Abstract
The HATCH score (hypertension <1 point>, age >75 years <1 point>, stroke or transient ischemic attack <2 points>, chronic obstructive pulmonary disease <1 point>, and heart failure <2 points>) was reported to be useful for predicting the progression of atrial fibrillation (AF) from paroxysmal to persistent or permanent AF for patients who participated in the Euro Heart Survey. The goal of the current study was to investigate whether the HATCH score was a useful scheme in predicting new-onset AF. Furthermore, we aimed to use the HATCH scoring system to estimate the individual risk in developing AF for patients with different comorbidities. We used the “Taiwan National Health Insurance Research Database.” From January 1, 2000, to December 31, 2001, a total of 670,804 patients older than 20 years old and who had no history of cardiac arrhythmias were enrolled. According to the calculation rule of the HATCH score, 599,780 (score 0), 46,661 (score 1), 12,892 (score 2), 7456 (score 3), 2944 (score 4), 802 (score 5), 202 (score 6), and 67 (score 7) patients were studied and followed for the new onset of AF. During a follow-up of 9.0 ± 2.2 years, there were 9174 (1.4%) patients experiencing new-onset AF. The incidence of AF was 1.5 per 1000 patient-years. The incidence increased from 0.8 per 1000 patient-years for patients with a HATCH score of 0 to 57.3 per 1000 patient-years for those with a HATCH score of 7. After an adjustment for the gender and comorbidities, the hazard ratio (95% confidence interval) of each increment of the HATCH score in predicting AF was 2.059 (2.027–2.093; P < 0.001). The HATCH score was useful in risk estimation and stratification of new-onset AF.
Keywords: atrial fibrillation, HATCH score, incidence
1. Introduction
Atrial fibrillation (AF) is an important risk factor for the ischemic stroke, and the AF-related stroke has a worse prognosis and a higher recurrence rate compared with the non-AF-related stroke.[1] It has been believed that AF was revealed as a paroxysmal state and then became sustained as a persistent state.[2] Recently, de Vos et al[3] reported the useful predicting factors of progression from paroxysmal to persistent as a HATCH score (hypertension <1 point>, age >75 years <1 point>, transient ischemic attack [TIA] or stroke <2 points>, chronic obstructive pulmonary disease [COPD] <1 point>, and heart failure <2 points>) in AF patients who participated in the Euro Heart Survey. The progressive mechanism is considered due to the electrical and structural remodeling of the atria based on those risk factors. However, the scoring system for predicting the new-onset of AF has not been well established. Since the risk factors of new-onset of AF could be close to the progression of AF from paroxysmal to persistent, we investigated whether the HATCH score was a useful scheme in predicting new-onset of AF using a large cohort in Taiwan. Furthermore, we aimed to use the HATCH scoring system to estimate the individual risk in developing AF for patients with different comorbidities.
2. Methods
The study design and methodology of the present study are similar to our previous studies.[4–12] This retrospective study used the National Health Insurance Research Database (NHIRD) of the Taiwan National Health Research Institutes. The National Health Insurance (NHI) system is a mandatory universal health insurance program that offers comprehensive medical care coverage to all Taiwanese residents. The NHIRD was a cohort dataset which contained all the medical claims data for 1,000,000 beneficiaries, who were randomly sampled from the 23 million enrollees under the NHI program. In this cohort dataset, the patients’ original identification numbers have been encrypted to protect their privacy, but the encrypting procedure was consistent, so that a linkage of the claims belonging to the same patient was feasible within the NHI database and can be followed continuously. The large database provided a good opportunity to study the usefulness of the HATCH score in predicting new-onset AF.
The HATCH score was calculated for each patient based on a point system (2 points for either a history of TIA/stroke or heart failure, respectively; 1 point for hypertension, age >75 years, or COPD, respectively). Information about important comorbid conditions of each individual was retrieved from the medical claims based on the International Classification of Diseases-Ninth Revision-Clinical Modification (ICD-9-CM) codes. We defined patients with a certain disease only when it was a discharge diagnosis or confirmed more than twice in an outpatient department. The diagnostic accuracies of important comorbidities in Taiwan NHIRD, such as hypertension, diabetes mellitus, heart failure, myocardial infarction, hyperlipidemia, and chronic obstructive pulmonary disease, have been validated before.[13,14]
2.1. Study cohort and clinical endpoint
From January 1, 2000, to December 31, 2011, a total of 670,804 patients older than 20 years and who had no history of cardiac arrhythmias were identified from the Taiwan NHIRD. The HATCH score was calculated for every patient. Finally, 599,780 (score 0), 46,661 (score 1), 12,892 (score 2), 7456 (score 3), 2944 (score 4), 802 (score 5), 202 (score 6), and 67 (score 7) patients were studied and followed for the occurrences of AF. A flowchart of the study enrollment is shown in Fig. 1.
