Abstract
Background:
A prospective, randomized, double-blind, placebo-controlled study was performed. The routine usage of TA in spinal surgery is controversial. Only a few studies have focused on patients undergoing posterior lumbar surgery for stenosis or spondylolisthesis, although a large clinical cohort exists in the population. This study aimed to evaluate the effect and safety of TA in reducing perioperative blood loss in posterior lumbar surgery for stenosis or spondylolisthesis.
Methods:
100 eligible patients out of 126 were randomized to receive either a bolus dose of 30 mg/kg TA i.v, a maintenance dosage of 2 mg/kg/h TA, or an equivalent volume of normal saline. The pedicle screw system was used for fixing in all the patients, followed by decompression and posterior lumbar interbody fusion. The primary outcomes were intraoperative estimated blood loss and total blood loss. The secondary outcomes were receiving packed red blood cells and postoperative hemoglobin and hematocrit levels.
Results:
In total, 4 patients were excluded from the analyses, 50 patients were in the TA group, and 46 in the placebo group. The demographic and baseline data between the groups were not statistically different. The intraoperative estimated blood loss and the total blood loss were 33% and 41% lower in the TA group than the placebo group, respectively. The blood transfusion rate did not vary significantly (P = 0.191). Except a patient with a dural tear in the placebo group, no other complications were observed.
Conclusion:
TA significantly reduced the perioperative blood loss in patients undergoing posterior lumbar surgery for stenosis or spondylolisthesis.
Keywords: blood loss, decompression, interbody fusion, spinal fixation, spinal stenosis, spondylolisthesis, tranexamic acid
1. Introduction
Lumbar stenosis and spondylolisthesis are the most common diseases[1,2] in clinical practice. Many patients with this disease are required to undergo posterior decompression interbody fusion surgery,[3,4] and massive blood loss is inevitable in the perioperative.[5–7] Although several methods have been introduced to reduce the perioperative blood loss and transfusion requirements, including preoperative autologous blood donation, hypotensive anesthesia, normovolemic hemodilution, perioperative blood salvage, much of the blood loss was yet inevitable.[4,7,8] Such excessive blood loss may cause severe hypotension, metabolic acidosis, infections, acute lung injury, and cardiac arrest.[6,8,9] Some patients might also have to receive an allogeneic blood transfusion, which increases their risk of transfusion-acquired infectious diseases and immune suppression.[8–10]
Recently, a number of studies demonstrated that TA can efficiently decrease the perioperative blood loss and transfusion.[9,11–17] Most of the previous studies focused on patients undergoing spinal correction surgery,[8,9,11,12,16,17] and only a few studies were involved in the patients undergoing posterior lumbar surgery for stenosis or spondylolisthesis.[15,18] Since the surgical procedure for stenosis or spondylolisthesis is not identical to spinal correction surgery, it can be correlated with extensive exposure, long-segment fixation, spinal canal decompression, interbody fusion,[3–5] and massive blood loss.[5,7]
Whether TA efficiently reduces the blood loss in these surgical procedures is yet controversial. A large cohort of patients undergoes this surgery, and hence, the surgeon is required to pay more attention to the perioperative blood loss in the patients.
TA is a synthetic antifibrinolytic drug and can reversibly bind at lysine sites of plasmin and plasminogen. The proteolytic action of plasmin and plasminogen on fibrin is inhibited by this interaction, which further prevents fibrinolysis and protects the clots, thereby decreasing the blood loss from the surgical wound.[19]
The present study evaluated the effect and safety of TA on reducing perioperative blood loss in posterior lumbar surgery for stenosis or spondylolisthesis.
2. Materials and methods
The study was approved by the First Affiliated Hospital of Chongqing Medical University Review Board (20150401) and registered at WHO International Clinical Trials Registry Platform (ChiCTR-IPR-15006088). All the patients were enrolled between January 2015 and March 2016 at the First Affiliated Hospital of Chongqing Medical University.
2.1. Inclusion criteria
The basic inclusion criteria for recruitment were: (1) patients with lumbar spinal stenosis or lumbar spondylolisthesis who were scheduled to undergo posterior lumbar decompression interbody fusion; the conservative therapy had failed. (2) Patients aged 18 to 80 years. (3) Patients who provided written informed consent.
2.2. Exclusion criteria
The exclusion criteria were: (1) allergy to TA. (2) History of bleeding disorders or thromboembolic events. (3) Severe cardiac or respiratory disease and renal or hepatic dysfunction. (4) Platelet count <150,000/mm3. (5) Preoperative Hb <10 g/dL. (6) Uncontrolled hypertension; high blood pressure (BP >160/90 mm Hg). (7) ASA physical status >III. (8) Intake of nonsteroidal anti-inflammatory drugs within 7 days before surgery. (9) Pregnancy.
2.3. Randomization and masking
Each patient eligible for the study was designated a unique sequence number. All the patients were randomly stratified into 3 levels, based on the difficulty of the surgical procedure. Subsequently, the sequence numbers were allocated to either the TA group or the placebo group by the computer-generated random assignment. The protocol assigned for the enrolled patients was also coded. On the day of surgery, a nurse trained in performing the protocol before the study, who was not involved with the trial or was not a personnel caring for the patients, opened the sealed envelope in an isolated room away from the operating room, and prepared the treatment. The bottle with the bolus dose and the syringe with the maintenance intravenous infusion were marked only with the sequence number and the acronym of the patient's name. All the apparatus was indistinguishable between the TA and placebo groups. After the treatment preparation, the envelopes were resealed until disclosed by the statistician. The anesthesiologist, surgeons, researchers, and patients were blinded to the procedure.
