Abstract
The long-term clinical impact of premature ventricular complexes (PVCs) on mortality and morbidity has not been fully studied. This study aimed to investigate the association between the burden of PVCs and adverse clinical outcome.
A total of 5778 subjects, who were pacemaker-free and ventricular tachycardia-free at baseline, received 24-hour electrocardiography monitoring between January 1, 2002 and December 31, 2004. Clinical event data were retrieved from the Bureau of National Health Insurance of Taiwan. Multivariate Cox hazards regression models and propensity-score matching were applied to assess the association between PVCs and adverse clinical outcome.
Average follow-up time was 10�± 1 year. In all, 1403 subjects expired, 1301 subjects were hospitalized in the cardiovascular (CV) ward, 3384 were hospitalized for any reason, and 631 subjects developed new-onset heart failure (HF). The optimal cut-off PVC frequency (12 beats per day) was obtained through receiver operator characteristic curves, with a sensitivity of 58.4% and specificity of 59.8%. Upon multivariate analysis, a PVC frequency >12 beats per day was an independent predictor for all mortality (hazard ratio [HR]: 1.429, 95% confidence interval [CI]: 1.284–1.590), CV hospitalization (HR: 1.127, 95% CI: 1.008–1.260), all-cause hospitalization (HR 1.094, 95% CI: 1.021–1.173), and new-onset HF (HR: 1.411, 95% CI: 1.203–1.655). Subjects with a PVC frequency >12 beats per day had an increased risk of cardiac death attributable to HF and sudden cardiac death. The incidence rates for mortality and HF were significantly increased in cases of raised PVC frequency. Propensity-score matching analysis also echoed the main findings.
Increased PVC burden was associated with a higher incidence of all-cause mortality, CV hospitalization, all-cause hospitalization, and new-onset HF which was independent of other clinical risk factors.
Keywords: heart failure, mortality, premature ventricular complexes
1. Introduction
A premature ventricular complex (PVC) is an early depolarization of ventricular myocardium. PVCs are common findings on electrocardiography (ECG) in the general population and are associated with structural heart disease and increased risk of sudden cardiac death.[1,2] The effect of PVC frequency on the incidence of congestive heart failure (HF) or mortality from PVCs, in the general population, remains unknown. Reports have suggested that frequent PVCs increase the risk of sudden cardiac death, cardiovascular (CV) events, and left ventricular systolic dysfunction.[3–11] The definition of high frequency PVCs can vary from 10,000 to 20,000�PVCs/day based on the particular study consulted.[11–13] Because of the pervasiveness of PVCs in the general population,[2,14] it is important to understand the association between PVC frequency and clinical outcome.
The aim of this study was to determine the optimal cut-off of PVC frequency for predicting mortality. In addition, this study sought to assess the impact of low frequency PVCs, based on 24-hour ECG monitoring, on mortality, CV hospitalization, all-cause hospitalization, and HF during long-term clinical follow-up.
2. Methods
2.1. Study design
The Institutional Review Board at Taipei Veterans General Hospital, Taipei, Taiwan approved this study (VGH-IRB Number: 2013-08-002AC#1). This retrospective, observational study was based on the Registry of 24-hour Electrocardiographic Monitoring at Taipei Veterans General Hospital (REMOTE database). The study population included consecutive clinic patients who received 24-hour ECG monitoring between January 1, 2002 and December 31, 2004.
2.2. Subjects
The indication for 24-hour Holter monitoring included palpitations, syncope, and clinical follow-up. The International Classification of Diseases, Ninth Revision codes were used to code baseline diseases present before enrollment. Variables (including comorbidities and medications) were collected from diagnoses of hospital discharge, outpatient department, emergency department visits, and the Collaboration Center of Health Information Application (CCHIA), Ministry of Health and Welfare in Taiwan. The diagnoses used for analysis were recorded twice in the outpatient department records, or at least once in the discharge summary. Patients with sustained ventricular tachycardia, permanent pacemaker, or history of catheter ablation were excluded. The final cohort included 5778 patients for analysis. Baseline disease was confirmed by history and physical examination, and medical record review. The methodology was validated in previous publications.[15–17]
2.3. Risk factors/variables
Data were collected based on demographic characteristics (age and gender) from the medical records. Target comorbidities, such as diabetes mellitus, hypertension, coronary artery disease, HF, chronic kidney disease, liver disease, history of myocardial infarction, and valvular heart disease, were determined using the International Classification of Diseases, Ninth Revision codes from the medical charts at the time of examination. All target comorbidities were confirmed based on verification of the medical records. Left ventricular ejection fraction data were collected from the echocardiography report in every documented HF patient. The New York Heart Association functional classification and medication data were determined from the medical and nursing charts. Baseline atrial fibrillation (AF) was based on baseline 12-lead ECG or on baseline Holter monitoring. The use of antiarrhythmic drugs (class I or III) and antihypertensive medication (including beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors [ACEI]/angiotensin II receptor blockers [ARB], diuretics, and alpha-blockers) was determined by medical chart review. All target comorbidities were validated by physical examination, physician report, medical record review, and CCHIA.
2.4. Follow-up and event determination
Patients with regular medication received regular follow-up at 1 to 3 month intervals. Patients without regular medication received follow-up every year or after new events depending on the clinical course. The follow-up data were retrieved from Taipei Veterans General Hospital, Taiwan National Health Insurance Research Database, and the database of the CCHIA which had been previously validated.[16,18,19] The primary endpoint of this study was all-cause mortality. The secondary endpoints were hospitalization for CV-related conditions, all-cause hospitalization, new-onset AF, and new-onset HF. Mortality data were retrieved from the CCHIA and further confirmed by linking with the National Death Registry.[19] New-onset HF and new-onset AF were identified and validated by echocardiographic result and ECG report. Follow-up was defined from the beginning of the registry to February 28, 2013.