Figure 1.
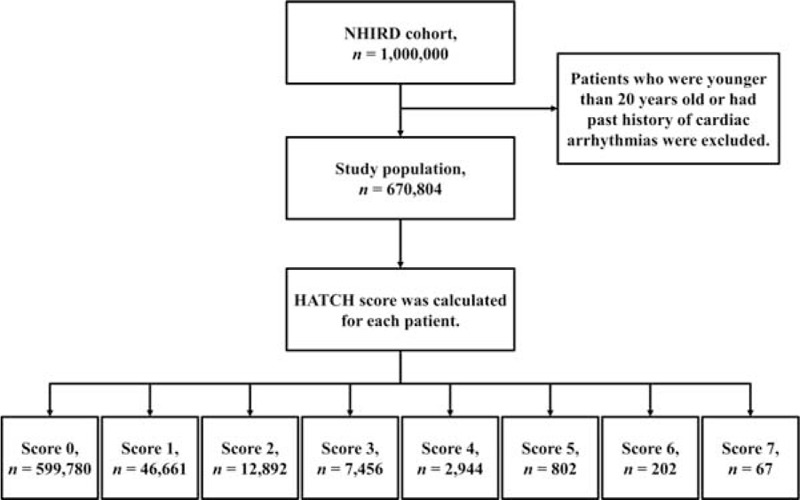
The flowchart of the enrollment of the study patients.
The clinical endpoint was the occurrence of new-onset AF (ICD-9-CM code 427.31) during the follow-up period. To ensure the accuracy of diagnosis, we defined patients as having AF only when it was a discharge diagnosis or confirmed more than twice in the outpatient department.[15] The diagnostic accuracy of AF using definition in NHIRD has been validated previously.[16,17]
2.2. Statistical analysis
Data were presented as the mean ± SD for continuous variables and proportions for categorical variables. An unpaired 2-tailed Student's t test was used for the analysis of continuous variables, and the differences between nominal variables were compared by the chi-square test. The incidence rate of new-onset of AF was calculated by dividing the number of events by person-time at risk with the 95% confidence interval (CI) estimated by exact binomial probabilities. The risk of incidence of AF was assessed using the Cox regression analysis. Statistical significance was set at P<0.05, and all statistical analyses were carried out by using SPSS 17.0 (SPSS, Chicago, IL). The present study was approved by the Institutional Review Board (IRB) at Taipei Veterans General Hospital, Taipei, Taiwan.
3. Results
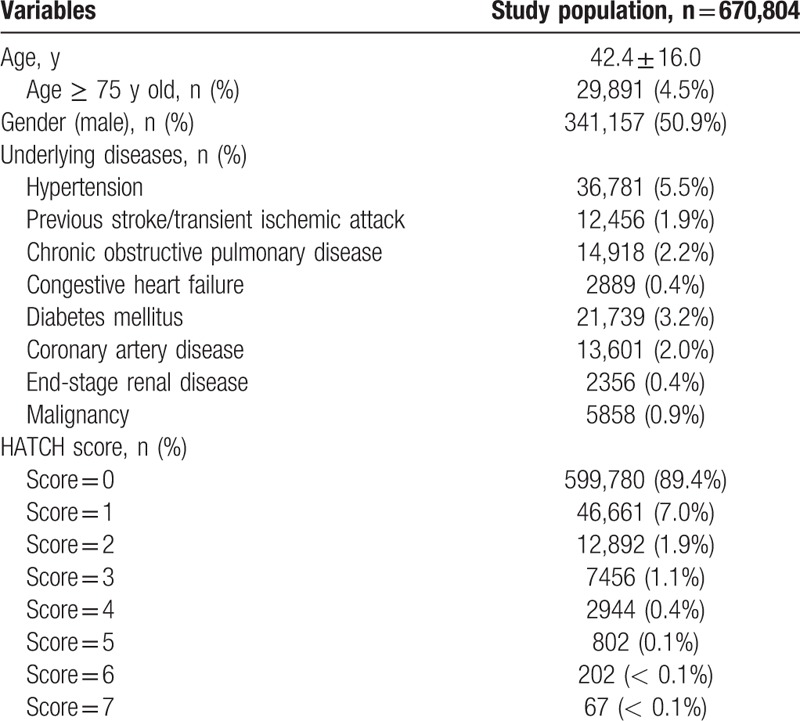
The age, percentage of each risk factor, and HATCH score of the study patients are shown in Table 1. The mean ages were 42.4 ± 16.0 years, and most of the patients (89.4%) have a HATCH score 0.
