Abstract
The incidence of colorectal cancer is rapidly increasing in South Korea. It is important to clarify the association between colorectal cancer and diet, being one of the main modifiable risk factors, as such studies in the Korean population are lacking.
A cross-sectional study was performed using data from participants who had undergone a screening colonoscopy and a nutritional assessment during a routine health check-up from January 2008 to December 2011. Dietary intake data were derived from 1-day food records; colorectal adenoma was histopathologically confirmed by biopsy during colonoscopy. Eventually, 2604 participants were included in the analysis. The risk of colorectal adenoma by quintile of dietary fat intake was analyzed using logistic regression. Subgroup analyses by degree of risk and by location of colorectal adenoma were additionally performed.
In men, total fat intake was not associated with risk of colorectal adenoma. However, risk of colorectal adenoma increased with higher saturated fatty acid (SFA) intake. The adjusted odds ratio in the highest quintile was 1.71 (95% confidence interval, 1.01–2.91) compared with that in the lowest quintile. There was no significant association between fat intake and risk of colorectal adenoma characterized by subsite. In female participants, total fat and specific fatty acid intake were not associated with risk of colorectal adenoma.
These data support that high SFA intake is associated with risk of colorectal adenoma in Korean men.
Keywords: colorectal adenoma, colorectal cancer, dietary fat intake, saturated fatty acid
1. Introduction
Colorectal cancer accounts for a significant portion of the global burden of cancer morbidity and mortality due to cancer.[1] More than half of the cases occur in developed countries and incidence rates vary nearly 10-fold by region.[1] Colorectal cancer has been reported to be strongly associated with westernization, and much has been learned about the lifestyle-, dietary-, metabolism-, and medication-related risk factors for colorectal cancer.[2]
Epidemiologic studies have suggested that among various environmental factors, diet may play the most important role in the risk of colorectal cancer. The Health Professionals Follow-up cohort study showed that high red meat intake was associated with an elevated risk of colon cancer.[3] A pooled analysis of case-control studies suggested that high fiber intake was inversely associated with risk of colorectal cancer.[4] Some case-control studies have shown that high saturated fatty acid (SFA) intake is associated with increased risk of colorectal cancer[5] or adenoma.[6] In addition, animal fat intake showed a positive association with risk of colorectal cancer.[7,8] There have been ongoing efforts to prove the association between the risk of colorectal cancer and dietary customs or specific nutrient intake. The significance of sex-specific biological and sociocultural differences in colorectal cancer risk has also been considered. Previous research has identified that differences in dietary pattern between the sexes affect the risk of cancer, as well as sex-specific characteristics of colorectal cancer in terms of hormonal and epigenetic factors.[9–13] However, to date, consistent results have not been obtained, and the causality remains uncertain.
Recently, the incidence of colorectal cancer has sharply increased in South Korea. This trend may be because of the transition of risk factors as well as the effect of colorectal cancer screening.[14] The major change in dietary pattern in South Korea includes a large increase in the consumption of animal food products and a fall in total cereal intake.[15] However, the amount and rate of increase in fat intake have remained relatively low in South Korea, which may be because of traditional dietary patterns.[15]
Most colorectal cancers arise from benign adenomas that develop over several years through an adenoma-carcinoma sequence.[16] Hence, for colorectal cancer prevention, it is important to clarify the role of dietary risk factors for colorectal adenoma. However, owing to differences between the typical dietary customs of Korea and other western countries, results from western data should not directly be applied to Korea.
The objective of this study was to determine the association between dietary fat intake, distributed by its specific types, and colorectal adenomatous polyps in Korean men and women.
2. Method
2.1. Study population
The subjects of our study included Korean adults aged 30 to 79 years who had undergone screening colonoscopy and a nutritional assessment during a comprehensive health screening examination in Seoul National University Hospital, Healthcare System Gangnam Center, from January 1st, 2008 to December 31st, 2011. National colorectal cancer screening guidelines recommend that all Korean adults aged 50 years and older undertake a fecal occult blood test (FOBT). Individuals with a positive FOBT result are referred for colonoscopy for further confirmation. However, the Korean Guidelines for Colorectal Cancer Screening and Polyp Detection recommend colonoscopy as the first strategy for colorectal cancer screening and polyp detection.[17] Thus, during personal screening, as opposed to national health screening, most medical institutions recommend colonoscopy for average-risk patients aged 50 years and older regardless of FOBT results.
