver disease or coagulation abnormalities; history of valvular heart disease, cardiomyopathy, myocarditis, congenital heart disease, peripheral vascular disease, or infective endocarditis; chronic kidney disease stage >III; cancer patients with a life expectancy <1 year; chronic heart failure; or any serious disease that could affect the short-term prognosis.
After screening the 1000 eligible subjects of the original prospective study, 300 were excluded (210 patients with ACS symptoms but angiography findings did not meet the stenosis criteria, 30 patients underwent coronary artery bypass graft, 10 patients refused PCI, and 50 patients declined participation) and 700 patients met the criteria and were included in the present retrospective study. All these subjects had received secondary prevention for CAD and were followed up for 1 year after PCI. Sixty-seven (9.6%) patients were lost to follow-up and 1-year follow-up data were available for 633 patients (90.4%).
2.3. Data collection
Data were prospectively recorded and included gender, age, and risk factors (diabetes, obesity, hypertension, and family history) at baseline. Serum levels of total cholesterol, LDL-C, high-density lipoprotein cholesterol, and triglycerides (TG) were collected at admission and after 1 year of treatment. Hypertension was defined as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg, or taking antihypertensive medication.[17] Diabetes was defined as fasting plasma glucose levels ≥7.0 mmol/L, postprandial glucose levels ≥11.1 mmol/L, and/or glycated hemoglobin ≥6.5%, or taking antidiabetes medication.[18] Obesity was defined as a body mass index ≥30.0 kg/m2.[19] Smoking was defined as using tobacco at admission.
2.4. Treatments
PCI was performed in a standard manner. Patients received aspirin (100–300 mg/day) and clopidogrel (300–600 mg) at least 2 hours before PCI. Glycoprotein IIb/IIIa inhibitors were used at the surgeon's discretion. Angiographic success was defined as a final stenosis <20%.
An aspirin dose of 300 mg/day was prescribed for 1 to 3 months, followed by 100 mg/day lifelong. Clopidogrel (75 mg/day) was given for at least 1 year after PCI.
Patients received statin treatment according to the clinical opinion of their treating physician and according to current guidelines.[1–3,10] Treatment included atorvastatin 20 mg/day, rosuvastatin 10 mg/day, pravastatin 40 mg/day, fluvastatin 80 mg/day, and simvastatin 20 mg/day. Compliance was self-reported.
2.5. LDL-C goal
The LDL-C goal of ≤1.81 mmol/L was selected according to the 2007 Chinese adult dyslipidemia Prevention Guidelines.[20] LDL-C levels were determined using the Friedewald equation.[21] Patients were grouped according to whether they had reached the <1.81 mmol/L goal by 1 year after PCI or not.
2.6. Statistical analysis
Results were presented as mean ± standard deviation or median with interquartile range, as appropriate. Non-normally distributed data (according to the Kolmogorov–Smirnov test) were log-transformed to normalize their distribution. One-way ANOVA with the LSD post hoc test was used for data analysis. Categorical data were presented as proportions and analyzed using the Chi-square test. Variables that were significantly associated with the outcome in univariate analyses were entered in a multiple logistic regression model to examine the association of the baseline parameters with reaching the LDL-C target. Statistical analyses were performed with SPSS 17.0 (IBM, Armonk, NY). Two-sided P-values <0.05 were considered statistically significant.
3. Results
3.1. Characteristics of the patients
Table 1 presents the characteristics of the patients. Mean age was 67.7 years, and 43.5% of the patients were female. Among the 633 patients, only 48% reached the LDL-C goal after 1 year of post-PCI statin treatment. Compared with those who reached the LDL-C goal, patients who did not reach the goal displayed a higher proportion of females (37.9% vs 29.7%, P = 0.02) and these patients were younger (66.7 ± 10.6 vs 68.9 ± 10.1 years, P = 0.009). There were no significant differences in body mass index, hypertension, smoking, hyperlipidemia, diabetes, and stroke between the 2 groups. There was a higher proportion of patients with a history PCI among the patients reaching the goal (24.8% vs 17.6%, P = 0.03). The baseline values of TG, total cholesterol, and LDL-C were higher among the patients who did not reach the goal compared with those who did (all P < 0.001).
Table 1.
Baseline characteristics of the patients according to whether or not they reached the LDL-C target of ≤1.81 mmol/L by one year after PCI.