Table 1.
Baseline characteristics of the patients (n = 670,804).
During a follow-up of 9.0 ± 2.2 years, there were 9174 (1.4%) patients experiencing new-onset AF. The overall incidence of AF was 1.5 per 1000 patient-years. The incidence rate increased from 0.8 per 1000 patient-years for patients with a HATCH score of 0 to 57.3 per 1000 patient-years for those with a HATCH score of 7 (Table 2 and Fig. 2).
Table 2.
Incidence of new-onset AF in patients with different HATCH scores.
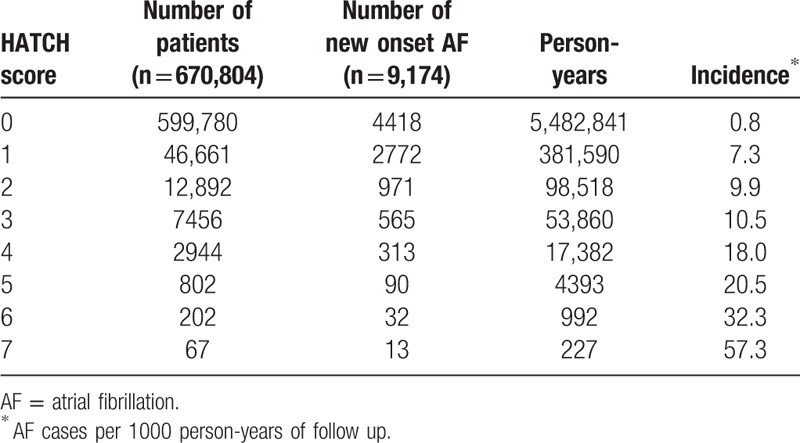
Figure 2.
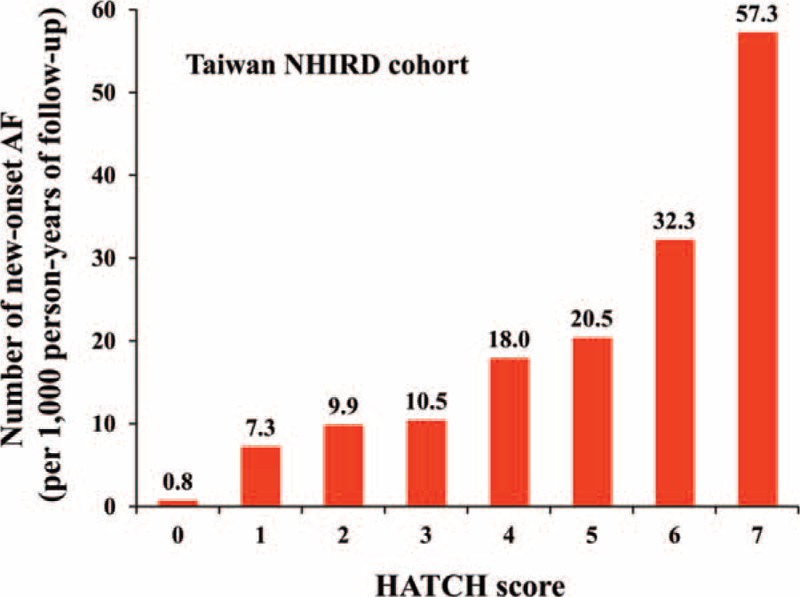
Incidence of new-onset AF in patients with different HATCH scores. AF = atrial fibrillation.