Of 3346 participants, 37 were excluded for one of the following reasons: history of colorectal cancer (n = 14); diagnosis with colorectal cancer during the study period (n = 10); history of chronic inflammatory bowel disease such as ulcerative colitis or Crohn disease (n = 6); and incomplete evaluation owing to bleeding tendency or poor cooperation (n = 7). The quality of bowel preparation was assessed by endoscopists using a 5-point scale: participants with poor preparation—presence of liquid and solid stool preventing total aspiration (score 2) or solid stool preventing visualization (score 1)—were excluded for thorough assessment (n = 366). Furthermore, 10 participants who had total energy intake >5000 kcal/day or <500 kcal/day were excluded because extreme nutrient intake distributions may not represent habitual diet.
Among eligible participants (n = 2933), we further excluded those with missing values in any of the study variables (11.2%): anthropometric data (n = 11), laboratory data (n = 2), questionnaire data on lifestyle behaviors, and medication use and family history (n = 316). Eventually, 2604 participants were included into the analysis. This study was approved by the Institutional Review Board of Seoul National University Hospital in Seoul, Korea (IRB number: 1507–090–689).
2.2. Baseline data collection
Clinical data including medical history, family history, current medication, and lifestyle behaviors were obtained using self-reported questionnaires. Medical records were thoroughly reviewed to complement a potential limited accuracy of self-reports. Participants were classified as never, past, or current smokers. With respect to alcohol consumption, problem drinkers were defined as men consuming >15 drinks per week and women consuming >10 drinks per week on average. People who exercise >150 minutes weekly with vigorous or moderate intensity were considered physically active. Data regarding history of diabetes or colonoscopic polypectomy, and first-degree family history of colorectal cancer, as well as medication use such as aspirin, calcium supplement, and nonsteroidal anti-inflammatory drugs (NSAIDs) were obtained from a self-reported questionnaire.
Body weight, height, and waist circumference were measured in a consistent manner. Serum glucose and HbA1C levels were measured after 12 hours of fasting. People who had serum glucose levels >126 mg/dL, people with HbA1C levels >6.5%, and those had already been treated with hypoglycemic medication were all defined as in diabetic state.
Dietary intake was derived from 1-day food records. Participants were requested to record what they ate over the course of this day, which is similar with their habitual diet. Extracted data were confirmed through individual interviews by well-trained dietitians and then analyzed using a program called CAN-Pro 3.0 (Computer-Aided Nutrient Analysis Program; Korea Nutrition Society, Seoul, Korea), that is based on Korean nutritional standards.
2.3. Colorectal adenomatous polyps
Adenomas were confirmed by pathologists after endoscopic examination of the bowel between the rectum and the cecum. Colorectal adenomatous polyps were described by size, multiplicity, histologic grade, and location. Participants with >3 adenomas or any adenoma >1 cm, with high-grade dysplasia or with villous component were defined as a high-risk group.[18] Locations of adenomatous polyps were described as proximal or distal, and the reference point for classification was determined by the splenic flexure.
2.4. Statistical analysis
Baseline characteristics of the study population were presented as mean with standard deviation for continuous variables and number with proportion for categorical variables. The χ2 test for categorical variables and student t test for continuous variables were performed for statistical comparisons in the study population. All nutrient intakes were adjusted by total energy intake using the residual regression method.[19]
Each type of fat and fatty acid intake was stratified into quintiles to examine trends in risk by level of exposure. Odds ratios (ORs) for colorectal adenomatous polyps in the higher intake groups (Q2–Q5) were calculated using multivariable logistic regression analysis by using the lowest (Q1) as the reference group. Age; body mass index; waist circumference; diabetic state; first-degree family history of colorectal cancer; history of colonoscopic polypectomy; smoking status; alcohol intake; physical activity; total energy intake; intake of protein, fiber, folate, or calcium; use of aspirin, calcium supplements, or NSAIDs; and specific types of fat were adjusted for. Sex-specific analyses were performed to control for differences in baseline characteristics between men and women. In addition, subgroups were created according to the risk of cancer and the location of the adenoma. Furthermore, regression analyses were conducted by adjusting variables in the same way as in the main analysis.
