Abstract
Background:
Management of pancreatic cysts is based on neoplastic–nonneoplastic discrimination. Endoscopic ultrasound (EUS) enables to differentiate neoplastic–nonneoplastic lesions and also allows fine-needle aspiration (FNA). In this study, we aim to assess feasibility and clinical relevance of cytological and biochemical analysis in differential diagnosis of cystic pancreatic lesions in patients who had undergone endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) due to pancreatic cysts.
Methods:
Participants were 96 patients who had undergone EUS-FNA for differential diagnosis of pancreatic cysts. Pancreatic cysts were classified as benign-mucinous, nonmucinous, and malignant according to patient history, physical examination, EUS appearance, and cystic fluid assessment. Tumor markers (CEA, CA(cancer antigens) 72.4, CA 19-9) , amylase, lipase and cytological assesment were compared between 3 different groups. Receiver-operating characteristics (ROC) curves were constructed to identify appropriate cut-off values.
Results:
Fluid CEA and CA 72.4 levels for benign-mucinous and malignant cysts were significantly higher than for nonmucinous cysts (P ≤ 0.04). A cut-off CEA level of 207 ng/mL differentiated mucinous etiology with a sensitivity of 72.7%, specificity of 97.7%, and accuracy of 89.5%. The sensitivity, specificity, and accuracy of the CA 72.4 cut-off level of 3.32 ng/mL were 80%, 69.5%, and 73.6%, respectively.
Conclusion:
Cyst fluid CEA and CA 72.4 levels have a high accuracy in discriminating mucinous from nonmucinous cysts. When combined with cytology their accuracy rate increases.
Keywords: cytology, endoscopic ultrasound, fluid analysis, mucinous, nonmucinous, pancreatic cysts
1. Introduction
Pancreatic cysts are mostly identified incidentally during diagnostic imaging for other conditions. Moreover, the increased use of endoscopic ultrasound (EUS) and cross-sectional imaging for the diagnosis of gastrointestinal lesions as yielded a 10-fold increase in the identification of pancreatic cysts, as well as resulting in a 2-fold increase in the resection of pancreatic cystic tumors.[1,2] In their review of 24,039 computed tomography (CT) and magnetic resonance (MR) images, Spinelli et al[3] identified a prevalence rate of pancreatic cysts of 1.2%. In their review of MR images, de jong et al[4] reported a prevalence rate of pancreatic cysts of 2.4%.
Pancreatic cysts are classified as either being mucinous and nonmucinous, this differentiation being important for treatment and follow-up strategies. Moreover, mucinous cystic neoplasia (MCN) and intraductal papillary mucinous neoplasia (IPMN) are mucinous cysts that have the potential for malignancy, even at the time of diagnosis, with the risk for malignancy ranging between 11% and 38% for MCNs,[5,6] 6.3% and 46.5% for branch-duct IPMNs,[7,8] and 35.7% and 100% for main duct IPMNs.[7,9] Due to their high risk for malignancy, patients with mucinous lesions require either frequent follow-up or early surgery. Comparatively, the risk for malignancy for nonmucinous lesions, such as pseudocysts and serous cystadenoma (SCA), is considered to be very low or nonexistent, with asemptomatic patients requiring follow-up.
Several algorithms have been developed to differentiate malignant and benign cysts, combining clinical history with clinical and radiological findings. However, none of these algorithms provide sufficiently conclusive results for certain use in practice. Abdominal ultrasound has a limited use in this indication while CT and MR images can provide a diagnosis only when characteristic features are present. Therefore, overall, these imaging techniques do not have sufficient specificity and sensitivity to evaluate the malignancy risk of a lesion.[10]
In recent years, EUS has come to play an important role in the differential diagnosis of pancreatic cysts, providing information on the inner structure of these lesions, as well as offering the possibility of using fine-needle aspiration (FNA) biopsy for cytology. EUS has been shown to have a sensitivity of 71% and specificity of 30% in identifying pancreatic cystic lesions requiring surgical resection. This sensitivity and specificity can be increased to 97% and 100%, respectively, by combining EUS with FNA.[11] Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is used to aspirate cystic fluid for the analysis of tumor markers (carcinoembryonic antigen [CEA]; cancer antigens [CA] 19–9, 15–3, and 72.4), pancreatic enzymes (amylase and lipase), and cytology. Of these different markers and enzymes, CEA is the most useful for diagnostic purposes, but with some limitations.[12,13] Specifically, a high CEA level differentiates mucinous from nonmucinous cysts but cannot reliably differentiate benign lesions from malignant ones.[14] Therefore, the aim of our study was to evaluate the clinical relevance of CEA, CA 19–9, CA 72–4, and amylase levels and cytology, obtained by EUS-FNA, for the differential diagnosis of pancreatic cysts.