2.5. PVC assessment
The details of Holter monitoring (Medilog FD4, Oxford Instruments) were reported in the previous publication.[17] The sampling rate was 2048 Hz. PVC was identified by a simultaneous 3-channel 24 hour Holter monitoring and manually edited by 2 experienced technicians. All the arrhythmic episodes and unknown strips were reviewed by 2 cardiologists again and confirmed by 1 cardiac electrophysiologist.
2.6. Statistical analysis
All analyses were performed using SPSS statistical software, version 20.0. Baseline patient characteristics are reported as means ± standard deviations for continuous variables, and as percentages for categorical variables. Receiver operator characteristic (ROC) curves and areas under the curves (AUCs) were analyzed to determine the optimal cut-off point for all-cause of death (Youden index). The final result of the ROC indicated an optimal cut-off point of 12 PVCs in a 24-hour ECG monitoring period (12 PVCs per day) as a predictor for adverse outcome. Baseline characteristics between the 2 groups (PVC frequency >12/day and ≦12/day) were compared using Student t test for continuous variables. The chi-square test with Yates correction was used to analyze the categorical variables.
Crude event rates from the 10-year Kaplan–Meier survival curves were compared between the 2 groups using the log-rank test for a given endpoint.
The relative risk for a given endpoint associated with PVC burden was estimated by calculating the hazard ratio (HR) using a Cox proportional hazards regression model. This model was run for all parameters that had a P-value < 0.05 at baseline (ie, age, gender, hypertension, coronary artery disease, previous myocardial infarction, diabetes mellitus, valvular heart disease, HF, and medication with ACEI/ARB or diuretics). Comparisons between the 2 groups for cause of death were performed using the chi-square test for categorical variables. The HRs of PVCs in different subgroups of patients with individual risk factors are shown in a forest plot (Fig. 1). Propensity-score matching by nearest-neighbor matching was also used to control all confounders.[20,21] A Cox proportional hazards model was applied to examine the risk of frequent PVCs. To determine whether inclusion of PVCs in the model improved the predictive power, discrimination tests were performed with the integrated discrimination index.[22,23] For the old model, markers for the outcome (ie, age, gender, hypertension, coronary artery disease, previous myocardial infarction, diabetes mellitus, HF, valvular heart disease, and medication with ACEI/ARB or diuretics) were used.
Figure 1.
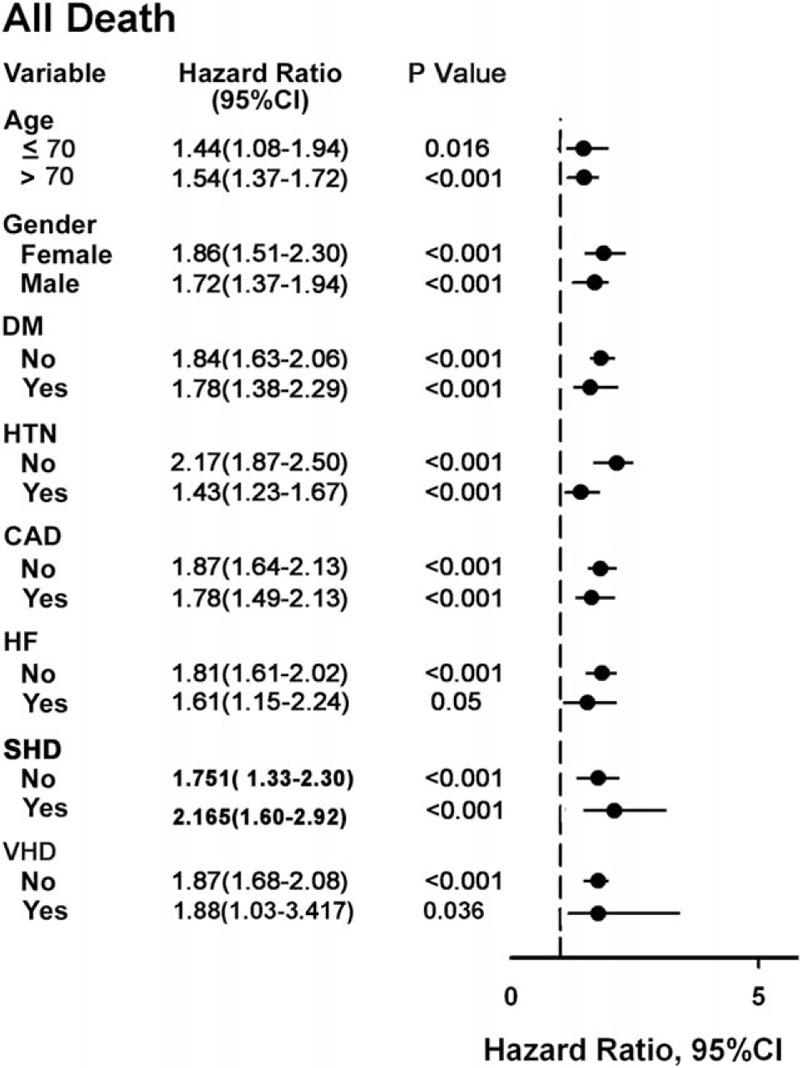
Forest plot for subgroup analysis for all-cause mortality. CAD�=�coronary artery disease, CI�=�confidence interval, DM�=�diabetes mellitus, HTN�=�hypertension, SHD�=�structural heart disease, VHD�=�valvular heart disease.