All statistical analyses were performed using STATA version 14.1 (Stata Co., College Station, TX). A P value <0.05 was considered to represent a statistically significant outcome.
3. Results
3.1. Baseline characteristics of the study population
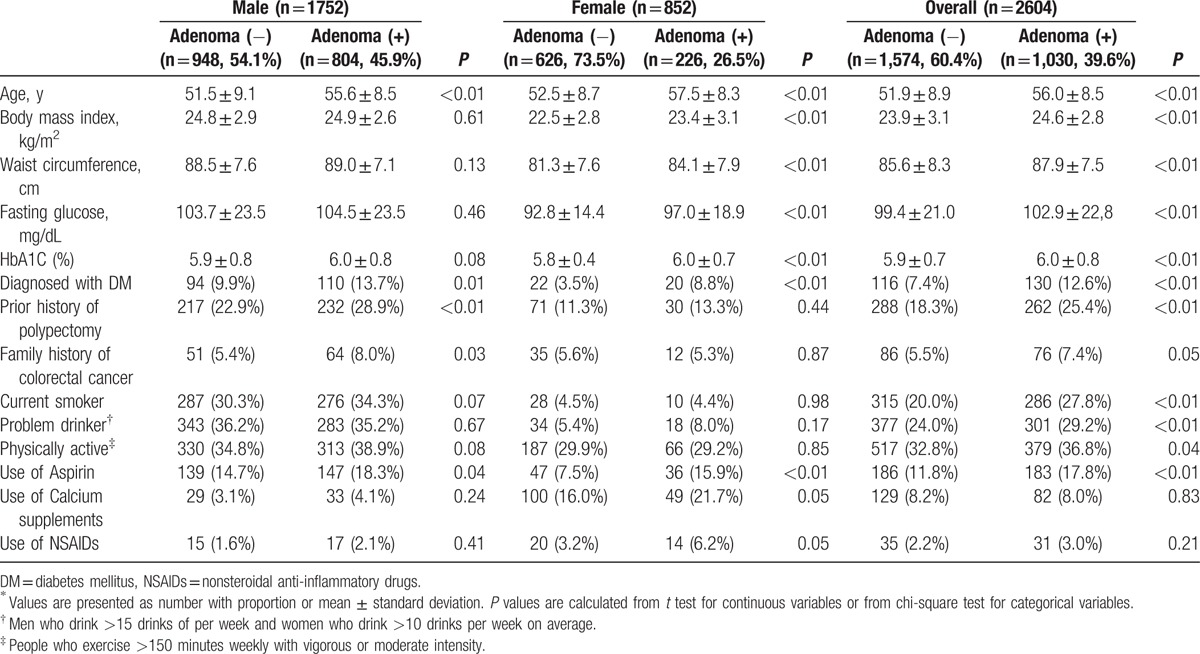
Table 1 presents the baseline characteristics of the study population in detail. In total, 1030 participants (39.6%) were diagnosed with colorectal adenoma. In both sexes, the mean age of participants with adenoma was significantly higher than that of adenoma-free participants. Mean body mass index (BMI) and waist circumference were higher in the adenoma group, especially in female participants. The proportion of participants in a diabetic state was also higher in the adenoma group, and again, this was observed in both sexes (19.0% vs. 17.3% in male participants, 15.0% vs. 6.1% in female participants). With regard to current use of medication, the adenoma group had a higher proportion of aspirin users than the adenoma-free group.
Table 1.
Baseline characteristics of the study population∗.