2.4. Unblinding for medical emergencies
In case, the anesthesiologist or surgeon suspected medical emergencies associated with TA, such as allergic shock, pulmonary embolism, deep vein thrombosis, stroke, or ischemic heart disease, the nature of the treatment was disclosed.
2.5. Administration program
Patients receiving a loading dose of 30 mg/kg TA over 15 minutes before skin incision followed by a maintenance infusion of TA (2 mg/kg/h) were continued until skin closure in the TA group. Equivalent normal saline was administered in the placebo group.
2.6. Anesthesia and monitoring
The standard general anesthesia applied to all patients was induced with intravenous drugs, including midazolam, sufentanil, propofol, and atracurium. The anesthesia was maintained using sevoflurane and atracurium. Consecutive to standard monitoring, the arterial blood pressure was also continuously monitored by a radial arterial line during operations. The mean arterial blood pressure (MAP) was controlled at about 20% below the preoperative baseline pressure in both the groups, until the procedures of decompression, fusion, and instrumentation were completed following which it was returned to the baseline.
The maintenance fluid requirements and third-space losses were regulated by colloid and crystal solution.
The transfusion threshold for the intraoperative Hb level was <7.0 g/dL, which was guided by arterial blood gas analysis, arterial blood pressure, central venous catheter, and urinary output.
2.7. Surgical procedure
All the surgeries in both the TA group and placebo group patients were performed by 2 surgeons: one with 12 years’ experience in spinal surgery and the other with 17 years. The attending surgeons completed a short questionnaire about the difficulty of surgery, a day before the operation. The operations were divided into 3 levels based on surgical difficulty (level I: low difficulty; level II: medium difficulty; level III: high difficulty). Conventionally, the decompression fusion and fixation of 1–2 segments were defined as level I, 3–4 segments as level II, and more than 4 segments as level III.[14,20,21]
Surgeries involved revision, extensive decompression, severe ASA physical status, and corpulent patients, which would increase the surgical difficulty,[7,14,22] requiring the surgeons to adjust the assessment of surgical difficulty.
All patients were placed in a prone position on the Hall–Relton frame or the Jackson table with abdomen free and underwent posterior decompression interbody fusion and fixation with the pedicle screw. Using the posterior midline approach, the lamina, facet joints, and spinous processes of fixation segment were exposed. After the pedicle screws system had been implanted, and the appropriate location confirmed by radiography, the 2 connecting rods were installed. Next, semilaminectomy and removal of the disc in the decompression segment and a CAGE with autogenous bone granules tamponade was placed into the intervertebral space. Then, radiography was used to confirm the position of CAGE at the appropriate region. The iliac crest bone was not used for grafting. During the procedure of decompression and fusion, it is essential that surgeon carefully protects the nerve root and dura mater spinalis, and avert any injury to venous plexus. Before closing the wound, the bleeding is cautiously stanched, and drainage placed routinely.
2.8. Data collection
The primary outcomes were intraoperative estimated blood loss and total blood loss. The intraoperative estimated blood loss was estimated by a trained nurse before starting the trial and was blinded to the treatment and patient groups. The intraoperative estimated blood loss was evaluated by adding the weight of gauzes and sponges to the volume of blood in the negative pressure suction bottles, and subtracting the volume of irrigation fluids. The postoperative drainage was recorded daily from the volume of blood accumulated in the negative pressure drainage bottles until the drainage was removed (when the volume of drainage was less than 30 mL/d). The total amount of bleeding was equal to the intraoperative estimated blood loss plus postoperative drainage. The second outcome was receiving packed red blood cells, as well as postoperative hemoglobin, hematocrit levels.
Postoperatively, the laboratory parameters such as Hb, HCT, PLT, PT, APTT, as well as, fibrinogen and D-dimer were estimated on postoperative days 1 and 3.
When the postoperative Hb level was <70 g/L, 7.0 to10.0 g/dL and patients exhibited symptomatic anemia, such as tachycardia, fatigue, lethargy, poor appetite, and pallor, packed red blood cells were administered.
Data were collected on patients’ demographics, laboratory values, intra- and postoperative blood loss, the surgical procedure to be performed, and blood products transfused perioperatively. The inpatients were daily observed for putative complications and unexpected events associated with treatment, such as the clinical symptoms of ischemic heart disease, stroke, deep vein thrombosis, and pulmonary embolism. All discharged patients were followed-up each week until the day 35 after the operation.
2.9. Statistical methods
The data were analyzed by SPSS software version 17 (SPSS Inc., Chicago, IL). A previous study showed that the mean perioperative blood loss for lumbar posterior interbody fusion was 500 ± 350 mL when treated at our institution. Presuming a 30% reduction in the perioperative blood loss, 2-tailed test with 0.05 alpha error, and a statistical power of 0.8, 46 patients were needed for each group. Therefore, we strategized to enroll 50 patients in each group.
The qualitative data were expressed numerically and also as a percentage. The parametric quantitative data were expressed as mean and standard deviation, whereas the nonparametric quantitative data were expressed as median (range). The qualitative data were compared with the χ2 test or Fisher's exact test. The parametric quantitative data were analyzed using an unpaired t-test, whereas the nonparametric quantitative data were compared with the Mann–Whitney U test. Correlation analysis was used to explore the potential impact factors for total blood loss. Then, in order to find the weight of potential risk factors for significant bleeding, we used multiple logistic regression analysis to construct the formula model. P < 0.05 was considered statistically significant.