2. Material and methods
2.1. Patients
We performed a single center retrospective cohort study of patients who had undergone evaluation for pancreatic cysts in the Department of Gastroenterohepatology, Istanbul Faculty of Medicine, Istanbul University, between January, 2010 and January, 2013. The study protocol was carried out in accordance with the 1975 Helsinki Declaration and was approved by the ethics committee of the Istanbul Faculty of Medicine (Reference number: 2013/697). All patients underwent EUS-FNA and pancreatic cyst aspiration. Clinical records were reviewed to extract clinical and demographic characteristics, as well as imaging, biochemical, and pathological test results.
2.2. Endoscopic ultrasound
EUS-FNAs were performed by 2 experienced endoscopic ultrasonographers (CK, FA) using a curvilinear echo-endoscope (Fujinon, NY). FNA was performed using a 22 G needle (Cook Medical Inc., Bloomington, IN). Pancreatic cysts were described and assessed based on localization, number, dimension, and characteristic features (wall thickness, presence of septation and/or mural nodule, communication with pancreatic duct, and calcification). The color, transparency, and viscosity of the cystic fluid were also recorded.
2.3. Cyst fluid analysis
Cyst fluid samples were preserved at −82 °C for biochemical analyses. When sufficient aspiration material was obtained, CEA, CA 19–9, CA 72–4, and amylase levels were determined. Analyzes were performed using the Roche Diagnostic Modular System (Roche Diagnostics Corporation, Indianapolis, IN). For cytological assessment, aspirated material was smeared and fixed by pure alcohol for PAP staining. The remaining volume of aspirated material was smeared and air dried for Giemsa staining. When sufficient aspirated material was available, tissue sections were obtained from prepared cell blocks for hematoxylin-eosin (HE) staining.
2.4. Classification of lesions
Pancreatic cysts were classified as benign nonmucinous, benign-mucinous, and malignant-mucinous cysts based on patient history, findings on physical examination, appearance on EUS, and analysis of cyst fluid. The diagnostic criteria for a benign nonmucinous cyst were: a typical appearance of the serous cyst on EUS; presence of nonmucoid cystic fluid; and absence of extracellular mucin, mucinous epithelium, and atypical epithelium on cytological examination. EUS findings characteristic of IPMNs or MCNs, in combination with aspiration of a thick, viscous and mucoid cystic fluid, and/or presence of an extracellular mucin on cytological examination was accepted to be diagnostic of a benign-mucinous pancreatic cyst. Cysts were identified as malignant based on the following criteria: localized in the head of the pancreas combined with obstructive jaundice; presence of a solid component, dilatation of the main pancreatic duct, mural nodules, and suspicious involvement of the main pancreatic duct; or the presence of suspicious or positive cytology results. Those 3 groups were compared in terms of clinical and biochemical parameters. In addition, another comparison is made in order to pinpoint differential diagnostic sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and a cut-off value of serum and fluid tumor markers in differentiating mucinous lesions from nonmucinous lesions. This was done by comparing benign nonmucinous cases with another group which combined both benign mucinous and malignant cases.
2.5. Statistical analysis
For statistical analysis, NCSS (Number Cruncher Statistical System) 2007 and PASS (Power Analysis and Sample Size) 2008 Statistical Software (NCSS LLC, Kaysville, UT) program was used. Descriptive statistics were calculated for all outcomes variables (mean, standard deviation, frequency, and percentage). Between-group comparisons were evaluated by one-way analysis of variance (ANOVA), with a Tukey post-hoc analysis used to evaluate the significance of identified between-group differences, for normally distributed variables and Kruskal–Wallis and Mann–Whitney U tests for variables with a nonnormal distribution. Between-group comparisons of qualitative data were evaluated using Pearson correlation, chi-squared test, and Fisher–Freeman–Halton test, as appropriate for the variable type. Receiver-operating characteristics (ROC) curves were constructed to determine optimal cut-off values of tumor marker levels to differentiate mucinous and nonmucinous lesions. For all analyzes, 95% confidence intervals were calculated and a P-value < 0.05 was considered to be significant.