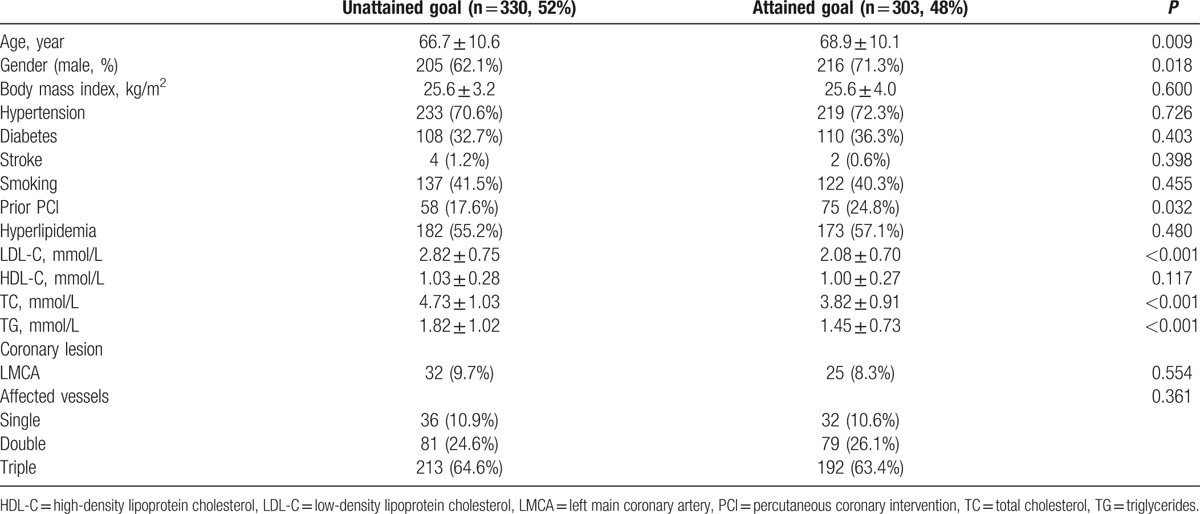
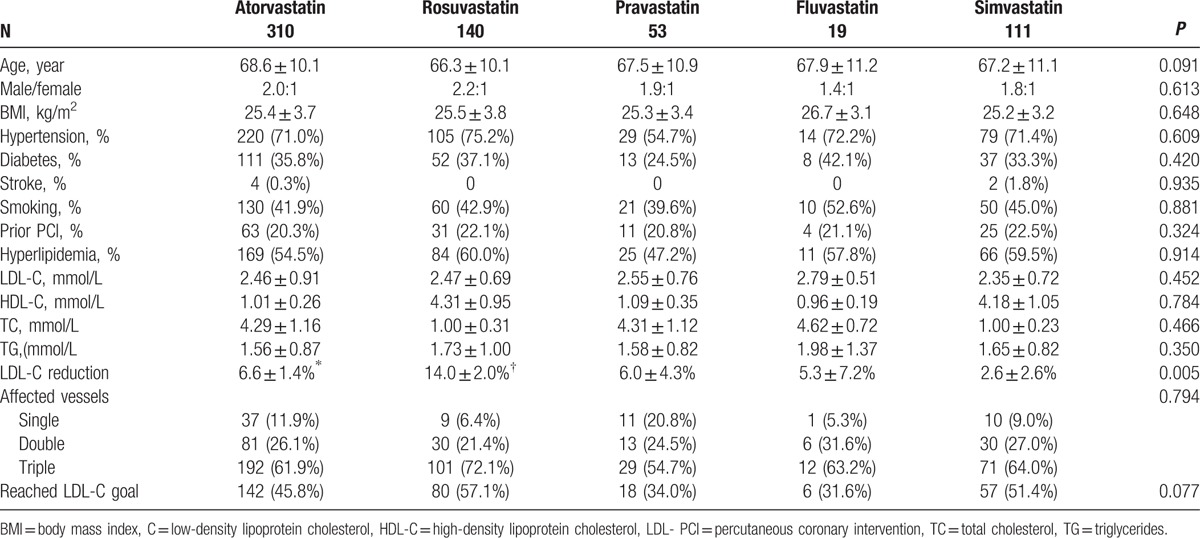
Table 2 shows that there were no differences among patients undergoing different statin treatments (all P > 0.05), except that rosuvastatin led to a higher reduction of LDL-C levels than the other statins (P < 0.05). There were no differences in the proportion of patients reaching the LDL-C goal among the different treatments (P > 0.05).
Table 2.
Characteristics of the patients according to the statin therapy.
3.2. Predictors of reaching the LDL-C target
The multivariate analysis showed that lower LDL-C levels on admission were predictive of reaching the LDL-C goal (odds ratio [OR] = 4.81; 95% confidence interval [CI]: 3.46–6.70; P < 0.001), together with older age (OR: 0.98; 95% CI: 0.96–0.997; P = 0.026), and male gender (OR: 0.64; 95% CI: 0.42–0.98; P = 0.040) (Table 3).
Table 3.
Multivariate logistic regression analysis showing the baseline factors independently associated with therapeutic target achievement after one year of statin treatment.