3. Results
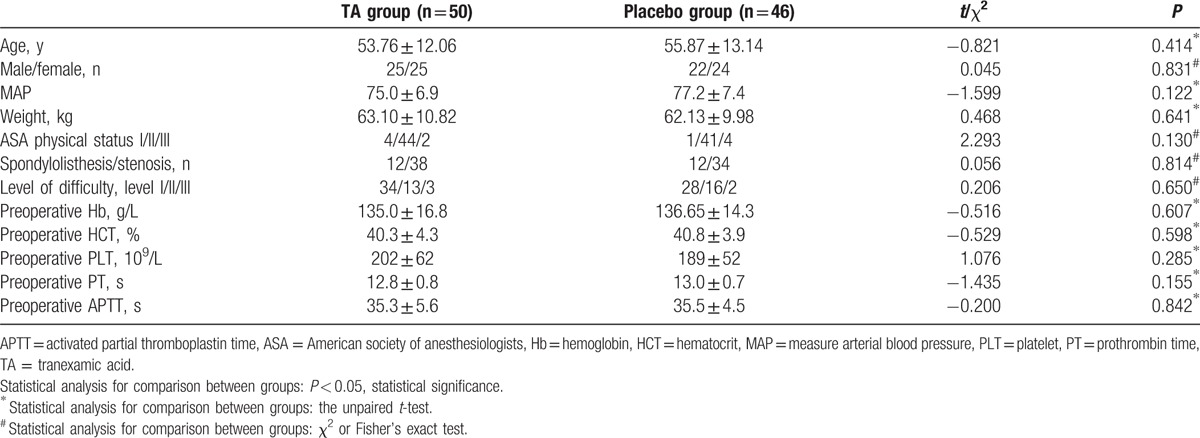
A cohort of 126 consecutive patients was screened, and 100 patients aged 22 to 78 years were enrolled. These patients presented lumbar spinal stenosis or lumbar spondylolisthesis and were undergoing posterior decompression interbody fusion fixation with the pedicle screw. In total, 51 patients were randomly assigned in the TA group and 49 in the placebo group (mean ages, 56 and 54 years, respectively). During the operation, 3 patients changed to the simple procedure (1 in the TA group and 2 in the placebo group), and dural tear occurred in 1 patient in the placebo group. Thus, 4 patients were excluded from all the analyses (Fig. 1). Moreover, any statistical differences were not observed between the 2 groups with respect to age, sex, MAP, weight, ASA physical status, and diagnosis. Additionally, the preoperative parameters of Hb, HCT, PLT, PT, and APTT were also not remarkably different between the 2 groups (Table 1).
Figure 1.
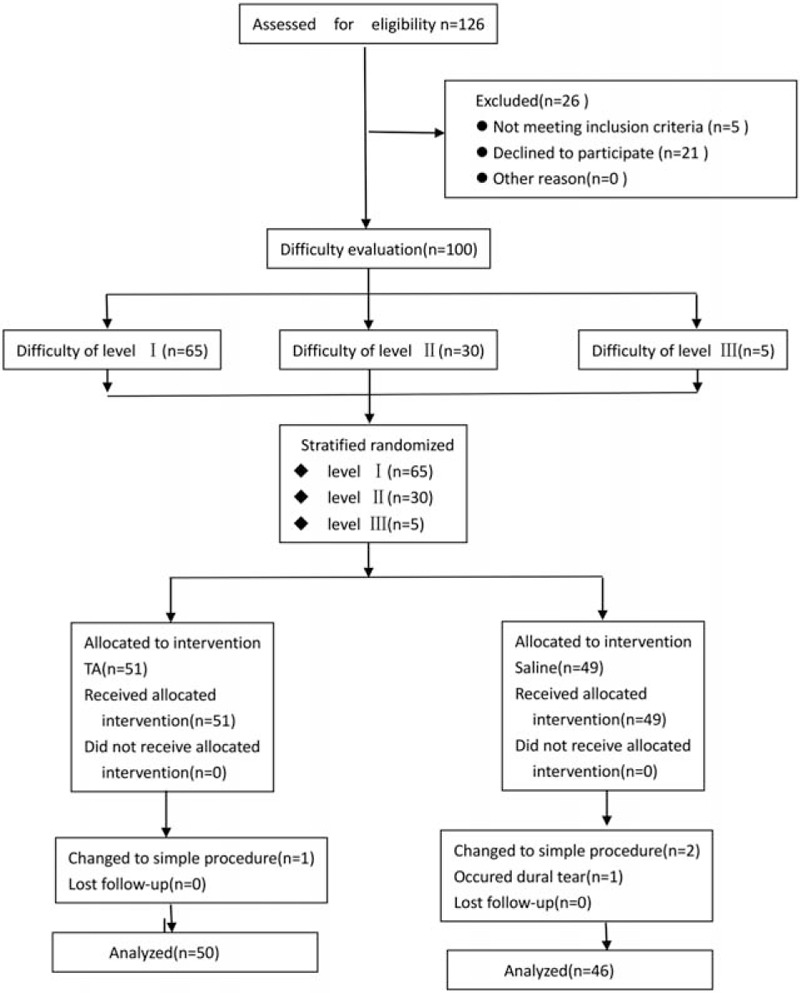
Schematic of the study design. TA indicates tranexamic acid. TA = tranexamic acid.
Table 1.
Baseline profile of the 2 study groups.