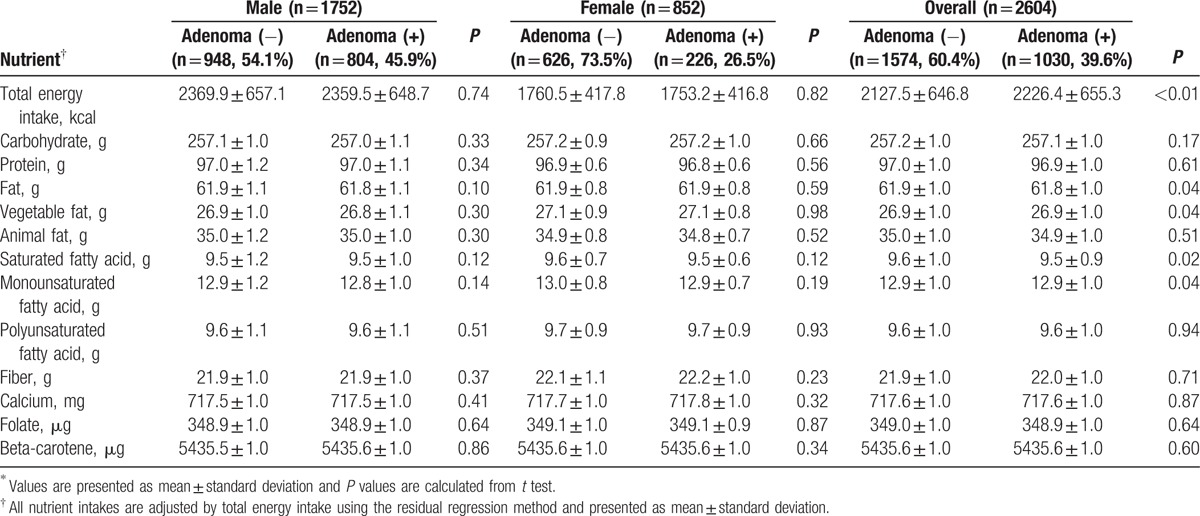
In both sexes, there was no statistically significant difference in mean daily intake of calories, macronutrients, specific fatty acids, and other nutrients in the adenoma-free group compared with the adenoma group (Table 2).
Table 2.
Mean nutrient intake of the study population∗.
3.2. Risk of colorectal adenoma according to dietary fat intake
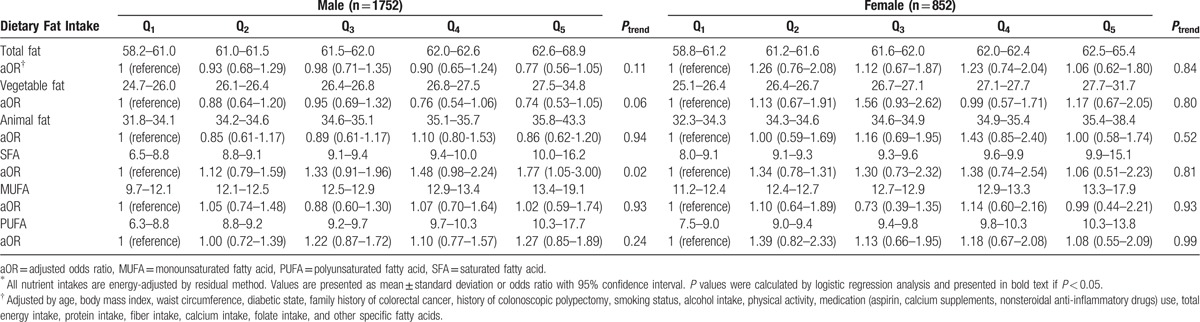
The risk of colorectal adenoma was analyzed by quintile for dietary intake of fat and specific fatty acids (Table 3). There was no statistically significant association between total fat intake and the risk of colorectal adenoma in men. With respect to specific fatty acids, the risk of colorectal adenoma increased with increasing SFA intake in the male group, after adjustment for confounders (Ptrend = 0.027). The OR of the highest quintile of SFA intake was 1.71 (95% confidence interval, 1.01–2.91). There was no association between colorectal adenoma and total fat or specific fatty acid intake in women.
Table 3.
Risk of colorectal adenoma by quintile of dietary fat intake∗.