For the cumulative effect of PVC burden, patients were further classified into no PVC group and PVC group. The PVC group was further classified into 4 quartiles according to their PVC burdens. The 3 cut-off points for the PVC group were 25 quintile, 50 quintile, and 75 quintile. Comparisons among incidence of mortality, all-caused hospitalization, CV hospitalization, and new-onset HF were performed.
3. Results
3.1. Baseline characteristics
All 5778 patients were followed up for 10 ± 1 year by outpatient clinical visits, emergency room visit records, hospitalization medical records, and the CCHIA. During follow-up, 1403 (24.3%) patients expired, 1301 (22.5%) patients were hospitalized in the CV ward, 3384 (58.6%) patients were hospitalized for any reason, and 631 (10.9%) patients were newly diagnosed with HF.
The optimal cut-off for PVC beats per 24 hours for predicting all-cause mortality was 12 PVCs per day, with a sensitivity of 58.3% and specificity of 59.8% (area under the ROC curve: 59.6%, Fig. 2).
Figure 2.
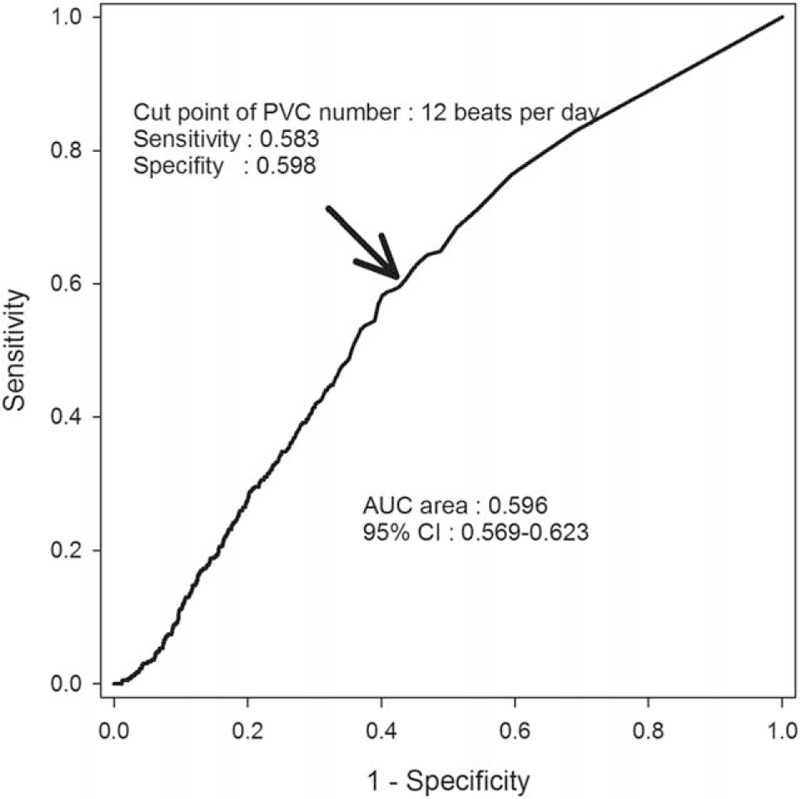
ROC curve survival analysis by PVC numbers. PVC indicates premature ventricular complex. PVC = premature ventricular complex, ROC�=�receiver operator characteristic.
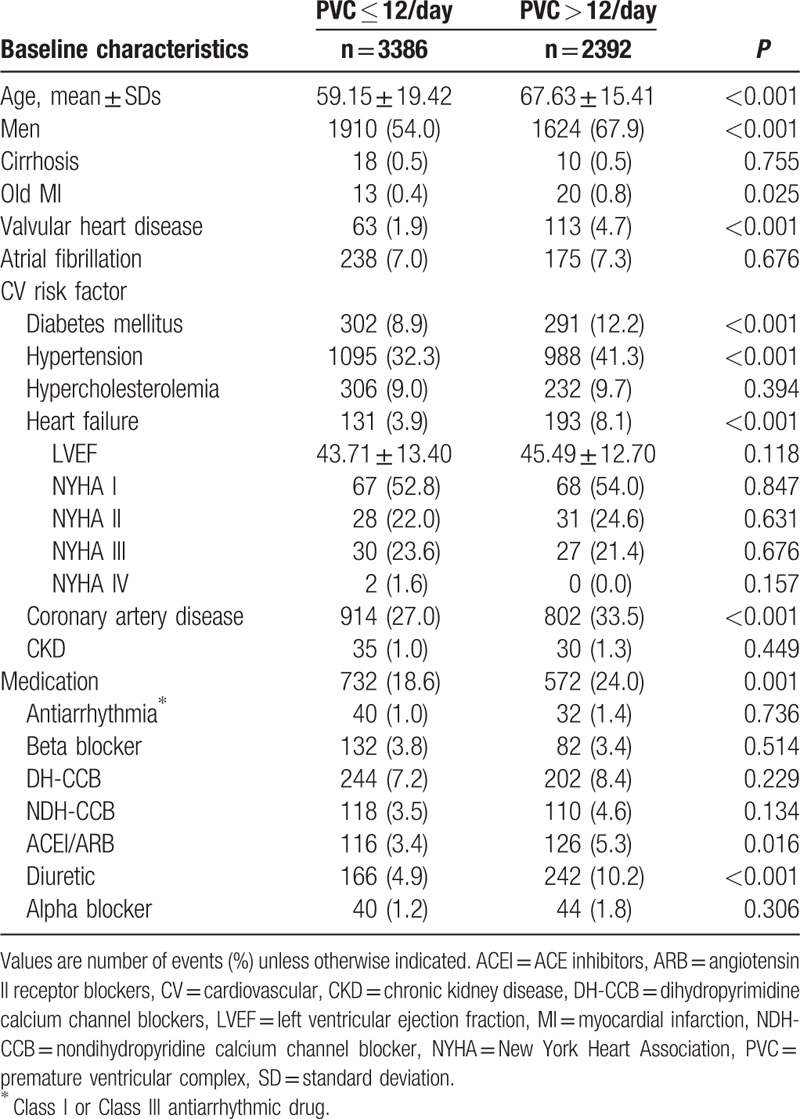
The baseline characteristics of patients with or without PVCs > 12/day are presented in Table 1. Patients with PVCs > 12/day were generally older, male, with a higher incidence of diabetes mellitus, hypertension, HF, coronary artery disease, valvular heart disease, history of myocardial infarction, and were prescribed more medications (ie, ACEI/ARB or diuretics) compared with the group with PVCs ≤ 12/day.