3. Results
3.1. Patient characteristics
A total of 96 patients formed our study group. Of this group, the data from 4 patients were not included in the analysis, as their lesions could not be classified. The remaining 92 patients had a mean age of 57.9 ± 15.1 years and included 42.4% (39/92) males. Among these patients, 51 were classified as having benign nonmucinous cysts, 26 benign mucinous cysts, and 15 malignant cysts. Age and sex distribution was comparable between groups (P > 0.05).
3.2. Pancreatic cyst characteristics
The most common locations of cysts was in the head of the pancreas, with 52.2% located in the head compared to 28.3% in the corpus, 13.0% in the tail, and 6.5% in the uncinate process. The mean cyst diameter was 31.4 ± 15.2 mm. Tumor location and size were comparable between the 3 groups (P > 0.05).
3.3. CEA, CA 19–9, CA 72–4, amylase, and lipase assessments
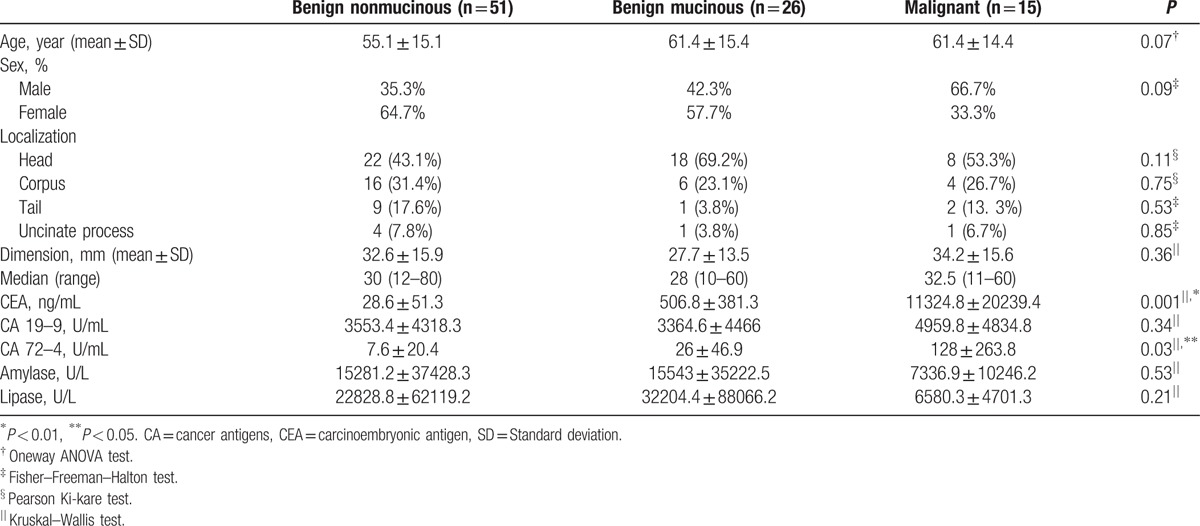
Results of cyst fluid analysis are reported in Table 1. The distribution of mean CEA and CA 72–4 levels for the 3 types of cysts was as follows: benign, nonmucinous group, 28.6 ± 51.3 ng/mL and 7.6 ± 20.4 U/mL, respectively; benign mucinous group, 506.8 ± 381.3 ng/mL and 26 ± 46.9 U/ml, respectively; and malignant group 11324.8 ± 20239.4 ng/mL and 128 ± 263.8 U/mL, respectively. There were significant between-group differences in CEA (P = 0.001) and CA 72–4 (P = 0.03) levels. Compared to the benign nonmucinous cysts group, levels of CEA and CA 72–4 were higher for the benign mucinous cysts group (CEA, P = 0.001; CA 72–4, P = 0.04) and malignant cysts group (CEA, P = 0.001; CA 72–4, P = 0.04). Levels were comparable between benign mucinous and malignant cysts groups (CEA, P = 0.36; CA 72–4, P = 0.46). There were no between-group differences in levels of CA 19.9 (P = 0.34), amylase (P = 0.53), and lipase (P = 0.21).
Table 1.
Characteristics of patients with benign mucinous, benign nonmucinous, and malignant pancreatic cystic lesions.