3.3. Subgroup analysis by adenoma characteristic
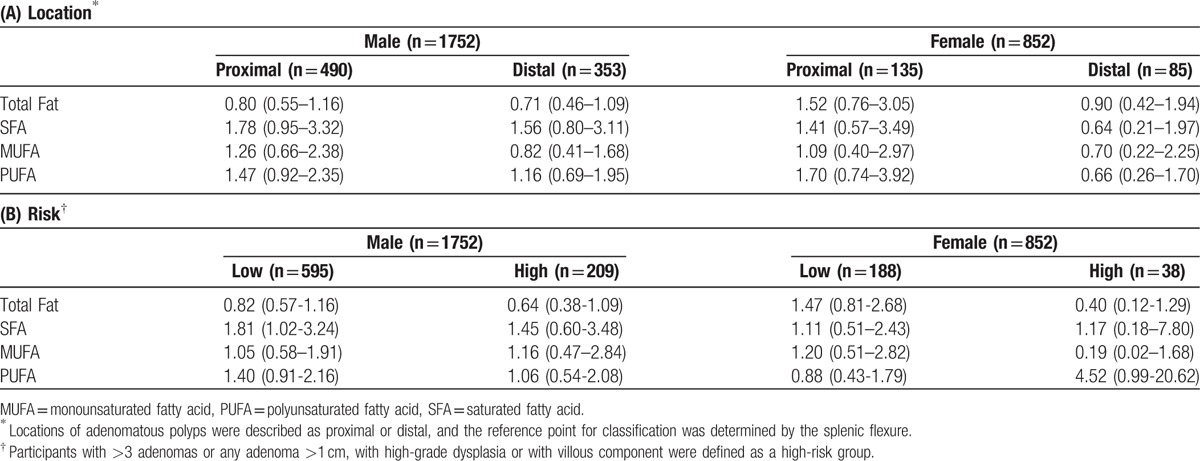
Additional analyses were performed to identify specific characteristics related to the risk of colorectal adenoma (Table 4). Colorectal adenomatous polyps were classified as proximal or distal, determined by the location of the most advanced or largest polyp. There were 625 participants with an adenoma in the proximal colon, and 448 had an adenoma in the distal colon; in both sexes, no significant associations were found between fat intake and the risk of proximal or distal colorectal adenoma. The association between SFA and colorectal adenoma in men remained statistically significant for the low-risk adenoma group, whereas, in both sexes, there was no apparent association between any type of fatty acid intake and risk of adenoma.
Table 4.
Subgroup analysis—adjusted odds ratio (OR) of the highest quintile compared with the lowest quintile of fat intake for colorectal adenoma characterized by (A) location and (B) risk.
4. Discussion
We conducted a cross-sectional study to assess the association between dietary fat intake and colorectal adenoma. We found a positive association between energy-adjusted SFA intake and risk of colorectal adenoma in men. No significant associations were found between any type of fat intake and risk of colorectal adenoma in women.
Although some epidemiological studies have supported the hypothesis that dietary fat increases the risk of colorectal cancer independently of its overall energy intake, these data failed to establish a definite conclusion. Our study results suggest that there is no association between total fat intake and risk of colorectal adenoma, consistent with former case–control studies.[20–23]
Previous studies showed a positive association between total fat intake and risk of colon cancer; this was usually attributable to fat from an animal source or SFA.[24–26] Some case-control studies have suggested that animal fat intake shows a positive association with risk of colorectal cancer[7,8]; moreover, other studies have shown that high SFA intake is associated with increased risk of colorectal cancer[5] or adenoma[6,23] Our study analyzed both animal fat and SFA intake; only SFA intake was positively associated with colorectal adenoma, in part consistent with the results of prior studies.
There have been many experimental studies to verify the epidemiological evidence from studies about the association between dietary fat and cancer. An early animal study demonstrated that tumor-promoting effects of dietary fat might be partly independent to its caloric density.[27] With respect to SFA, a previous study suggested that diglycerides containing SFA chains induce mitogenesis of adenomas and some carcinomas in the colon, and may enhance the invasive capacity of carcinomas.[28] Another experimental study demonstrated that SFA, essential for lipopolysaccharide (LPS) and toll-like receptor 4 (TLR4) activation, induces nuclear factor B activation, as well as expression of cyclooxygenase-2 (COX-2) and other inflammatory markers.[29]
Our study focused on results from intake of specific fatty acids rather than total fat, as recent studies showed that different types of fat and fatty acid are likely to influence the risk of cancer differentially, regardless of total amount of fat intake or calories from total fat intake. Our study results suggested that high SFA intake was positively associated with colorectal adenoma, but there was no significant association between monounsaturated fat (MUFA) or polyunsaturated fat (PUFA) and colorectal adenoma. Recent studies have shown variable effects of specific subtypes of PUFA, such as eicosanopentaenoic acid (EPA), on colorectal cancer. It is suggested that substituting food for high medium-chain fatty acids, arachidonic acids, or an EPA-rich diet may contribute to reduced risk of colorectal cancer.[30] Also, increased consumption of EPAs and docosahexaenoic acids was associated with reduced risk of colorectal cancer.[31] A positive association between oleic acid intake and risk of colorectal adenoma has also been described.[20] Many studies aimed to verify the possible mechanisms of the effect of intake of such fatty acids on the risk of colorectal cancer to support the epidemiological evidence.[32] However, our study only provided a limited assessment owing to absence of data about more detailed subtypes of MUFA or PUFA.