Figure 3 shows that the cumulative incidence curves of AF for patients with different HATCH scores. It demonstrated that patients with higher HATCH scores were associated with a higher rate of new-onset AF during the follow-up period. After an adjustment for the gender and comorbidities other than the components of the HATCH scoring system (diabetes mellitus, coronary artery disease, end-stage renal disease, and malignancy) in the multivariate regression analysis, the hazard ratio (95% confidence interval) of each increment of the HATCH score in predicting AF was 2.059 (2.027–2.093; P <0.001). The relative risk of the development of AF in each group of patients with different HATCH scores compared to those with a HATCH score of 0 represented by hazard ratios is shown in Table 3 and Fig. 4. The c-index on the basis of area under the receiver operating characteristic curve for the HATCH score in predicting new-onset AF was 0.716 (95% confidence interval = 0.710–0.723, P value < 0.001).
Figure 3.
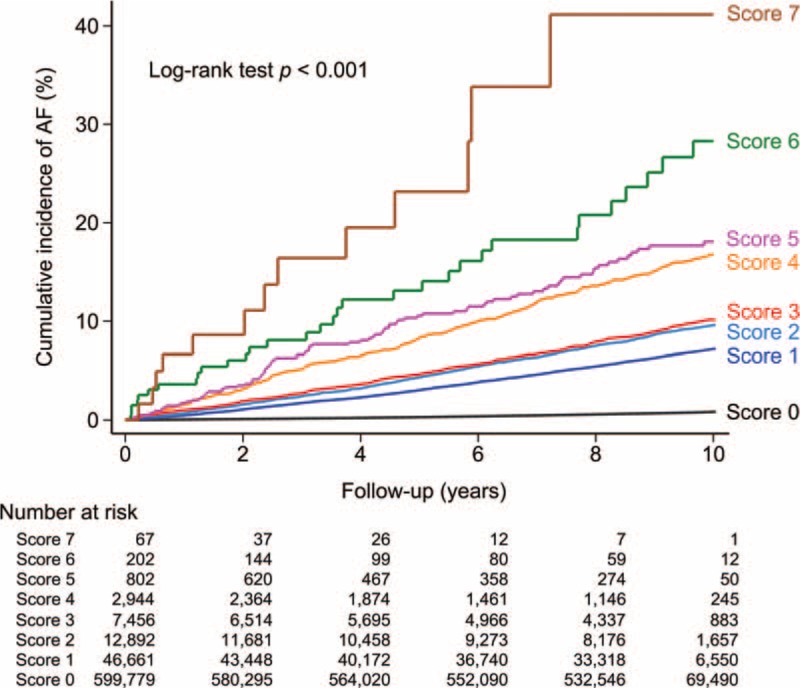
The cumulative incidence curves with a log-rank test demonstrated that patients with higher HATCH scores were associated with a higher rate of new-onset AF during the follow-up period. AF = atrial fibrillation.
Table 3.
Hazard ratio of new onset AF in patients with different HATCH scores in comparison to the patients with a HATCH score of zero.
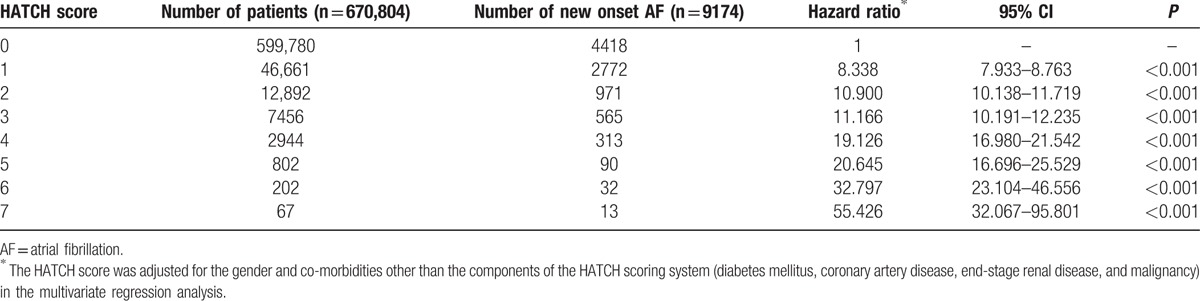
Figure 4.