3.4. Cytological assessment
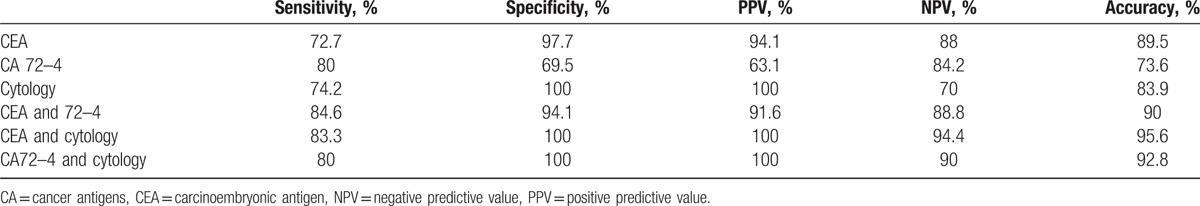
In 38.5% of cases, cytology was nondiagnostic. The sensitivity, specificity, PPV, NPV, and accuracy of cytology in differentiating mucinous cysts was 74.2%, 100%, 100%, 70%, and 83.9%, respectively.
3.5. ROC analysis
The calculated area under the ROC curve (AUC) for cysts was as follows: 0.888 for CEA (95%CI, 0.734–0.998; P = 0.001); 0.754 for CA 72–4 (%95 CI, 0.575–0.932; P = 0.01); 0.564 for CA 19–9 (95% CI, 0.369–0.760; P = 0.54); and 0.458 for amylase (95% CI, 0.236–0.681; P = 0.69). CEA and CA 72–4 levels differentiated mucinous and nonmucinous lesions (P < 0.05; Fig. 1). For a cut-off CEA level of 207 ng/mL, the sensitivity, specificity, PPV, NPV, and accuracy of levels of CEA in differentiating a mucinous etiology were 72.7%, 97.7%, 94.1%, 88%, and 89.5%, respectively. The sensitivity, specificity, PPV, NPV, and accuracy for a cut-off CA 72.4 level of 3.32 ng/mL were 80%, 69.5%, 63.1%, 84.2%, and 73.6%, respectively.
Figure 1.

ROC curve for CEA, CA 19–9, CA 72–4, and amylase. CA = cancer antigens, CEA = carcinoembryonic antigen, ROC = receiver-operating characteristics.
3.6. Combination of tests
The diagnostic validity of combining CEA and CA 72.4, as well as of combining cytology with CEA or CA 72.4, is reported in Table 2. The combination of CEA and CA 72.4 provided a sensitivity, specificity, PPV, NPV, and accuracy for differentiating a mucinous cyst etiology of 84.6%, 94.1%, 91.6%, 88.8%, and 90%, respectively. Combining cytology with CEA levels resulted in a sensitivity of 83.3%, specificity of 100%, PPV of 100%, NPV of 94.4%, and accuracy of 95.6%. Similarly, combining cytology with CA 72–4 level yielded a sensitivity of 80%, specificity of 100%, PPV of 100 T, NPV of 90%, and accuracy of 92.8%.
Table 2.
Value of tumor markers and cytology in determining mucinous etiology.
3.7. Clinical outcome of the patients with pancreatic cysts
Although 86% of the cases in the benign nonmucinous group were followed up through surveillance, 14% underwent surgical treatment. Among cases in the benign mucinous group, 76% were followed up through surveillance, with 24% undergoing surgical treatment. In malignant group 40% of patients were operable but 60% were nonoperable.