The subsite-specific etiology of colorectal cancer is poorly understood and the various dietary factors are considered to play a role in different mechanisms. One Swedish study suggested that high consumption of red meat was associated with risk of distal colon cancer rather than risk of proximal colon or rectal cancer.[33] In addition, a large European cohort study demonstrated that the association between red meat intake and colorectal cancer was stronger for rectal and left-sided colon cancer than for right-sided colon cancer, although differences were not statistically significant.[34] However, a retrospective study from the Korean National Health System cohort suggested that meat consumption is associated with risk of proximal colon cancer in men.[35] In our study, in which the location of the adenoma was analyzed, high SFA intake was not significantly associated with adenomas at the proximal or distal colon. There was insufficient evidence for an association between macronutrient intake and subsite-specific colorectal cancer, and the risk of proximal or distal adenoma in particular was rarely known. The subsite-specific difference may be attributed to N-nitroso compound being higher in the distal colon and rectum than in the proximal colon,[36] but colorectal adenomas might be less affected by the carcinogenic fecal composition than colorectal carcinomas, owing to shorter exposure times. Further studies about dietary risk factors and site-specific colorectal adenomas need to demonstrate the diet-related mechanism of colorectal cancer. In our study, high-risk adenoma was defined by its size, histologic grade, and multiplicity. Differences with respect to the degree of risk might suggest how dietary risk factors influence each phase of the cancer development pathway, but few studies have reported about these aspects. In our study, statistical significance of the association between SFA and colorectal adenoma in men was only maintained in the low-risk adenoma group.
Dietary patterns and clinical characteristics of colorectal cancer were known to be different in men and women. In both sexes, diet has been considered as a risk factor for developing colorectal cancer and previous studies have reported correlation with diet and colon cancer. This is particularly evident in women[11,37]; however, this may be because of reduced exposure to risk factors such as cigarettes and alcohol compared to their male counterparts. In this study, risk of colorectal adenoma increased in male participants with higher SFA intake. However, analyses in females might be difficult to interpret because of hormone-related factors, which were not adjusted for because of limited data. There were also relatively fewer female subjects in our study and further research on the sex-specific dietary risk factors of colorectal adenoma is needed.
Epidemiologic studies about dietary risk factors for cancer in the Asian population have found that a westernized diet is associated with increased risk of colorectal cancer; however, an association between each macronutrient and cancer of the colon and rectum has not been confirmed.[38–40] A case-control study in Shanghai found an inverse association between colon cancer and micronutrients that are common in vegetables, but there was no consistent trend for fat consumption.[39] A large prospective study in Japan also suggested that high processed meat intake increased the risk of colon cancer, but specific fatty acid intakes were not related.[40] Unlike previous studies, our study adjusted total energy intake to control for confounding and reduce extraneous variation, and we also considered a larger variety of confounding factors such as previous history of polypectomy and medication; these methodological differences might account for discrepancies found between our and previous studies.
Studies in Korea examining dietary risk factors of colorectal cancer have been lacking because of the many difficulties in collecting and analyzing nutrition-related data. A former study by our center had investigated dietary risk factors of colorectal adenoma including fat intake, but data of specific fatty acid intake were not included.[41] These reasons highlight the clinical significance of our study in which we analyzed dietary fat intakes by source and specific fatty acids using a large sample size in the Korean population.
Our study has several limitations. First, a cross-sectional study design cannot explicate the causal relationship between nutrition and colorectal adenoma. However, our study was conducted in asymptomatic, healthy participants who are more likely to maintain a consistent dietary habit. Second, the nutritional assessment through a 1-day food record was somewhat limited in terms of reflecting the usual dietary behaviors, although persons exhibiting an extreme range of total calorie intake were excluded. Third, we could not analyze specific subtypes of unsaturated fatty acid or trans-fatty acid associated with risk of colorectal cancer in more detail, owing to the limited dietary information in the Korean database. For the same reason, we could not conduct additional analyses by food group, such as red or processed meat, fruit, and vegetables. Fourth, potential confounding factors, such as hormonal status in female participants, were not adjusted for because of the absence of data.