Table 1.
Baseline characteristics of all patients.
3.2. PVCs and long-term outcomes
Patients with PVCs > 12/day had higher rates of all-cause mortality, all-cause hospitalization, CV hospitalization, and new-onset HF compared to patients with PVCs ≤ 12/day (Table 2). After multivariate adjustment for baseline risk factors, the clinical event rates remained higher in patients with PVCs > 12/day, with an estimated HR (95% confidence interval) of 1.429 (1.284–1.590) for all deaths, 1.127 (1.008–1.260) for CV hospitalization, 1.094 (1.021–1.173) for all-cause hospitalization, and 1.411 (1.203–1.655) for new-onset HF.
Table 2.
Ten-year event rates in patients with and without PVC more than 12 beats per day.
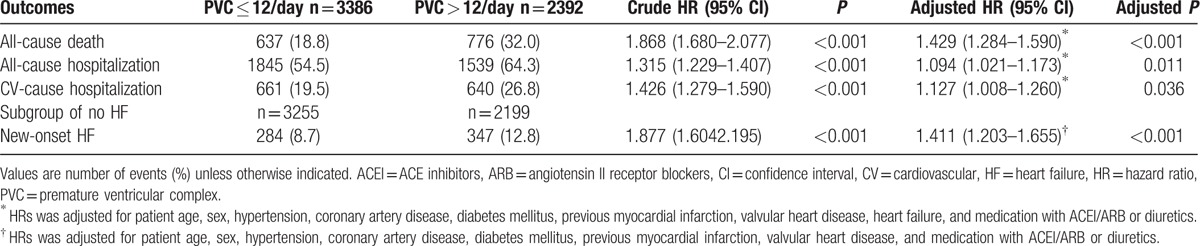
Figure 3 shows the Kaplan–Meier survival curve, CV hospitalization-free survival curve, and HF-free survival curve in patients with or without PVCs > 12/day. The patients with PVCs > 12/day had a higher incidence of adverse events.
Figure 3.
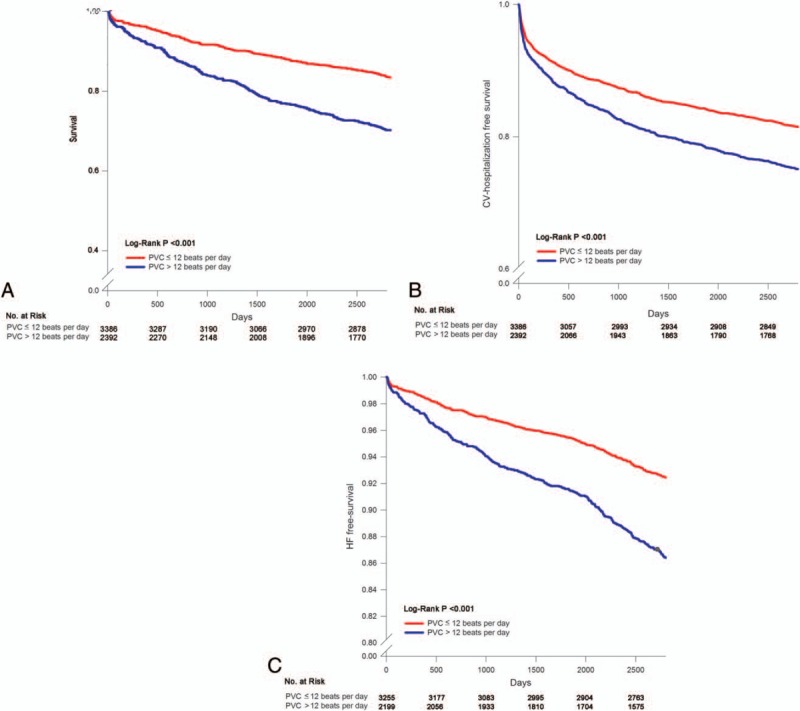
Kaplan–Meier curve of survival in patients with PVCs. Panel A shows Kaplan–Meier curve of survival in patients with PVC ≦12 beats per day or PVC >12 beats per day. Panel B shows Kaplan–Meier curve of CV hospitalization-free survival in patients with PVC ≦12 beats per day or PVC >12 beats per day. Panel C shows Kaplan–Meier curve of occurrence of new-onset HF-free survival in patients with PVC ≦12 beats per day or PVC >12 beats per day. CV = cardiovascular, HF = heart failure, PVC = premature ventricular complex.