4. Discussion
Reducing LDL-C to ≤1.81 mmol/L is a common therapeutic goal after ACS,[1–3,10] but there is a lack of real-world data about the proportion of patients with ACS reaching this LDL-C goal after 1 year of post-PCI statin therapy. Therefore, the aim of the present study was to examine the rate of patients reaching the LCL-C target of ≤1.81 mmol/L of post-PCI statin treatment and the factors associated with goal fulfillment. Results showed that lower LDL-C levels at admission were predictive of LDL-C goal achievement, together with older age and male gender.
The present study showed that after 1 year of post-PCI conventional statin therapy in high-risk patients, the proportion of patients reaching the LDL-C goal was 48%. This rate was higher than that of the DYSIS-China study (38%)[15] and a study from Hong Kong,[16] but these previous studies used a primary goal of 2.6 mmol/L instead of 1.81 mmol/L, but the Hong Kong study suggested that further LDL-C reduction below 2.6 mmol/L did not result in any additional benefit.[16] Indeed, based on the survival curves in relation to LDL-C levels, no additional benefit was observed below 2.4 mmol/L.
There are a number of possible reasons for discrepancies among studies. First, with the implementation of recent guidelines, physicians have an improved awareness of the LDL-C goals for very high-risk patients. Second, differences in follow-up time could play a role. Third, the proportion of high-risk patients in the DYSIS-China study was lower than in the present study. Fourth, the distributions of statin treatments were different among studies. Finally, the differences among studies could be due to study population and target LDL-C levels. Indeed, this could be due to ethnic differences between the Chinese and Caucasian populations, leading to the inapplicability of the thresholds used in Western populations.[16] Additional studies are still necessary to examine the exact outcomes of LDL-C reduction in these patients.
Different statins have different LDL-C reduction potency. The magnitude of LDL-C reduction observed in the present study is comparable to that reported by a meta-analysis.[22] The present study showed that rosuvastatin had the best LDL-C-lowering effect compared with the other statins. Nevertheless, the rates of achieving the LDL-C goal were similar among the different statin treatments. A possible explanation is that patients with a greater number of factors of poor prognosis could be more aggressively treated with more potent statins, but the present study was not designed to assess this factor and additional studies are necessary.
The present study showed that there was a higher proportion of females among the patients not reaching the LDL-C goals, which is supported by a previous study.[23] Therefore, it could be hypothesized that female patients with high-risk CAD should receive more attention during postoperative management. Female gender is generally considered a protective factor against CHD, but this protective effect disappears after menopause. Since the mean age was around 67 to 69 years in the present study, it could be supposed that most of these women were menopausal,[24] but this specific factor was not examined in the present study.
The rates of reaching the LDL-C goal was higher in patients with a PCI history, which could be related to these patients being more aggressively treated, as previously suggested.[25,26] Indeed, previous studies showed that target achievement was associated with compliance and that compliance was associated with a higher number of follow-up visits.[25,26] TG levels in patients who did not reach the LDL-C goal were higher than those in patients who reached the goal (P < 0.001). European guidelines[23] recommend that TG-targeting treatment should be started when TG levels are >1.7 mmol/L in high-risk CAD patients. Diabetes mellitus is often combined with hypertriglyceridemia[25,26] and some studies showed that the rates of achieving the LDL-C goal was lower in ACS patients with diabetes mellitus combined with hypertriglyceridemia,[27] but the present study and a recent study[22] did not reach this conclusion. Additional studies are necessary to clarify these points.
The present study is not without limitations. First, it was from a single hospital and only involved patients who were managed by cardiologists. Therefore, these findings may not reflect the trends in achieving lipid goals in other Asian countries or the practices of primary care physicians. Second, because of the retrospective nature of the study, some data were not available. Menopause has a strong influence on the CAD risk in women, but it was not collected. The exact dose of statin was not collected either, which constitutes a major limitation. Third, only patients who had complete lipid profile data were included. Patients without recent lipid profiles could be more likely to be undertreated, and the present study may have underestimated the actual number of patients who are achieving their lipid goals in clinical practice. Fourth, patients could not be stratified according to the type of ACS they experienced before PCI. Finally, the patients were followed up for 1 year only, preventing the observation of the long-term effects of statin therapy after PCI, as well as the prognosis of the patients. The results of the present study need to be confirmed by multicenter studies and larger sample size.
5. Conclusions
In conclusion, among patients treated with statin after PCI for ACS, 48% did not reach the LDL-C target of <1.81 mmol/L after 1 year of optimal post-PCI statin treatment. The multivariate analysis suggested that higher LDL-C levels at admission, younger age, and female gender were independently associated with not reaching the LDL-C target after 1 year of optimal statin therapy after PCI. Additional multicenter studies are necessary to confirm and refine these results, but young or female patients should be given a closer follow-up after PCI and more aggressive lipid-lowering could be necessary, including diet and a combination of drugs.