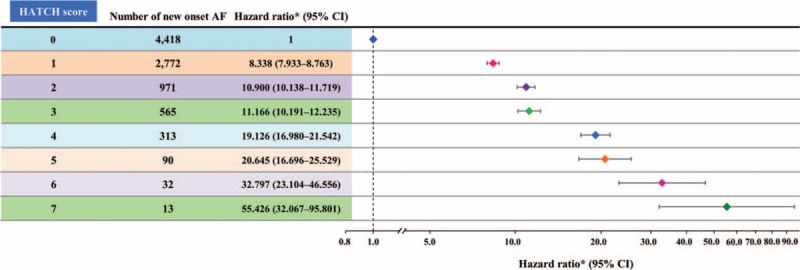
Risk of new-onset AF in patients with different HATCH scores. AF = atrial fibrillation.
4. Discussion
The present study is the first large-scale nationwide population-based investigation analyzing the risk of new-onset of AF stratified by the HATCH score. We demonstrated that the overall incidence rate of AF was 1.5 per 1000 patient-years in Taiwan, and the HATCH score could be used to estimate the individual risk of new-onset AF for patients with different comorbidities.
4.1. HATCH score and risk of incident AF
It is well known that an abnormally rapid firing from the pulmonary veins can trigger and initiate AF.[18,19] Moreover, an arrhythmogenic atrial substrate could be related to AF maintenance rotor with the generation of wave splitting and stabilized re-entry.[20] The AF substrate can result from the electrical and structural remodeling due to an aging effect and several systemic diseases such as hypertension and heart failure.[21,22] Therefore, AF might easily occur and become perpetuated without self-termination to SR among patients having a high HATCH score.
COPD, a pulmonary rather than cardiovascular disorder, represents a unique component of the HATCH scheme. AF and COPD frequently coexist and complicate treatment of both conditions. Previous researches reported that COPD had been present in more than one-tenth of the patients with AF and were associated with increased mortality and incidence of major adverse events.[23–25] Recent studies have demonstrated that reduced forced expiratory volume in 1 second (FEV1) and obstructive respiratory disease were associated with an increased risk of incident AF.[26,27] Li et al[26] analyzed the data of 15,004 middle-aged subjects enrolled in the Atherosclerosis Risk in Communities (ARIC) cohort study; the rate of incident AF was inversely associated with FEV1. There were several possible mechanisms which may explain for the relationship between COPD and AF. Impaired lung function could trigger ectopic beats by deterioration of gas composition and pulmonary hypertension,[28] resulting in elevated atrial pressure and altering the electrophysiological properties of atrial tissues, which in turn trigger AF.[29] Besides, hypercapnia, which was not uncommon in COPD patients, could alter atrial electrophysiology and increase the vulnerability to AF.[30]
4.2. Clinical applications
Although the reported incidence and prevalence of AF is lower in Asians than Caucasians,[31,32] Asia has a higher overall disease burden because of its proportionally larger aged population.[33] For example, on the basis of reported age-adjusted prevalence rates and projected population figures in China, there will be an estimated 5.2 million men and 3.1 million women with AF older than 60 years by year 2050.[33] Therefore, how to estimate the risk of incidental AF among Asians is an important issue. There were several scoring schemes which have been proposed to predict the risk of new-onset AF.[34,35] However, the data from which these clinical risk stratification schemes derive were acquired in cohorts, which mainly consisted of Caucasian patients, such as ARIC and Framingham Heart Studies. In the present study, we showed that an easily calculated clinical scheme, the HATCH score, can be used to estimate the individual risk of incidental AF for patients with different comorbidities. As contrasted with the HATCH score, both of CHADS2 and CHA2DS2-VASc scores include a diabetes mellitus (DM).[7] Several studies show that type 2 DM could play an important role of AF induction and maintenance[22,36] and lead to cerebrovascular complications due to even brief episodes of AF.[37] Moreover, patients with chronic heart failure (CHF) are also reported to have a potential of having subclinical AF.[38] Therefore, DM and CHF patients with cryptogenic stroke and transient ischemic attack who showed high risk of new onset of AF based on the HATCH score should be strongly considered to implant a continuous monitoring device for detecting subclinical AF. The c-index on the basis of area under the receiver operating characteristic curve of HATCH score in predicting new-onset AF (0.716) was similar to, but slightly higher than that of, the CHADS2 score (c-index = 0.713) reported in the previous publication.[5] Furthermore, when patients having a high HATCH score without previously documented AF exhibit acute stroke, we may have to survey for “subclinical” AF more aggressively to reduce the risk of recurrent ischemic events through the use of oral anticoagulants.