A propensity score matched analysis was performed to control for all confounders by nearest neighbor method. Table 3 shows propensity-matched sample and baseline characteristics. A Cox proportional hazards model was applied to examine the risk of PVCs > 12/day in the matched group (Table 4). The HR (95% confidence interval) of PVCs > 12/day group was 1.322 (1.125-1.553) for all-cause death, 1.092 (0.99-1.205) for all-cause hospitalization, 1.239 (1.059-1.448) for CV hospitalization, and 1.385 (1.105-1.736) for new-onset HF.
Table 3.
Baseline characteristics of all patients (propensity core matched).
Table 4.
Ten-year event rates in patients with and without PVC more than 12 beats per day (propensity matched).
The 5778 patients were divided into no-PVC group and PVC group. The PVC group was further divided to quartiles, according to their PVC frequencies. Cut-off points for the 1st, 2nd, and 3rd quartiles were 3, 26, and 324 PVCs per day, respectively. Subgroup comparisons for incidence of mortality and new-onset HF are shown in Fig. 4. The incidence rates for mortality and HF were significantly increased with increased PVC frequency.
Figure 4.
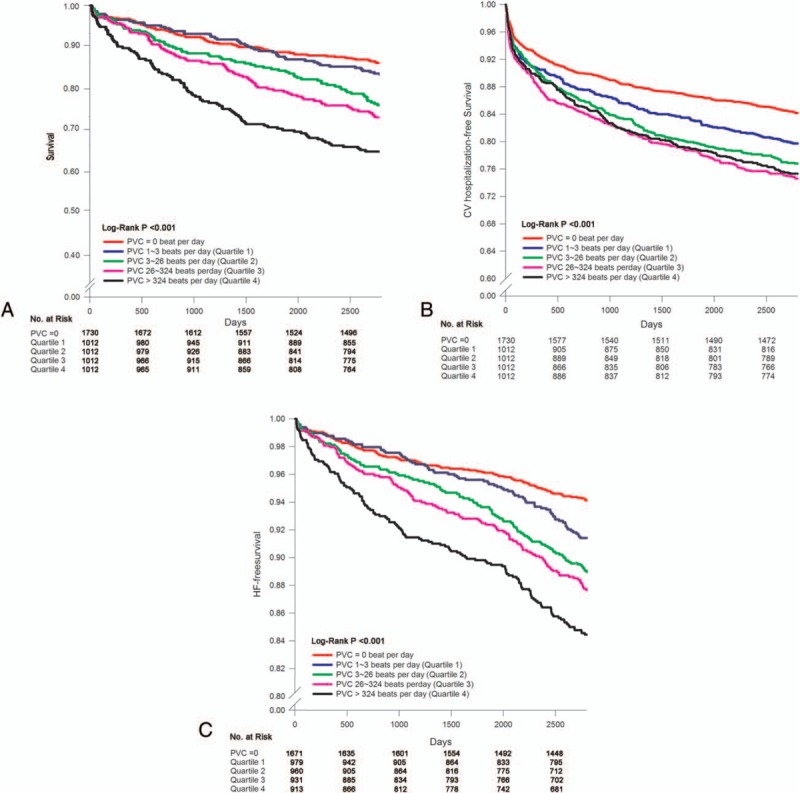
Kaplan–Meier curve of quartile comparison. Panel A shows Kaplan–Meier curve of quartile comparison for all mortality. Panel B demonstrates Kaplan–Meier curve of quartile comparison for CV hospitalization. Panel C shows Kaplan–Meier curve of quartile comparison for occurrence of new-onset HF. CV = cardiovascular, HF = heart failure, PVC = premature ventricular complex.
3.3. Discrimination
Markers were used for the outcome (ie, age, gender, hypertension, coronary artery disease, previous myocardial infarction, diabetes mellitus, valvular heart disease, HF, and medication with ACEI/ARB or diuretics) in our study as the old model. When PVC was added to other variables for estimates of risk, the integrated discrimination index was 0.0025 (P = 0.0043) for mortality, 0.0019 (P = 0.0026) for CV hospitalization, and 0.0038 (P = 0.00045) for new-onset HF.
3.4. Cause of mortality analysis
Regarding analysis of all mortality, PVCs > 12/day was associated with death due to infection and CV events (Fig. 5). When CV events were further divided into myocardial infarction, HF, and sudden cardiac death, PVC > 12/day was associated with HF and sudden cardiac death.
Figure 5.
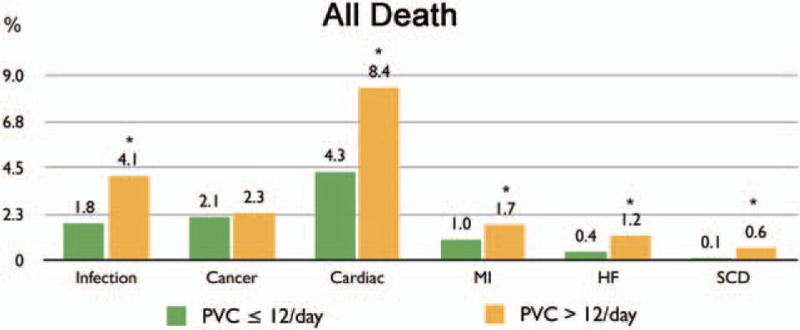
Cause of all-cause death. ∗P�<�0.05. HF�=�heart failure, MI�=�myocardial infarction, PVC�=�premature ventricular complex, SCD�=�sudden cardiac death.