4. Discussion
Management of pancreatic cysts is based on discriminating between neoplastic and nonneoplastic cysts. Thus, in patients with pancreatic cysts, a correct preoperative diagnosis is vital in preventing unnecessary surgical procedures. Although CT and MR imaging are widely used for this purpose, these imaging techniques have insufficient sensitivity and specificity to characterize cystic lesions of the pancreas, with Fisher et al[15] reporting an accuracy rate of 39% for CT in their assessment of 48 neoplastic cysts. EUS provides a superior alternative to CT and MR imaging to characterize cystic lesions, with the capacity to identify ductal communication and the presence of mural nodule or solid component.[16] However, EUS imaging of cysts alone is insufficient as an independent predictor for malignancy. The accuracy of discriminating premalignant lesions from malignant lesions based on EUS-detected morphology alone was reported to range between 40% and 93%.[17] In a multicenter study, the diagnostic accuracy of EUS morphology was reported to be 51%.[18] The diagnostic accuracy rate was substantively increased by combining EUS with FNA. A meta-analysis[19] reported a sensitivity and specificity of EUS-FNA for the diagnosis of solid pancreatic neoplasia of 91% and 94%, respectively. However, the diagnostic value of EUS-FNA for pancreatic cystic lesions has not yet been established, with variable results having been reported. The sensitivity of EUS-FNA has been reported to range between 22% and 95% for the diagnosis of pancreatic cystic neoplasia (PCN).[11,18,20] A recent meta-analysis of EUS-FNA for the diagnosis of PCN reported a sensitivity of 54% and specificity of 93%.[21] In another meta-analysis, the sensitivity and specificity of cytology in discriminating serous lesions from mucinous cystic lesions were reported to be 63% and 88%, respectively.[22] In our study, cytology was found to have a sensitivity of 74%, specificity of 100%, and accuracy of 83.9% for discriminating mucinous lesions from nonmucinous lesions. The most important problem encountered in EUS-FNA is inadequate sampling, which can result in false-negative findings or nondiagnosis. Frossard et al found that FNA is inadequate for diagnosis in 23% of patients. In our study, 38.5% of cytological assessments were nondiagnostic.
Pancreatic cyst fluid may contain glycoproteins secreted from epithelium, such as CEA, CA 19–9, CA 125, CA 15–3, and CA 72–4. Therefore, a number of researchers have sought to determine cut-off levels of these glycoproteins for accurate differentiation of mucinous and nonmucinous lesions. In their study of 112 patients with a confirmed histological diagnosis, Brugge et al[18] reported a cut-off level of CEA > 192 ng/mL provided a sensitivity of 73%, specificity of 84%, and accuracy of 79% in discriminating mucinous lesions from nonmucinous cysts. Another study reported a cut-off CEA level of 5 ng/mL in nonmucinous cysts provided 95% specificity, with a cut-off value of 800 ng/mL in mucinous cysts providing a 98% specificity.[23]
In a meta-analysis performed by Thornton et al[21] the sensitivity of CEA for discriminating PCN was 63% and its specificity 88%. A 2013 meta-analysis reported CEA to have a sensitivity and specificity of 63% in predicting malignant cysts.[24] The cut-off value for CEA to discriminate mucinous and nonmucinous pancreatic cysts has not yet been determined. In various studies, values ranging between 30 and 800 ng/mL have been reported.[13,23,25] In our study, we did not identify a difference between benign mucinous and malignant cysts in terms of the level of CEA in cyst fluid. However, the level of CEA was found to be significantly higher in these 2 groups than in the benign nonmucinous group (benign mucinous, 506.8 ± 381.3; malignant cyst, 11324.8 ± 20239.4; benign nonmucinous, 28.6 ± 51.3 ng/mL; P = 0.001). Our findings are in agreement with previous research indicating that CEA level is the most important tumor marker for discriminating mucinous etiology from nonmucinous cystic lesions, but with limited usefulness in differentiating benign and malignant mucinous lesions. Based on our ROC analysis, a cut-off CEA level of 207 ng/mL provided a sensitivity of 72.7%, specificity of 97.7%, and accuracy of 89.5% for the diagnosis of mucinous lesions. When CEA was combined with cytology, the sensitivity increased to 83.3%, specificity to 100%, and accuracy to 95.6%.