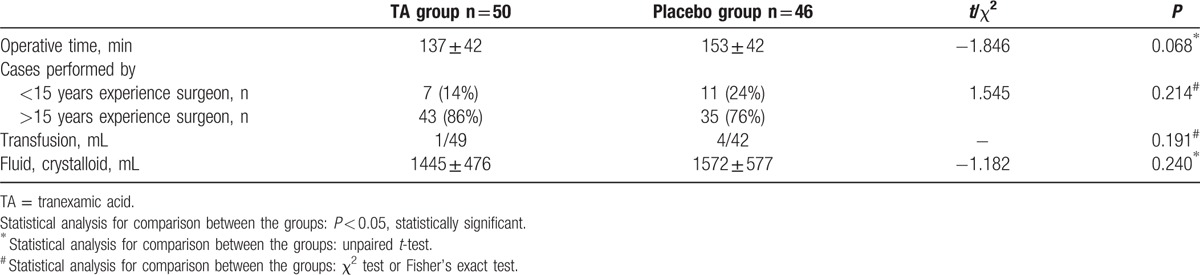
However, intraoperative estimated blood loss (170 ± 153 mL vs 255 ± 188 mL, P = 0.016), postoperative drainage (145 ± 84 mL vs 281 ± 165 mL, P ≤ 0.001), and total blood loss (315 ± 205 mL vs 536 ± 261 mL, P < 0.001) were significantly different between the TA and placebo groups (Fig. 2). On the other hand, the operative time, surgeons, transfusion, and total liquid volume of fluid and crystalloid did not exhibit distinct differences between the TA and placebo groups (Table 2).
Figure 2.
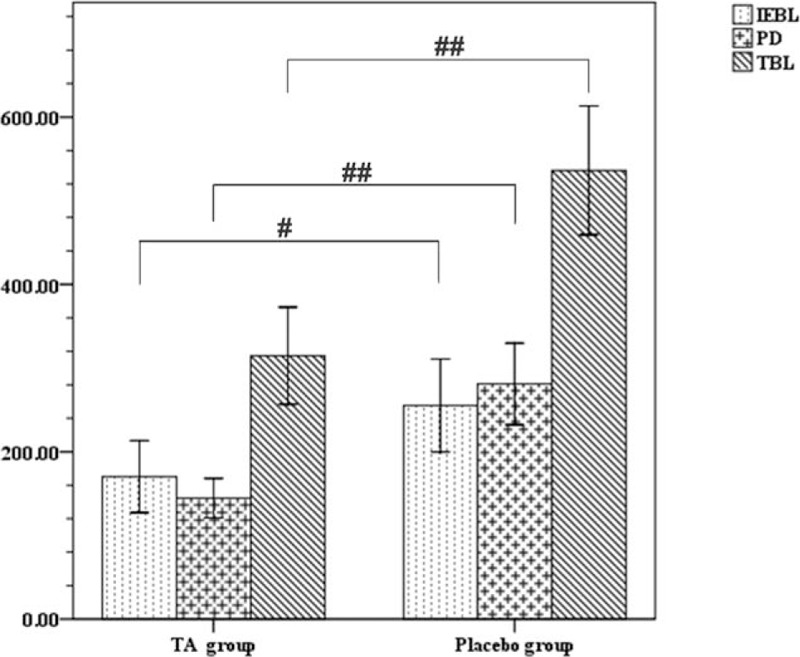
The differences between the 2 groups on IEBL, PD, TBL. Data were analyzed by 1-way ANOVA. #P < 0.05; ##P < 0.001, IEBL = intraoperative estimated blood loss, PD = postoperative drainage, TBL = total blood loss.
Table 2.
Operative data.
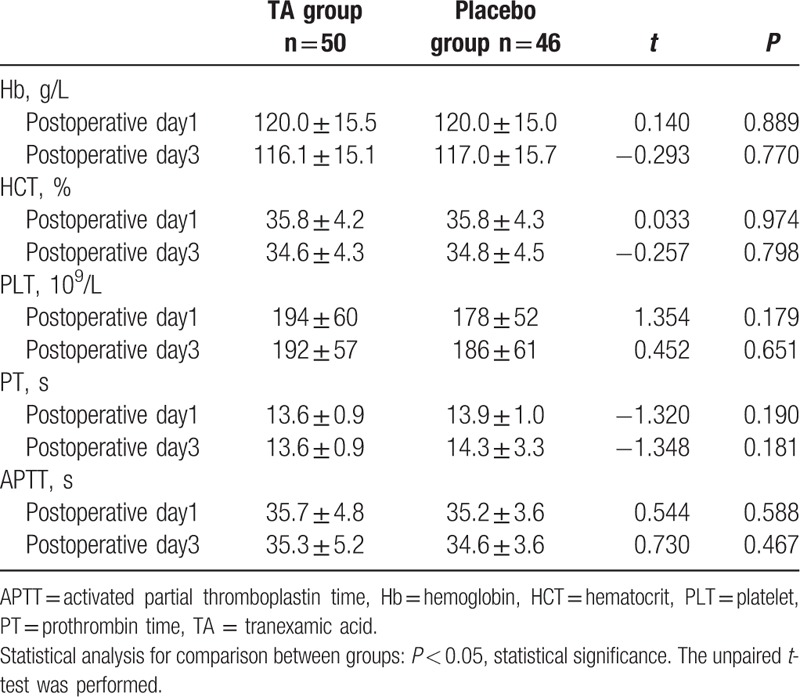
Similar to the preoperative parameters, the postoperative Hb, HCT, PLT, PT, and APTT were also not statistically different between the groups (Table 3). Except a patient with dural tear in the placebo group, which was excluded from the statistical analysis, no other major complications, such as deep vein thrombosis, pulmonary embolism, stroke, ischemic heart disease, epidural hematoma formation, allergic reaction, liver and kidney dysfunction were observed.