4. Discussion
This study demonstrated that patients with a PVC frequency >12 per day were at increased risk for mortality, CV hospitalization, and development of HF which was independent of gender, age, or structural heart disease. Based on an analysis of all-cause mortality, a PVC frequency >12 per day was associated with death due to infection and CV events, including HF and sudden cardiac death. The incidence rates for mortality and HF were significantly increased in patients with high PVC burden.
4.1. High PVC burden is associated with poor clinical outcome
After adjusting for various potential confounding factors and propensity score matching, we found that a PVC frequency >12 per day was associated with increased incidence of all-cause mortality, CV hospitalization, and new-onset HF.
In a previous study,[3] the risks of cardiac death were significantly increased in subjects (with or without baseline coronary heart disease) with a PVC frequency >720 per day (presence of PVCs on 2-minute rhythm strip) during a 6.3-year follow-up. Another 16.4-year follow-up analysis of subjects from the general population, without a history of stroke or coronary heart disease, found that the presence of PVCs > 720/day (presence of PVC on 2-minute rhythm strip) correlated with sudden cardiac death.[4] Abdalla et al[24] characterized PVCs (ie, 2 or more uniform PVCs every 2 minutes) and their complexity (multiform, pairs, runs, R-on-T) and reported that frequent or complex PVCs placed the patient at a significantly increased risk of sudden cardiac death during a 6.5-year follow-up study in healthy men.
Findings from both observational studies performed on the general population and meta-analyses have indicated that frequent PVCs were associated with a substantial increase in the risk for CV events and poor prognosis and such results are compatible with our findings.[25,26] However, one of the limitations of these studies was the use of the 2-minute ECG “rhythm strip,” the 12-second ECG, the 30-second ECG, and the 1-hour ECG which may not have been long enough to identify patients with low frequency PVCs. Our study was based on 24-hour Holter monitoring which can detect low frequency PVCs. To the best of our knowledge, our study is the first to demonstrate that low frequency PVCs based on Holter monitoring are significantly associated with all-cause mortality and CV hospitalization independent of gender, age, and structural heart disease.
Early prevention and treatment of the diseases underlying low frequency PVCs may alter the poor prognosis. Continuous monitoring also plays an important role in follow-up, especially in cases of HF or arrhythmia after specific treatment or intervention.[27] Further studies investigating the effect of ventricular arrhythmia burden (with or without medication) using continuous monitoring is warranted.
4.2. Increased PVC burden contributes to HF
After adjusting for various potential confounding factors and propensity score matching, we found that a PVC frequency >12/day was associated with an increased incidence of new-onset HF. In the quartile subgroup comparison, the incidence of HF increased with PVC frequency to a greater extent than previously reported.
Niwano et al[11] demonstrated progressive worsening of left ventricular function in patients with PVCs >1000/day over a follow-up period of 4 to 8 years. In addition, a significant association between frequent PVCs and left ventricular dysfunction has been shown to exist in patients without structural heart disease.[12] Reversal of left ventricular dysfunction was documented after ablation of PVCs.[28–35] PVC-induced cardiomyopathy was originally thought to be a type of tachycardia-induced cardiomyopathy. We hypothesize that a low frequency of PVCs will cause a vicious cycle that enhances the progressive development of PVCs, called “PVC begets PVC”, which may increase the frequency of PVCs and result in left ventricular dysfunction. Regular follow-up with Holter monitoring in a PVC patient without structural heart disease is warranted for early treatment and prevention.
Calcium released from the sarcoplasmic reticulum is crucial for excitation–contraction coupling. Previous data have demonstrated that an abnormality in RyR2, which is a calcium release channel on cardiac sarcoplasmic reticulum, causes mitochondrial calcium overload, oxidative stress, and worsening of HF or cardiac arrhythmia.[36,37]
Changes in PVC frequency have been found in HF patients under treatment, which might correlate with changes in the perfusion state of the patient.[38] Thus, further prospective trials with routine follow-up using Holter Monitoring may be warranted.
4.3. PVC burden and cardiac death
Based on our results, patients with frequent PVCs have a greater risk of cardiac death attributed to HF and sudden cardiac death. High PVC burden has been shown to predispose to left ventricular dysfunction in previous studies.[11,12,28,29,35] Our study disclosed that a low frequency of PVCs also predisposes to cardiac dysfunction. Patients with decreased left ventricular function had a greater PVC burden than patients with normal left ventricular function.[10,35] A low frequency of PVCs might beget more PVC burden. Unexpected sudden cardiac death during episodes of clinical worsening of HF accounts for approximately one-third of deaths in HF patients.[39] The increase in incidence of HF may contribute to sudden cardiac death, based on our study.
4.4. Ventricular arrhythmia mechanism
Cardiac arrhythmia is related to systemic oxidative stress, which is associated with reactive oxygen species. The homeostasis of cellular metabolism and ion channel was important in cardiac cells. Reactive oxygen species are responsible for the regulation of homeostasis in the excitable cardiac cell. Previous publication suggested that cardiac arrhythmia is associated with cellular reactive oxygen species through the effect on alteration in ion channel, mitochondrial function, and gap junction.[40] Furthermore, Santulli et al[41] using knocking of single amino acid missense mutations, provided strong genetic evidence supporting a central role for mutant leaky RyR2 in the pathogenesis of cardiac arrhythmias. In addition, recent studies showed the microRNAs might play important roles in the adaptive process of the failing heart and affect the treatment response of cardiac arrhythmia via electrical membrane signaling and ion channel activation.[42–44] A previous study also reported that metabolic syndrome was associated with poor outcome after PVC ablation.[45] This result emphasizes the role of metabolic, inflammatory, and redox processes, and ionic channel conduction properties on cardiac arrhythmia. The above findings also provide physicians with important information regarding clinical evaluation of patients with PVCs. Further studies investigating the role of metabolic, inflammatory, and redox processes, and ionic channel function in generating cardiac arrhythmias are warranted.