The incidence of colorectal cancer in Korea is rising, and the transition in dietary habits is considered to be one of the main causes. Meanwhile, despite rapid industrialization, Korean dining remains a unique custom; hence, it will be important to identify dietary risk factors for the Korean population to reduce the burden of disease. Future studies might include analysis on micronutrients and macronutrients, as well as typical dining and cooking habits. Also, prospective studies and experimental investigations need to be performed to support the epidemiological evidence.
Footnotes
Abbreviations: CI = confidence interval, MUFA = monounsaturated fatty acid, OR = odds ratio, PUFA = polyunsaturated fatty acid, SFA = saturated fatty acid.
The authors report no conflicts of interest.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology 2010;138:2029–43. e2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Giovannucci E, Rimm EB, Stampfer MJ, et al. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res 1994;54:2390–7. [PubMed] [Google Scholar]
- [4].Howe GR, Benito E, Castelleto R, et al. Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst 1992;84:1887–96. [DOI] [PubMed] [Google Scholar]
- [5].Whittemore AS, Wu-Williams AH, Lee M, et al. Diet, physical activity, and colorectal cancer among Chinese in North America and China. J Natl Cancer Institute 1990;82:915–26. [DOI] [PubMed] [Google Scholar]
- [6].Giovannucci E, Stampfer MJ, Colditz G, et al. Relationship of diet to risk of colorectal adenoma in men. J Natl Cancer Inst 1992;84:91–8. [DOI] [PubMed] [Google Scholar]
- [7].Willett WC, Stampfer MJ, Colditz GA, et al. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med 1990;323:1664–72. [DOI] [PubMed] [Google Scholar]
- [8].Le Marchand L, Wilkens LR, Hankin JH, et al. A case-control study of diet and colorectal cancer in a multiethnic population in Hawaii (United States): lipids and foods of animal origin. Cancer Causes Control 1997;8:637–48. [DOI] [PubMed] [Google Scholar]
- [9].McCashland TM, Brand R, Lyden E, et al. Gender differences in colorectal polyps and tumors. Am J Gastroenterol 2001;96:882–6. [DOI] [PubMed] [Google Scholar]
- [10].DeCosse J, Ngoi S, Jacobson J, et al. Gender and colorectal cancer. Eur J Cancer Prev 1993;2:105–16. [DOI] [PubMed] [Google Scholar]
- [11].West DW, Slattery ML, Robison LM, et al. Dietary intake and colon cancer: sex-and anatomic site-specific associations. Am J Epidemiol 1989;130:883–94. [DOI] [PubMed] [Google Scholar]
- [12].Foley EF, Jazaeri AA, Shupnik MA, et al. Selective loss of estrogen receptor β in malignant human colon. Cancer Res 2000;60:245–8. [PubMed] [Google Scholar]
- [13].Nguyen SP, Bent S, Chen Y-H, et al. Gender as a risk factor for advanced neoplasia and colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2009;7:676–81. e673. [DOI] [PubMed] [Google Scholar]
- [14].Shin A, Kim KZ, Jung KW, et al. Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat 2012;44:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim S, Moon S, Popkin BM. The nutrition transition in South Korea. Am J Clin Nutr 2000;71:44–53. [DOI] [PubMed] [Google Scholar]
- [16].Muto T, Bussey H, Morson B. The evolution of cancer of the colon and rectum. Cancer 1975;36:2251–70. [DOI] [PubMed] [Google Scholar]
- [17].Lee BI, Hong SP, Kim S-E, et al. Korean guidelines for colorectal cancer screening and polyp detection. Intest Res 2012;10:67–88. [Google Scholar]
- [18].Levin B, Lieberman DA, McFarland B, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Cancer J Clin 2008;58:130–60. [DOI] [PubMed] [Google Scholar]
- [19].Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S. [DOI] [PubMed] [Google Scholar]
- [20].Mathew A, Peters U, Chatterjee N, et al. Fat, fiber, fruits, vegetables, and risk of colorectal adenomas. Int J Cancer 2004;108:287–92. [DOI] [PubMed] [Google Scholar]