Table 3.
Postoperative hematocrit and coagulation test.
Correlation analysis showed that TA, operative time, difficult level of surgery and prothrombin time were of potential impact on total blood loss (Table 4). Furthermore, the formula model conducted with multiple logistic regression analysis showed that only the operation time and TA were the important factors for significant bleeding (total blood loss >500 mL). TA showed a distinctive protective effect for blood loss (OR 0.002, 95% CI 2.483–25.371, P < 0.001) (Table 5).
Table 4.
Correlation analysis for potential impact factors to total blood loss.
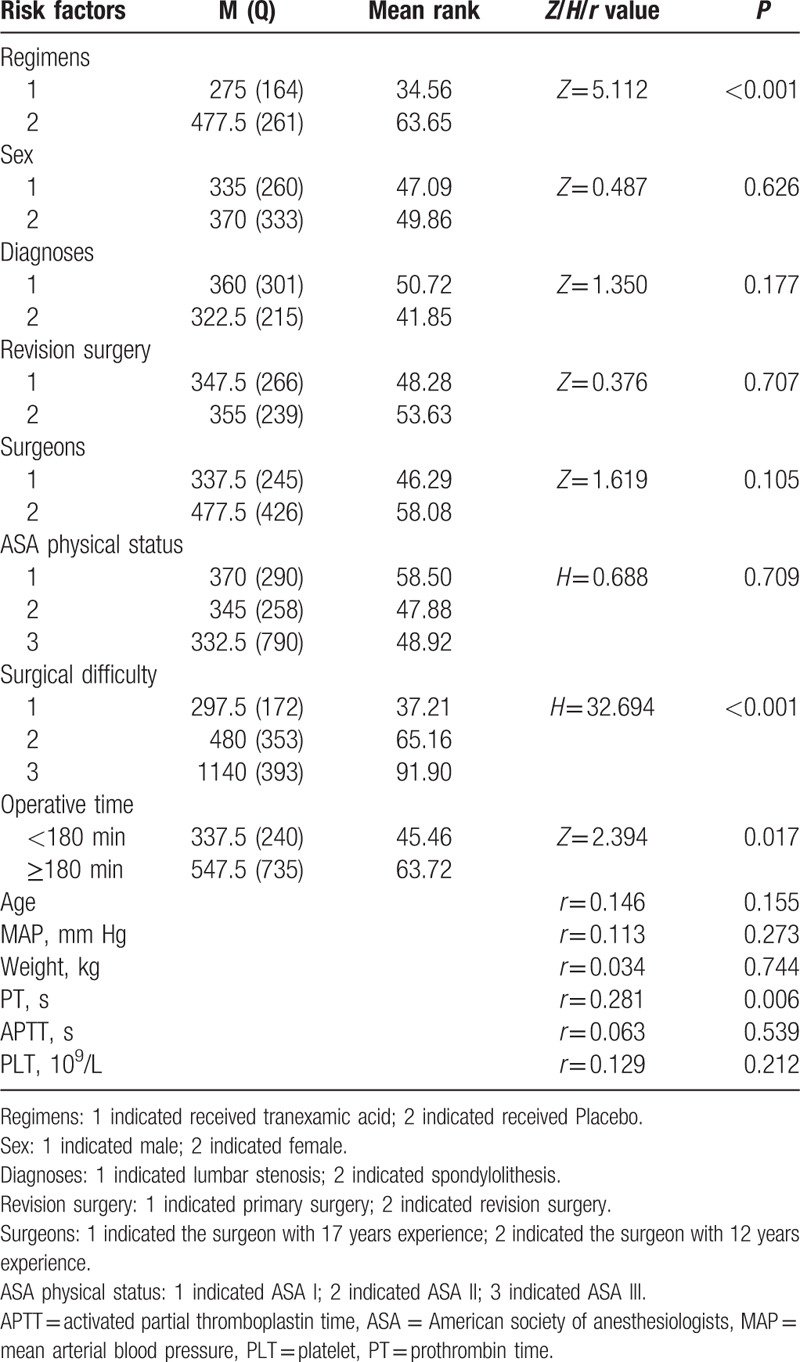
Table 5.
Risk factors for significant bleeding (>500 mL) from multiple logistic regression analysis.

4. Discussion
The present study aimed to evaluate the efficacy and safety of TA in reducing perioperative blood loss in posterior lumbar surgery for stenosis or spondylolisthesis. Our results showed 33% reduced intraoperative estimated blood loss in the TA group and 41% total blood loss as compared to the placebo group. Only 1 patient showed the occurrence of a dural tear in the placebo group; no other adverse events with the use of TA were seen in our study.
Previous studies showed that ASA physical status, body weight, different surgical techniques, the number of fusion segment, attending surgeons, duration of operation, and heterogeneous patient populations for various diagnosis may impact the perioperative blood loss.[9,13,20,22,23] In our study, the patient profile, diagnosis, ASA physical status, and level of difficulty did not differ between the 2 groups. Moreover, the operation time and the patients treated by the 2 attending surgeons between the 2 groups did not exhibit any statistical difference. All the surgical procedures were similar among the patients undergoing posterior decompression lumbar interbody fusion fixation with the pedicle screw. Hence, the baseline was identical between the TA and placebo groups.