5. Limitations
Our study had several limitations. Our results may have been related to the baseline conditions of the patients rather than to an effect of PVC burden. Regarding baseline characteristics, the patient group with PVC frequency >12/day was older, predominantly male, and had a higher incidence of hypertension, diabetes mellitus, coronary artery disease, valvular heart disease, and previous myocardial infarction compared with the group with a PVC frequency ≦12/day. In addition, there were more deaths related to infection in the group with PVC frequency >12/day. Although we used Cox regression hazard model and propensity-matched adjustment for extensive risk to decrease the effect of the potential confounders, selection bias may still have existed. In addition, the sensitivity and specificity were low in our study population. Our study could not determine whether treating or preventing PVCs would reduce adverse events. Furthermore, our study population was not representative of the general population. The study population had a higher CV risk and was referred for cardiac symptoms in the initial setting. Holter monitoring indication may stratify PVC frequency or study outcomes. Additionally, the PVC burden may be higher in the setting of subclinical heart disease. Frequent PVCs might be a marker of an underdiagnosed physical condition that can lead to ventricular dysfunction. Electrolyte disorder associated with the side effects of several antihypertensive agents was not evaluated in this study. Further prospective studies may be necessary before applying these results to a clinical setting.
6. Conclusions
Elevated PVC burden was associated with a higher risk of mortality, CV hospitalization, and new-onset HF, independent of the clinical risk factors, in this ten years follow-up study.
Acknowledgments
The authors thank National Yang Ming University Hospital grant RD 2016-005, MOST104-2314-B-010-055-MY3, MOST104–2314-B-075–070, Taipei Veterans General Hospital grant V104E7-002, V105C-116, V105C-121, VGHUST104-G7-3-1 and -2, VGHUST105-G7-9-1 and -2, and Taipei Veterans General Hospital-National Yang-Ming University Excellent Physicians Scientists Cultivation Program (105-V-B-021) for the support.
Footnotes
Abbreviations: ACEI = angiotensin-converting enzyme inhibitors, AF = atrial fibrillation, ARB = angiotensin II receptor blockers, CCHIA = Collaboration Center of Health Information Application, CV = cardiovascular, ECG = electrocardiography, HF = heart failure, HR = hazard ratio, PVC = premature ventricular complex, ROC = receiver operator characteristic.
Funding/support: This study was supported by National Yang Ming University Hospital grant RD 2016-005, MOST104-2314-B-010-055-MY3, MOST104–2314-B-075–070, Taipei Veterans General Hospital grant V104E7-002, V105C-116, V105C-121, VGHUST104-G7-3-1 and -2, VGHUST105-G7-9-1 and -2, and Taipei Veterans General Hospital-National Yang-Ming University Excellent Physicians Scientists Cultivation Program (105-V-B-021).
The authors have no conflicts of interest to disclose.
References
- [1].Messineo FC. Ventricular ectopic activity: prevalence and risk. Am J Cardiol 1989;64:53J–6J. [DOI] [PubMed] [Google Scholar]
- [2].Simpson RJ, Jr, Cascio WE, Schreiner PJ, et al. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002;143:535–40. [DOI] [PubMed] [Google Scholar]
- [3].Massing MW, Simpson RJ, Jr, Rautaharju PM, et al. Usefulness of ventricular premature complexes to predict coronary heart disease events and mortality (from the Atherosclerosis Risk in Communities cohort). Am J Cardiol 2006;98:1609–12. [DOI] [PubMed] [Google Scholar]
- [4].Cheriyath P, He F, Peters I, et al. Relation of atrial and/or ventricular premature complexes on a two-minute rhythm strip to the risk of sudden cardiac death (the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 2011;107:151–5. [DOI] [PubMed] [Google Scholar]
- [5].Ofoma U, He F, Shaffer ML, et al. Premature cardiac contractions and risk of incident ischemic stroke. J Am Heart Assoc 2012;1:e002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fisher FD, Tyroler HA. Relationship between ventricular premature contractions on routine electrocardiography and subsequent sudden death from coronary heart disease. Circulation 1973;47:712–9. [DOI] [PubMed] [Google Scholar]
- [7].Rabkin SW. Electrocardiographic abnormalities in apparently healthy men and the risk of sudden death. Drugs 1984;28(Suppl 1):28–45. [DOI] [PubMed] [Google Scholar]
- [8].Stein PK, Sanghavi D, Sotoodehnia N, et al. Association of Holter-based measures including T-wave alternans with risk of sudden cardiac death in the community-dwelling elderly: the Cardiovascular Health Study. J Electrocardiol 2010;43:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Duffee DF, Shen WK, Smith HC. Suppression of frequent premature ventricular contractions and improvement of left ventricular function in patients with presumed idiopathic dilated cardiomyopathy. Mayo Clinic Proc 1998;73:430–3. [DOI] [PubMed] [Google Scholar]
- [10].Lee GK, Klarich KW, Grogan M, et al. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circul Arrhyth Electrophysiol 2012;5:229–36. [DOI] [PubMed] [Google Scholar]
- [11].Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009;95:1230–7. [DOI] [PubMed] [Google Scholar]
- [12].Kanei Y, Friedman M, Ogawa N, et al. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008;13:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010;7:865–9. [DOI] [PubMed] [Google Scholar]