In terms of the diagnostic value of CA 72–4, Hammel et al[26] reported a cut-off level >40 U/mL could discriminate mucinous cysts and cystic adenocarcinomas from SCA and pseudocyst, with a sensitivity and specificity of 63% and 98%, respectively. Sperti et al[27] reported CA 72–4 to be higher in mucinous cystic tumors, providing a sensitivity of 80% and a specificity of 95% in differentiating mucinous and malignant cystic tumors. Brugge et al[18] reported a CA 72–4 cut-off level of 7 U/mL as providing a sensitivity of 80%, specificity of 61%, and accuracy of 72% in discriminating mucinous cysts from nonmucinous cysts. In our study, CA 72–4 levels were found to be significantly higher in patients with benign mucinous and malignant cysts, compared to benign nonmucinous cysts: benign mucinous, 26 ± 46.9 U/mL; malignant, 128 ± 263.8 U/mL; and benign nonmucinous, 7.6 ± 20.4 U/mL; P = 0.03. Based on our ROC analysis, a cut-off value of 3.32 U/mL provided a sensitivity of 80%, a specificity of 69.5%, and an accuracy of 73.6% for discriminating mucinous from nonmucinous lesions. The combination of CA 72–4 with CEA or cytology increased the sensitivity, specificity, and diagnostic accuracy in patients with PCN: CA 72.4 + CEA, sensitivity of 84.6%, specificity of 94.1%, and accuracy of 90%; CA 72.4 + cytology, 80%, 100%, and 92.8%, respectively. Clinically, the combination of CA 72–4 and CEA may not make a significant contribution to diagnosis compared to the interpretation of CEA results on their own. Therefore, although the combination of CA 72–4 with cytology results provides a sensitivity, specificity, and accuracy which are comparable to those of CEA, CEA is still considered to be the most appropriate diagnostic marker to use in most cases.
CA 19–9 and amylase are other markers that may inform the classification of pancreatic cysts. Although not as valuable as CEA as a marker, the level of CA 19–9 <37 U/mL may predict benign lesions, such as pseudocyst and SCAs, with a sensitivity of 19% and specificity of 98%.[23] There is conflicting information regarding the utility of amylase in the discrimination of pancreatic cysts. Amylase levels are particularly useful in confirming the diagnosis of pseudocysts in the presence of a history of pancreatitis. According to Oh et al[28] a cut-off value of 6800 U/L discriminates pseudocysts from mucinous neoplasia with a sensitivity, specificity, and accuracy of 66.1%, 81.2%, and 69.3%, respectively. However, a high level of amylase is not specific to pseudocysts.[29] The effectiveness of amylase levels in discriminating benign-malignant lesions has not been shown. Even in our study, levels of CA 19–9 and amylase were comparable between groups.
There are several limitations in our study, which must be considered. Foremost, this is a retrospective study with absence of long-term follow-up patient data. Insufficient cellularity in cytological examination was also an important limitation.
Current approach to the multidisciplinary assessment of pancreatic cysts consists of a combination of routine clinical tests, typically including radiological appearance, EUS findings, cystic fluid analysis, and cytological analysis. However, the diagnosis commonly remains undetermined in the absence of surgical resection. Additional tests providing molecular analysis to the existing approach may increase the sensitivity and specificity of diagnosis. However, genetic examinations are costly and cannot be recommended for routine practice. Combining different tests is, therefore, an important strategy to achieve a more precise diagnosis. For proper treatment of pancreatic cysts, accurate diagnosis is essential to inform treatment, particularly as our knowledge regarding the biological behavior of pancreatic neoplastic cysts evolves.
Footnotes
Abbreviations: CA = cancer antigens , CEA = carcinoembryonic antigen, CT = computed tomography, EUS = endoscopic ultrasound, EUS-FNA = endoscopic ultrasound-guided fine needle aspiration, FNA = fine-needle aspiration, IPMN = intraductal papillary mucinous neoplasia, MCN = mucinous cystic neoplasia, MR = magnetic resonance, NPV = negative predictive value, PCN = pancreatic cystic neoplasia, PPV = positive predictive value, ROC = receiver-operating characteristics, SCA = serous cystadenoma.
The authors have no funding and conflicts of interests to disclose.