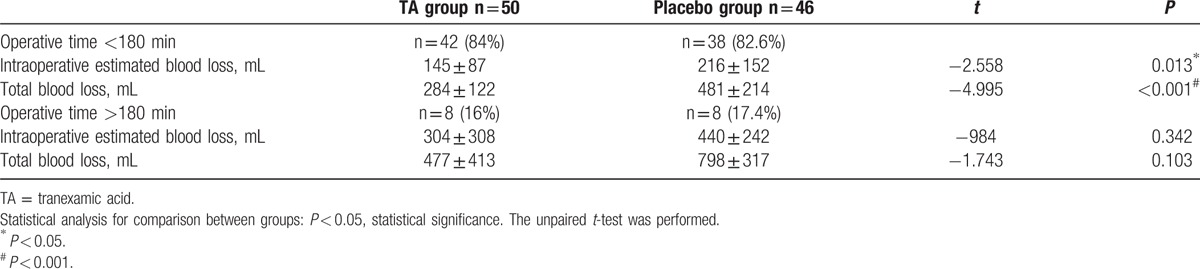
Although correlation analysis showed that tranexamica acid, operative time, surgical difficulty, PT were potential factors for blood loss; the logistic regression model predicted that only TA and operative time were the important factors for significant bleeding. TA was found to exert an impact on decreasing the perioperative blood loss (OR 0.002, 95% CI 2.483–25.371, P < 0.001), which was in agreement with the previous studies.[9,13,20] In order to eliminate the interference of operative time, we had divided patients into subgroups depending on whether their operative time was more than 180 minutes or not. There were 80 patients’ operative times less than 180 minutes, and the intraoperative estimated blood loss, total blood loss were significantly different between the TA group and the PL group (P = 0.013, P < 0.001). This meant TA still significantly reduced the blood loss in this subgroup. Nevertheless, the intraoperative time and total blood loss were not significantly different between the 2 groups when the operative time was more than 180 minutes (Table 6). Although TA did not lead to significant reduction in the perioperative blood loss, there were only 16 patients in this subgroup, which may be inadequate to detect a difference in the variables.
Table 6.
Subgroup analysis of perioperative blood loss in different operative time.
The etiology of the perioperative blood loss for spinal surgery is multifactorial.[3,5,8,19] The increased fibrinolytic activity and spinal venous plexus injury are critical factors contributing toward the perioperative blood loss.[8,19,24] TA can competitively inhibit plasmin and plasminogen, thereby impeding the fibrinolysis and stabilizing the blood clot.[19] Previous studies demonstrated that TA could inhibit the fibrinolytic activity and decrease blood loss in spinal deformity surgery or long segment fixation and fusion surgery.[9,11–13,25] Elwatidy et al[25] showed that TA reduced 49% blood loss in the spinal surgery. Berney et al[11] reported that TA decreased nearly 50% intraoperative estimated blood loss in adolescent idiopathic scoliosis surgery. The surgery for lumbar stenosis and spondylolisthesis is not identical to the deformity correction surgery or simple long segment fixation and fusion surgery. The vertebral venous plexus is common in patients with lumbar stenosis or spondylolisthesis,[24,26] when injured during the operation, renders hemostasis difficult.[4,14,27] TA can supposedly stabilize the blood clot to block the epidural venous plexus vascular rupture, thus, reducing the bleeding.[14,19,28]
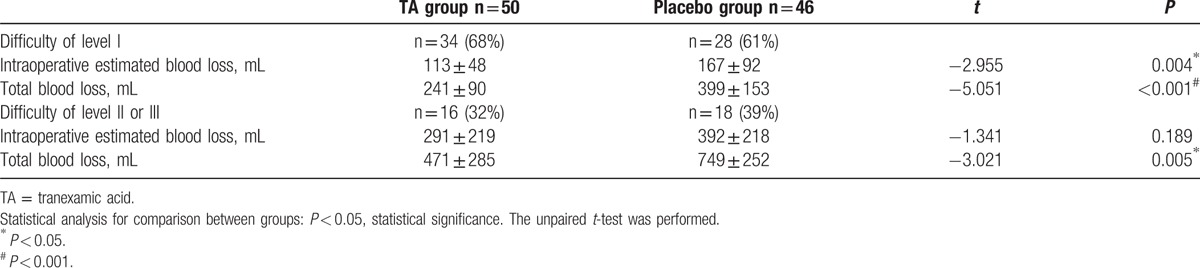
Nevertheless, the hemostatic efficacy of TA is yet unconfirmed in the patients undergoing posterior lumbar surgery for stenosis or spondylolisthesis. Wang et al[15] administered 15 mg/kg TA, which reduced 13% total blood loss in patients undergoing posterior lumbar interbody fusion for stenosis in a randomized controlled trial, and the total blood loss was statistically different between the TA and placebo groups. This study subtly assists the clinicians to make operative decisions. Although the study designed by Wang et al was rudimentary, it was composed on a well-balanced baseline index, and the blood loss was observed to be remarkably different between the treatment and control groups. Moreover, the study did not describe any sample size or test power analysis. Typically, any intervention evaluated in a randomized controlled trial only reduces more than 25%, which calls for attention.[11,13,14] The 13% reduction of the total blood loss could not provide reliable evidence for the usage of TA. The present study manifested administering 30 mg/kg initial dose and 2 mg/kg/h maintenance dosage, which lessened 41% total blood loss. This prospective, randomized, double-blind, placebo-controlled study differed from that of Wang et al as we assumed 30% reduction in blood loss, which was clinically significant. The sample size could be recalculated based on the study. The 41% reduction of total blood loss in the present study could provide reliable evidence for the standard use of TA in the surgery for lumbar stenosis or spondylolisthesis. Another difference between Wang et al and our study was the patient population. Only patients with surgical difficulty levels II and III were enrolled in their study, whereas our study also included the difficulty level I patients. The level I is a huge cohort in clinical practice, and any measures to reduce the bleeding should not be ignored in this population. Therefore, we used stratified randomization to prevent interference by the difficulty levels. The composition of the population between the groups was not statistically different. The subgroup analysis exhibited that TA not only lessened the perioperative blood loss in medium and high-level difficulty surgeries for stenosis or spondylolisthesis but also decreased the blood loss in the low difficulty surgeries for these diseases (Table 7). These results showed the similar effect to those in previous studies.[9,11–14,16] The third difference with Wang et al was the administered dose regimen. More than 15 mg/kg is considered as a high dose regimen.[19] Karski[29] conducted a comparison of different dose regimens of TA in cardiac surgery. They found that 100 mg/kg TA has better hemostasis effect than that of 50 mg/kg TA. Using a initial dose of 30 mg/kg TA i.v. and a maintenance dosage of 2 mg/kg/h TA, Elwatidy et al[25] had a similar result to our study. So our study maybe partly support that the high dose of TA have better effect than the low dose of TA in spinal surgeries.