- [14].Ng GA. Treating patients with ventricular ectopic beats. Heart 2006;92:1707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol 2012;60:1231–8. [DOI] [PubMed] [Google Scholar]
- [16].Chao TF, Hung CL, Chen SJ, et al. The association between hyperuricemia, left atrial size and new-onset atrial fibrillation. Int J Cardiol 2013;168:4027–32. [DOI] [PubMed] [Google Scholar]
- [17].Lin CY, Chang SL, Lin YJ, et al. Long-term outcome of multiform premature ventricular complexes in structurally normal heart. Int J Cardiol 2015;180:80–5. [DOI] [PubMed] [Google Scholar]
- [18].Hsieh CY, Chen CH, Li CY, et al. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc 2015;114:254–9. [DOI] [PubMed] [Google Scholar]
- [19].Lu TH, Lee MC, Chou MC. Accuracy of cause-of-death coding in Taiwan: types of miscoding and effects on mortality statistics. Int J Epidemiol 2000;29:336–43. [DOI] [PubMed] [Google Scholar]
- [20].Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2014. 15;33(6):1057-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annu Rev Public Health 2000;21:121–45. [DOI] [PubMed] [Google Scholar]
- [22].Kerr KF, McClelland RL, Brown ER, et al. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol 2011;174:364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- [24].Abdalla IS, Prineas RJ, Neaton JD, et al. Relation between ventricular premature complexes and sudden cardiac death in apparently healthy men. Am J Cardiol 1987;60:1036–42. [DOI] [PubMed] [Google Scholar]
- [25].Ataklte F, Erqou S, Laukkanen J, et al. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am J Cardiol 2013;112:1263–70. [DOI] [PubMed] [Google Scholar]
- [26].Lee V, Hemingway H, Harb R, et al. The prognostic significance of premature ventricular complexes in adults without clinically apparent heart disease: a meta-analysis and systematic review. Heart 2012;98:1290–8. [DOI] [PubMed] [Google Scholar]
- [27].Sardu C, Santamaria M, Rizzo MR, et al. Telemonitoring in heart failure patients treated by cardiac resynchronisation therapy with defibrillator (CRT-D): the TELECART Study. Int J Clin Pract 2016;70:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005;112:1092–7. [DOI] [PubMed] [Google Scholar]
- [29].Takemoto M, Yoshimura H, Ohba Y, et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol 2005;45:1259–65. [DOI] [PubMed] [Google Scholar]
- [30].Ezzat VA, Liew R, Ward DE. Catheter ablation of premature ventricular contraction-induced cardiomyopathy. Nat Clin Pract Cardiovasc Med 2008;5:289–93. [DOI] [PubMed] [Google Scholar]
- [31].Yokokawa M, Kim HM, Good E, et al. Relation of symptoms and symptom duration to premature ventricular complex-induced cardiomyopathy. Heart Rhythm 2012;9:92–5. [DOI] [PubMed] [Google Scholar]
- [32].Chugh SS, Shen WK, Luria DM, et al. First evidence of premature ventricular complex-induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol 2000;11:328–9. [DOI] [PubMed] [Google Scholar]
- [33].Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009;6:1543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ban JE, Park HC, Park JS, et al. Electrocardiographic and electrophysiological characteristics of premature ventricular complexes associated with left ventricular dysfunction in patients without structural heart disease. Europace 2013;15:735–41. [DOI] [PubMed] [Google Scholar]
- [35].Bogun F, Crawford T, Reich S, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm 2007;4:863–7. [DOI] [PubMed] [Google Scholar]
- [36].Santulli G, Xie W, Reiken SR, et al. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A 2015;112:11389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xie W, Santulli G, Reiken SR, et al. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep 2015;5:11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Asensio-Lafuente E, Castillo-Martínez L, Orea-Tejeda A, et al. Changes in the arrhythmic profile of patients treated for heart failure are associated with modifications in their myocardial perfusion conditions. Cardiol J 2008;15:261–7. [PubMed] [Google Scholar]
- [39].Narang R, Cleland JG, Erhardt L, et al. Mode of death in chronic heart failure. A request and proposition for more accurate classification. Eur Heart J 1996;17:1390–403. [DOI] [PubMed] [Google Scholar]
- [40].Jeong EM, Liu M, Sturdy M, et al. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol 2012;52:454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Santulli G, Pagano G, Sardu C, et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest 2015;125:4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sardu C, Marfella R, Santulli G, et al. Functional role of miRNA in cardiac resynchronization therapy. Pharmacogenomics 2014;15:1159–68. [DOI] [PubMed] [Google Scholar]
- [43].Sardu C, Santamaria M, Paolisso G. Marfella R. microRNA expression changes after atrial fibrillation catheter ablation. Pharmacogenomics 2015;16:1863–77. [DOI] [PubMed] [Google Scholar]
- [44].Santulli G, Iaccarino G, De Luca N, et al. Atrial fibrillation and microRNAs. Front Physiol 2014;24:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sardu C, Carreras G, Katsanos S, et al. Metabolic syndrome is associated with a poor outcome in patients affected by outflow tract premature ventricular contractions treated by catheter ablation. BMC Cardiovasc Disord 2014;14:176. [DOI] [PMC free article] [PubMed] [Google Scholar]