4.3. Study limitations
There were several limitations of the present study. First, the diagnosis of AF was based on the diagnostic codes registered by the physicians responsible for the treatments of patients, and was not further checked externally. However, the accuracy of diagnosis of AF in Taiwan's NHIRD has been previously well validated.[16,39] Second, the present study only enrolled Taiwanese patients, and whether the results can be extrapolated to other populations remains uncertain. A further prospective study is necessary to confirm the results of our study.
5. Conclusions
The HATCH score which consisted of an age >75 and several clinical risk factors was useful for the risk estimation and stratification of the development of AF.
Footnotes
Abbreviations: AF = atrial fibrillation, ARIC = atherosclerosis risk in communities, COPD = chronic obstructive pulmonary disease, FEV1 = forced expiratory volume in 1 second, NHIRD = National Health Insurance Research Database, TIA = transient ischemic attack.
Funding: This work was supported in part by grants from the Ministry of Science and Technology (MOST 104–2314-B-075–024-MY3), and Taipei Veterans General Hospital (V99C1–140, V99A-153, V100D-002–3, V101D-001–2, V102B-025, V103B-018, and V105B-023).
The authors have no conflicts of interest to disclose.
References
- [1].Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760–4. [DOI] [PubMed] [Google Scholar]
- [2].Takahashi N, Seki A, Imataka K, et al. Clinical features of paroxysmal atrial fibrillation. An observation of 94 patients. Jpn Heart J 1981;22:143–9. [DOI] [PubMed] [Google Scholar]
- [3].de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. [DOI] [PubMed] [Google Scholar]
- [4].Chang TY, Chao TF, Liu CJ, et al. The association between influenza infection, vaccination, and atrial fibrillation: a nationwide case-control study. Heart Rhythm 2016;13:1189–94. [DOI] [PubMed] [Google Scholar]
- [5].Chao TF, Liu CJ, Chen SJ, et al. CHADS2 score and risk of new-onset atrial fibrillation: a nationwide cohort study in Taiwan. Int J Cardiol 2013;168:1360–3. [DOI] [PubMed] [Google Scholar]
- [6].Chao TF, Liu CJ, Tuan TC, et al. Rate-control treatment and mortality in atrial fibrillation. Circulation 2015;132:1604–12. [DOI] [PubMed] [Google Scholar]
- [7].Chao TF, Liu CJ, Tuan TC, et al. Comparisons of CHADS2 and CHA2DS2-VASc scores for stroke risk stratification in atrial fibrillation: which scoring system should be used for Asians? Heart Rhythm 2016;13:46–53. [DOI] [PubMed] [Google Scholar]
- [8].Chao TF, Liu CJ, Tuan TC, et al. Impact on outcomes of changing treatment guideline recommendations for stroke prevention in atrial fibrillation: a nationwide cohort study. Mayo Clin Proc 2016;91:567–74. [DOI] [PubMed] [Google Scholar]
- [9].Chao TF, Liu CJ, Wang KL, et al. Using the CHA2DS2-VASc score for refining stroke risk stratification in ’low-risk’ Asian patients with atrial fibrillation. J Am Coll Cardiol 2014;64:1658–65. [DOI] [PubMed] [Google Scholar]
- [10].Chao TF, Liu CJ, Wang KL, et al. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol 2015;65:635–42. [DOI] [PubMed] [Google Scholar]
- [11].Chao TF, Wang KL, Liu CJ, et al. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol 2015;66:1339–47. [DOI] [PubMed] [Google Scholar]
- [12].Chao TF, Liu CJ, Liao JN, et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation 2016;133:1540–7. [DOI] [PubMed] [Google Scholar]
- [13].Lin CC, Lai MS, Syu CY, et al. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc 2005;104:157–63. [PubMed] [Google Scholar]
- [14].Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011;20:236–42. [DOI] [PubMed] [Google Scholar]
- [15].Chen SJ, Liu CJ, Chao TF, et al. Dental scaling and atrial fibrillation: a nationwide cohort study. Int J Cardiol 2013;168:2300–3. [DOI] [PubMed] [Google Scholar]
- [16].Lin LJ, Cheng MH, Lee CH, et al. Compliance with antithrombotic prescribing guidelines for patients with atrial fibrillation—a nationwide descriptive study in Taiwan. Clin Ther 2008;30:1726–36. [DOI] [PubMed] [Google Scholar]