Table 7.
Subgroup analysis of perioperative blood loss in different difficulty level surgeries.
There was an interesting phenomenon that the perioperative blood loss was significantly different between TA and PL groups, whereas postoperative Hb and HCT were not significantly different. We aboratively analyzed the data, and then found that although there was no statistically significant difference in the blood transfusion requirement between the 2 groups, the patients number received packed red blood cells in the placebo group was obviously larger than that of the TA group (4 patients in PL group vs 1 patient in TA group). This may lessen the difference of Hb/HCT between the 2 groups. This is in line with previous studies.[9,14]
The postoperative epidural hematoma is a severe complication of after lumbar decompression surgery.[14,30] TA inhibits fibrinolysis and stabilizes blood clot, thereby decreasing the epidural and subcutaneous hematoma formation.[14,19] All patients were followed-up for more than 35 days after the operation. Any hematoma formation in either of the groups, due to the low incidence of hematoma, was not seen[30]; since the sample size was small, the difference may be not discovered.
A common concern related to the use of TA is its potential for inducing thromboembolic events. Several studies have confirmed that TA does not increase the risk of deep vein thrombosis and pulmonary embolism.[8,9,11–16,19] In our study, no clinical symptoms of deep vein thrombosis or signs of pulmonary embolism were revealed. Any complications associated with TA, such as allergic reaction, stroke, ischemic heart disease, and dysfunction of liver and kidney, also did not occur. Thus, TA can be safely administered in patients undergoing posterior interbody fusion surgery for lumbar stenosis or spondylolisthesis.
However, the present study conferred some limitations. Due to more than 50% patients belonging to the population with low difficulty surgeries, the effect of the test for patients with the difficulty of level II and III would be lessened. We carried out a subgroup analysis based on the level of difficulty. Patients with only the difficulty levels II and III did not show any statistical difference between the 2 groups in intraoperative estimated blood loss, the reduction trend was visible (less 26%); the total blood loss in the TA group was significantly less than the placebo group (Table 7), which is in agreement with the previous studies.[15,18] Nevertheless, future studies would be designed to adequately decrease the number of patients with difficulty level I, and increase the population of level II and III. The second limitation was single dose treatment regimen, as the optimal dose regimen in this kind of spinal surgery is not known. A simultaneous efficacy comparison of different dose regimens of TA will also be investigated. The third limitation was that there were 2 surgeons included in our study. Some documents reported that surgeons may be a potential factor affecting the amount of bleeding during the perioperative period. However, the operative time, intraoperative estimated blood loss, and total blood loss were not statistically different and the correlation analysis showed the surgeons were not significant factor for total blood loss. The significance of surgeons’ effects may be minimal, given the fact the patients were equally distributed in both groups, and the surgeons equally taken part in surgeries between the groups, as well as all procedures were similarly performed in the same institution. The present study agrees with the previous documents.[13,14,25]
5. Conclusion
TA efficacy can safely reduce the perioperative blood loss in patients undergoing posterior decompression interbody instrumentation for stenosis or spondylolisthesis. A simultaneous comparison of different dose regimens of TA should be conducted in order to describe the efficiency of the drug for a wide application in the future.
Acknowledgments
The authors thank Zhang Junhui (Department of Statistics, Southwest Medical University) who provided statistical support.
Footnotes
Abbreviations: APTT = activated partial thromboplastin time, ASA = American society of anesthesiologists, BP = blood pressure, Hb = hemoglobin, HCT = hematocrit, MAP = mean arterial blood pressure, PLT = platelet, PT = prothrombin time, TA = tranexamic acid.
Funding: This study was funded by the First Affiliated Hospital of Chongqing Medical University.
The authors have no conflicts of interest to disclose.