References
- [1].Carpizo DR, Allen PJ, Brennan MF. Current management of cystic neoplasms of the pancreas. Surgeon 2008;6:298–307. [DOI] [PubMed] [Google Scholar]
- [2].Garud SS, Willingham FF. Molecular analysis of cyst fluid aspiration in the diagnosis and risk assessment of cystic lesions of the pancreas. Clin Transl Sci 2012;5:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg 2004;239:651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detectedby screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol 2010;8:806–11. [DOI] [PubMed] [Google Scholar]
- [5].Reddy RP, Smyrk TC, Zapiach M, et al. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol 2004;2:1026–31. [DOI] [PubMed] [Google Scholar]
- [6].Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 1999;23:410–22. [DOI] [PubMed] [Google Scholar]
- [7].Kim SC, Park KT, Lee YJ, et al. Intraductal papillary mucinous neoplasm of the pancreas: clinical characteristics and treatment outcomes of 118 consecutive patients from a single center. J Hepatobiliary Pancreat Surg 2008;15:183–8. [DOI] [PubMed] [Google Scholar]
- [8].Mimura T, Masuda A, Matsumoto I, et al. Predictors of malignant intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol 2010;44:224–9. [DOI] [PubMed] [Google Scholar]
- [9].Nara S, Onaya H, Hiraoka N, et al. Preoperative evaluation of invasive and noninvasive intraductal papillarymucinous neoplasms of the pancreas: clinical, radiological, and pathological analysis of 123 cases. Pancreas 2009;38:8–16. [DOI] [PubMed] [Google Scholar]
- [10].Chaudhari VV, Raman SS, Vuong NL, et al. Pancreatic cystic lesions: discrimination accuracy based on clinical data and high resolution CT features. J Comput Assist Tomogr 2007;31:860–7. [DOI] [PubMed] [Google Scholar]
- [11].Frossard JL, Amouyal P, Amouyal G, et al. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am J Gastroenterol 2003;98:1516–24. [DOI] [PubMed] [Google Scholar]
- [12].Sawhney MS, Devarajan S, O’Farrel P, et al. Comparison of carcinoembryonic antigen and molecular analysis in pancreatic cyst fluid. Gastroint Endosc 2009;69:1106–10. [DOI] [PubMed] [Google Scholar]
- [13].Attasaranya S, Pais S, LeBlanc J, et al. Endoscopic ultrasound-guided fine needle aspiration and cyst fluid analysis for pancreatic cancer. J Pancreas 2007;8:553–63. [PubMed] [Google Scholar]
- [14].Park WG, Mascarenhas R, Palaez-Luna M, et al. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas 2011;40:42–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fisher WE, Hodges SE, Yagnik V, et al. Accuracy of CT in predicting malignant potential of cystic pancreatic neoplasms. HPB (Oxford) 2008;10:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brugge WR. The use of EUS to diagnose cystic neoplasms of the pancreas. Gastrointest Endosc 2009;69:203–9. [DOI] [PubMed] [Google Scholar]
- [17].Ahmad NA, Kochman ML, Brensinger C, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc 2003;58:59–64. [DOI] [PubMed] [Google Scholar]
- [18].Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004;126:1330–6. [DOI] [PubMed] [Google Scholar]
- [19].Hewitt MJ, McPhail MJ, Possamai L, et al. EUS guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 2012;75:319–31. [DOI] [PubMed] [Google Scholar]
- [20].Sedlack R, Affi A, Vazquez-Sequeiros E, et al. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc 2002;56:543–7. [DOI] [PubMed] [Google Scholar]
- [21].Thornton GD, McPhail MJ, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology 2013;13:48–57. [DOI] [PubMed] [Google Scholar]
- [22].Thosani N, Thosani S, Qiao W, et al. Role of EUSFNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Dig Dis Sci 2010;55:2756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc 2005;62:383–9. [DOI] [PubMed] [Google Scholar]
- [24].Ngamruengphong S, Bartel MJ, Raimondo M. Cyst carcinoembryonic antigen in differentiating pancreatic cysts: a meta-analysis. Dig Liver Dis 2013;45:920–6. [DOI] [PubMed] [Google Scholar]
- [25].Snozek CL, Mascarenhas RC, O’Kane DJ. Use of cyst fluid CEA, CA19-9, and amylase for evaluation of pancreatic lesions. Clin Biochem 2009;42:1585–8. [DOI] [PubMed] [Google Scholar]
- [26].Hammel P, Voitot H, Vilgrain V, et al. Diagnostic value of CA 72-4 and carcinoembryonic antigen determination in the fluid of pancreatic cystic lesions. Eur J Gastroenterol Hepatol 1998;10:345–8. [DOI] [PubMed] [Google Scholar]
- [27].Sperti C, Pasquali C, Guolo P, et al. Serum tumor markers and cyst fluid analysis are useful for the diagnosis of pancreatic cystic tumors. Cancer 1996;78:237–43. [DOI] [PubMed] [Google Scholar]
- [28].Oh HC, Kang H, Brugge WR. Cyst fluid amylase and CEA levels in the differential diagnosis of pancreatic cysts: a single-center experience with histologically proven cysts. Dig Dis Sci 2014;59:3111–6. [DOI] [PubMed] [Google Scholar]
- [29].Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multi-institutional restrospective study of 398 cases. Ann Surg 1999;230:152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