- [17].Chang CH, Lee YC, Tsai CT, et al. Continuation of statin therapy and a decreased risk of atrial fibrillation/flutter in patients with and without chronic kidney disease. Atherosclerosis 2014;232:224–30. [DOI] [PubMed] [Google Scholar]
- [18].Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- [19].Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999;100:1879–86. [DOI] [PubMed] [Google Scholar]
- [20].Lin YJ, Suenari K, Lo MT, et al. Novel assessment of temporal variation in fractionated electrograms using histogram analysis of local fractionation interval in patients with persistent atrial fibrillation. Circ Arrhythm Electrophysiol 2012;5:949–56. [DOI] [PubMed] [Google Scholar]
- [21].Tuan TC, Chang SL, Tsao HM, et al. The impact of age on the electroanatomical characteristics and outcome of catheter ablation in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2010;21:966–72. [DOI] [PubMed] [Google Scholar]
- [22].Chao TF, Suenari K, Chang SL, et al. Atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation associated with diabetes mellitus or impaired fasting glucose. Am J Cardiol 2010;106:1615–20. [DOI] [PubMed] [Google Scholar]
- [23].Dankner R, Goldbourt U, Boyko V, et al. Predictors of cardiac and noncardiac mortality among 14,697 patients with coronary heart disease. Am J Cardiol 2003;91:121–7. [DOI] [PubMed] [Google Scholar]
- [24].Sidney S, Sorel M, Quesenberry CP, Jr, et al. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest 2005;128:2068–75. [DOI] [PubMed] [Google Scholar]
- [25].Huang B, Yang Y, Zhu J, et al. Clinical characteristics and prognostic significance of chronic obstructive pulmonary disease in patients with atrial fibrillation: results from a multicenter atrial fibrillation registry study. J Am Med Dir Assoc 2014;15:576–81. [DOI] [PubMed] [Google Scholar]
- [26].Li J, Agarwal SK, Alonso A, et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2014;129:971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Johnson LS, Juhlin T, Engstrom G, et al. Reduced forced expiratory volume is associated with increased incidence of atrial fibrillation: the Malmo Preventive Project. Europace 2014;16:182–8. [DOI] [PubMed] [Google Scholar]
- [28].Ryu JH, Krowka MJ, Pellikka PA, et al. Pulmonary hypertension in patients with interstitial lung diseases. Mayo Clin Proc 2007;82:342–50. [DOI] [PubMed] [Google Scholar]
- [29].Alonso A, Tang W, Agarwal SK, et al. Hemostatic markers are associated with the risk and prognosis of atrial fibrillation: the ARIC study. Int J Cardiol 2012;155:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stevenson IH, Roberts-Thomson KC, Kistler PM, et al. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010;7:1263–70. [DOI] [PubMed] [Google Scholar]
- [31].Ball J, Carrington MJ, McMurray JJ, et al. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol 2013;167:1807–24. [DOI] [PubMed] [Google Scholar]
- [32].Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tse HF, Wang YJ, Ahmed Ai-Abdullah M, et al. Stroke prevention in atrial fibrillation—an Asian stroke perspective. Heart Rhythm 2013;10:1082–8. [DOI] [PubMed] [Google Scholar]
- [34].Chamberlain AM, Agarwal SK, Folsom AR, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol 2011;107:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet 2009;373:739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rizzo MR, Sasso FC, Marfella R, et al. Autonomic dysfunction is associated with brief episodes of atrial fibrillation in type 2 diabetes. J Diabetes Complications 2015;29:88–92. [DOI] [PubMed] [Google Scholar]
- [37].Marfella R, Sasso FC, Siniscalchi M, et al. Brief episodes of silent atrial fibrillation predict clinical vascular brain disease in type 2 diabetic patients. J Am Coll Cardiol 2013;62:525–30. [DOI] [PubMed] [Google Scholar]
- [38].Sardu C, Santamaria M, Rizzo MR, et al. Telemonitoring in heart failure patients treated by cardiac resynchronisation therapy with defibrillator (CRT-D): the TELECART Study. Int J Clin Pract 2016;70:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jansen JW, Haverkate F, Koopman J, et al. Influence of factor XIIIa activity on human whole blood clot lysis in vitro. Thromb Haemost 1987;57:171–5. [PubMed] [Google Scholar]