Footnotes
Abbreviations: ACS = acute coronary syndrome, CHD = coronary heart disease, LDL-C = low-density lipoprotein cholesterol, PCI = percutaneous coronary intervention, TG = triglycerides.
Ethics approval and consent to participate: The study was approved by the ethics committee of the Beijing Hospital. All patients provided a written informed consent.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007;50:e1–57. [DOI] [PubMed] [Google Scholar]
- [2].Jneid H, Anderson JL, Wright RS, et al. 2012ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012;60:645–81. [DOI] [PubMed] [Google Scholar]
- [3].Amsterdam EA, Wenger NK, Brindis RG, et al. 2014AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:e344–426. [DOI] [PubMed] [Google Scholar]
- [4].Braunwald E. Unstable angina and non-ST elevation myocardial infarction. Am J Respir Crit Care Med 2012;185:924–32. [DOI] [PubMed] [Google Scholar]
- [5].Li H, Ge J. Cardiovascular diseases in China: current status and future perspectives. Ijc Heart & Vasculature 2015;6:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pinto X, Meco JF, Corbella E, et al. [Secondary Preventive Program of atherosclerosis in a university hospital. Results and predictors of clinical course]. Med Clin (Barc) 2003;120:768–72. [DOI] [PubMed] [Google Scholar]
- [7].Cannon CP, Maraganore JM, Loscalzo J, et al. Anticoagulant effects of hirulog, a novel thrombin inhibitor, in patients with coronary artery disease. Am J Cardiol 1993;71:778–82. [DOI] [PubMed] [Google Scholar]
- [8].Pedersen TR. Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9. [PubMed] [Google Scholar]
- [9].Hulten E, Jackson JL, Douglas K, et al. The effect of early, intensive statin therapy on acute coronary syndrome: a meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:1814–21. [DOI] [PubMed] [Google Scholar]
- [10].Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]
- [11].Melloni C, Shah BR, Ou FS, et al. Lipid-lowering intensification and low-density lipoprotein cholesterol achievement from hospital admission to 1-year follow-up after an acute coronary syndrome event: results from the Medications ApplIed aNd SusTAINed Over Time (MAINTAIN) registry. Am Heart J 2010;160: 1121-9, 9 e1. [DOI] [PubMed] [Google Scholar]
- [12].Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- [13].Baigent C, Blackwell L, et al. Cholesterol Treatment Trialists Collaboration Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kearney PM, Blackwell L, et al. Cholesterol Treatment Trialists Collaboration Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–25. [DOI] [PubMed] [Google Scholar]
- [15].Wang F, Ye P, Hu D, et al. Lipid-lowering therapy and lipid goal attainment in patients with metabolic syndrome in China: subgroup analysis of the Dyslipidemia International Study-China (DYSIS-China). Atherosclerosis 2014;237:99–105. [DOI] [PubMed] [Google Scholar]
- [16].Lee VW, Chau RY, Cheung HY, et al. How low should we target the LDL goal to improve survival for acute coronary syndrome patients in Hong Kong? BMC Cardiovasc Disord 2015;15:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- [18].American Diabetes Association Standards of medical care in diabetes-2016 abridged for primary care providers. Clin Diabetes 2016;34:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kushner RF, Ryan DH. Assessment and lifestyle management of patients with obesity: clinical recommendations from systematic reviews. JAMA 2014;312:943–52. [DOI] [PubMed] [Google Scholar]
- [20].Joint Committee for Developing Chinese Guidelines on Prevention and Treatment of Dyslipidemia in Adults Chinese guidelines on prevention and treatment of dyslipidemia in adults. Chin J Cardiol 2007;35:390–419. [PubMed] [Google Scholar]
- [21].Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- [22].Naci H, Brugts JJ, Fleurence R, et al. Dose-comparative effects of different statins on serum lipid levels: a network meta-analysis of 256,827 individuals in 181 randomized controlled trials. Eur J Prev Cardiol 2013;20:658–70. [DOI] [PubMed] [Google Scholar]
- [23].Santos RD, Waters DD, Tarasenko L, et al. Low- and high-density lipoprotein cholesterol goal attainment in dyslipidemic women: The Lipid Treatment Assessment Project (L-TAP) 2. Am Heart J 2009;158:860–6. [DOI] [PubMed] [Google Scholar]
- [24].Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- [25].Martin SS, Gosch K, Kulkarni KR, et al. Modifiable factors associated with failure to attain low-density lipoprotein cholesterol goal at 6 months after acute myocardial infarction. Am Heart J 2013;165:26–33.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA 2007;297:177–86. [DOI] [PubMed] [Google Scholar]
- [27].Chapman MJ, Ginsberg HN, Amarenco P, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011;32:1345–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