References
- [1].Ishimoto Y, Yoshimura N, Muraki S, et al. Associations between radiographic lumbar spinal stenosis and clinical symptoms in the general population: the Wakayama Spine Study. Osteoarthritis Cartilage 2013;21:783–8. [DOI] [PubMed] [Google Scholar]
- [2].Sakai T, Sairyo K, Takao S, et al. Incidence of lumbar spondylolysis in the general population in Japan based on multidetector computed tomography scans from two thousand subjects. Spine 2009;34:2346–50. [DOI] [PubMed] [Google Scholar]
- [3].Liu X, Wang Y, Qiu G, et al. A systematic review with meta-analysis of posterior interbody fusion versus posterolateral fusion in lumbar spondylolisthesis. Eur Spine J 2014;23:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Musluman AM, Yilmaz A, Cansever T, et al. Posterior lumbar interbody fusion versus posterolateral fusion with instrumentation in the treatment of low-grade isthmic spondylolisthesis: midterm clinical outcomes. J Neurosurg Spine 2011;14:488–96. [DOI] [PubMed] [Google Scholar]
- [5].Lee SY, Kim TH, Oh JK, et al. Lumbar stenosis: a recent update by review of literature. Asian Spine J 2015;9:818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Szpalski M, Gunzburg R, Sztern B. An overview of blood-sparing techniques used in spine surgery during the perioperative period. Eur Spine J 2004;13(suppl 1):S18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hu SS. Blood loss in adult spinal surgery. Eur Spine J 2004;13(suppl 1):S3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Neilipovitz DT. TA for major spinal surgery. Eur Spine J 2004;13(suppl 1):S62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sethna NF, Zurakowski D, Brustowicz RM, et al. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology 2005;102:727–32. [DOI] [PubMed] [Google Scholar]
- [10].Perel P, Clayton T, Altman DG, et al. Red blood cell transfusion and mortality in trauma patients: risk-stratified analysis of an observational study. PLoS Med 2014;11:e1001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Berney MJ, Dawson PH, Phillips M, et al. Eliminating the use of allogeneic blood products in adolescent idiopathic scoliosis surgery. Eur J Orthop Surg Traumatol 2015;25(suppl 1):S219–223. [DOI] [PubMed] [Google Scholar]
- [12].Peters A, Verma K, Slobodyanyuk K, et al. Antifibrinolytics reduce blood loss in adult spinal deformity surgery: a prospective, randomized controlled trial. Spine (Phila, PA 1976) 2015;40:E443–449. [DOI] [PubMed] [Google Scholar]
- [13].Raksakietisak M, Sathitkarnmanee B, Srisaen P, et al. Two doses of tranexamic acid reduce blood transfusion in complex spine surgery: a prospective randomized study. Spine (Phila, PA 1976) 2015;40:E1257–1263. [DOI] [PubMed] [Google Scholar]
- [14].Tsutsumimoto T, Shimogata M, Ohta H, et al. Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: a prospective randomized study. Spine (Phila, PA 1976) 2011;36:1913–8. [DOI] [PubMed] [Google Scholar]
- [15].Wang Q, Liu J, Fan R, et al. Tranexamic acid reduces postoperative blood loss of degenerative lumbar instability with stenosis in posterior approach lumbar surgery: a randomized controlled trial. Eur Spine J 2013;22:2035–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Winter SF, Santaguida C, Wong J, et al. Systemic and topical use of tranexamic acid in spinal surgery: a systematic review. Global Spine J 2016;6:284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang B, Li H, Wang D, et al. Systematic review and meta-analysis of perioperative intravenous tranexamic acid use in spinal surgery. PloS One 2013;8:e55436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Endres S, Heinz M, Wilke A. Efficacy of tranexamic acid in reducing blood loss in posterior lumbar spine surgery for degenerative spinal stenosis with instability: a retrospective case control study. BMC Surg 2011;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs 2012;72:585–617. [DOI] [PubMed] [Google Scholar]
- [20].Wong J, El Beheiry H, Rampersaud YR, et al. Tranexamic acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg 2008;107:1479–86. [DOI] [PubMed] [Google Scholar]
- [21].Neilipovitz DT, Murto K, Hall L, et al. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg 2001;93:82–7. [DOI] [PubMed] [Google Scholar]
- [22].Gill JB, Chin Y, Levin A, et al. The use of antifibrinolytic agents in spine surgery. A meta-analysis. J Bone Joint Surg Am 2008;90:2399–407. [DOI] [PubMed] [Google Scholar]
- [23].Cheriyan T, Maier SP, 2nd, Bianco K, et al. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J 2015;15:752–61. [DOI] [PubMed] [Google Scholar]
- [24].Ju JH, Ha HG, Jung CK, et al. Patterns of epidural venous varicosity in lumbar stenosis. Korean J Spine 2012;9:244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Elwatidy S, Jamjoom Z, Elgamal E, et al. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine (Phila, PA 1976) 2008;33:2577–80. [DOI] [PubMed] [Google Scholar]
- [26].Paksoy Y, Gormus N. Epidural venous plexus enlargements presenting with radiculopathy and back pain in patients with inferior vena cava obstruction or occlusion. Spine (Phila, PA 1976) 2004;29:2419–24. [DOI] [PubMed] [Google Scholar]
- [27].Choksey MS. Peri-operative lumbar epidural bleeding controlled by dural tube reflation. Brit J Neurosurg 2009;23:437–8. [DOI] [PubMed] [Google Scholar]
- [28].Taghaddomi RJ, Mashhadiezhad H, Attar ARS, et al. The effect of intravenous tranexamic acid on blood loss in lumbar hernial disc resection under inhalation and total intravenous anesthesia. Iranian Red Crescent Med J 2009;11:265–70. [Google Scholar]
- [29].Karski JM, Dowd NP, Joiner R, et al. The effect of three different doses of tranexamic acid on blood loss after cardiac surgery with mild systemic hypothermia (32 degrees C). J Cardiothorac Vasc Anesth 1998;12:642–6. [DOI] [PubMed] [Google Scholar]
- [30].Kao FC, Tsai TT, Chen LH, et al. Symptomatic epidural hematoma after lumbar decompression surgery. Eur Spine J 2015;24:348–57. [DOI] [PubMed] [Google Scholar]