- [21].Martínez ME, McPherson SR, Annegers FJ, et al. Association of diet and colorectal adenomatous polyps: dietary fiber, calcium, and total fat. Epidemiology 1996;7:264–8. [DOI] [PubMed] [Google Scholar]
- [22].Lieberman DA, Prindiville S, Weiss DG, et al. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. Jama 2003;290:2959–67. [DOI] [PubMed] [Google Scholar]
- [23].Neugut AI, Garbowski GC, Lee WC, et al. Dietary risk factors for the incidence and recurrence of colorectal adenomatous polyps: a case-control study. Ann Intern Med 1993;118:91–5. [DOI] [PubMed] [Google Scholar]
- [24].Jain M, Cook G, Davis F, et al. A case-control study of diet and colo-rectal cancer. Int J Cancer 1980;26:757–68. [DOI] [PubMed] [Google Scholar]
- [25].Peters RK, Pike MC, Garabrant D, et al. Diet and colon cancer in Los Angeles county, California. Cancer Causes Control 1992;3:457–73. [DOI] [PubMed] [Google Scholar]
- [26].Hursting SD, Thornquist M, Henderson MM. Types of dietary fat and the incidence of cancer at five sites. Prevent Med 1990;19:242–53. [DOI] [PubMed] [Google Scholar]
- [27].Klurfeld DM, Weber MM, Kritchevsky D. Inhibition of chemically induced mammary and colon tumor promotion by caloric restriction in rats fed increased dietary fat. Cancer Res 1987;47:2759–62. [PubMed] [Google Scholar]
- [28].Friedman E, Isaksson P, Rafter J, et al. Fecal diglycerides as selective endogenous mitogens for premalignant and malignant human colonic epithelial cells. Cancer Res 1989;49:544–8. [PubMed] [Google Scholar]
- [29].Lee JY, Sohn KH, Rhee SH, et al. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 2001;276:16683–9. [DOI] [PubMed] [Google Scholar]
- [30].Nkondjock A, Shatenstein B, Maisonneuve P, et al. Specific fatty acids and human colorectal cancer: an overview. Cancer Detect Prev 2003;27:55–66. [DOI] [PubMed] [Google Scholar]
- [31].Theodoratou E, McNeill G, Cetnarskyj R, et al. Dietary fatty acids and colorectal cancer: a case-control study. Am J Epidemiol 2007;166:181–95. [DOI] [PubMed] [Google Scholar]
- [32].Larsson SC, Kumlin M, Ingelman-Sundberg M, et al. Dietary long-chain n− 3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr 2004;79:935–45. [DOI] [PubMed] [Google Scholar]
- [33].Larsson SC, Rafter J, Holmberg L, et al. Red meat consumption and risk of cancers of the proximal colon, distal colon and rectum: the Swedish Mammography Cohort. Int J Cancer 2005;113:829–34. [DOI] [PubMed] [Google Scholar]
- [34].Norat T, Bingham S, Ferrari P, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J Natl Cancer Inst 2005;97:906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shin A, Joo J, Bak J, et al. Site-specific risk factors for colorectal cancer in a Korean population. PloS One 2011;6:e23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Povey AC, Hall CN, Badawi A, et al. Elevated levels of the pro-carcinogenic adduct, O 6-methylguanine, in normal DNA from the cancer prone regions of the large bowel. Gut 2000;47:362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jacobs ET, Thompson PA, Martínez ME. Diet, gender, and colorectal neoplasia. J Clin Gastroenterol 2007;41:731–46. [DOI] [PubMed] [Google Scholar]
- [38].Lee H, Gourley L, Duffy S, et al. Colorectal cancer and diet in an asian population—A case-control study among Singapore Chinese. Int J Cancer 1989;43:1007–16. [DOI] [PubMed] [Google Scholar]
- [39].Chiu BC, Ji B-T, Dai Q, et al. Dietary factors and risk of colon cancer in Shanghai, China. Cancer Epidemiol Biomarker Prev 2003;12:201–8. [PubMed] [Google Scholar]
- [40].Oba S, Shimizu N, Nagata C, et al. The relationship between the consumption of meat, fat, and coffee and the risk of colon cancer: a prospective study in Japan. Cancer Lett 2006;244:260–7. [DOI] [PubMed] [Google Scholar]
- [41].Yang SY, Kim YS, Song JH, et al. Dietary risk factors in relation to colorectal adenoma. Korean J Gastroenterol 2012;60:102. [DOI] [PubMed] [Google Scholar]


