Supplemental Digital Content is available in the text
Keywords: dexmedetomidine, meta-analysis, nausea, vomiting
Abstract
Background:
Postoperative nausea and vomiting (PONV) is a frequent complication in postoperative period. The aim of this article was to evaluate the effect of dexmedetomidine on PONV.
Method:
RevMan 5.3 software was applied for performing statistic analysis. Twenty-four trials with 2046 patients were included.
Results:
The PONV of the dexmedetomidine group was significantly lower compared with the placebo group (0.56, 95% CI: 0.46, 0.69). Subgroup analysis further confirmed the effect of dexmedetomidine (irrespective of administration mode) (P < 0.00001). Perioperative fentanyl consumption in dexmedetomidine group were also reduced significantly (P < 0.00001). Whereas, side effects such as bradycardia, hypotension increased in dexmedetomidine group (especially in loading dose mode and loading dose plus continuous infusion mode).
Conclusions:
Dexmedetomidine administrated in continuous infusion mode has the advantage to prevent PONV as well as reduce side effects such as bradycardia and hypotension.
1. Introduction
General anesthesia is widely used in several surgeries. It can cause some complications such as postoperative nausea and vomiting (PONV) and cognitive dysfunction. PONV is more common in general anesthesia than spinal anesthesia.[1,2] Also, it can cause electrolyte imbalance and aggravate bleeding that delay hospital discharge.[3] It is reported that PONV is even higher especially after gynecologic surgery, ranging from 24% to 75%, even up to 90%.[4] Some clear risks including female gender, postoperative opioid treatment, the history of motion sickness and/or PONV and nonsmoker have been shown to independently predict PONV.[5,6]
Dexmedetomidine is a potent and highly selective α2-adrenoceptor agonist, which binds to transmembrane G protein-binding receptor located in the brain and spinal cord. It affects the functions of central nervous, circulatory systems and exhibits sedative, analgesic, sympatholytic properties.[7] It has been widely used in different clinical settings like department of anesthesiology and intensive care unit (ICU).[8] Recently, the effect of dexmedetomidine on PONV has been the focus of clinical researchers. Nevertheless, controversy about the effectiveness of dexmedetomidine for PONV is still ongoing, for different results reported in associated literature.
To our knowledge, there was no updated analysis done for combination of related data during general anesthesia. Therefore, we performed this meta-analysis to investigate the antiemetic effect of dexmedetomidine in patients undergoing general anesthesia.
2. Materials and methods
2.1. Ethical statement
All results and analyses were from previous published studies, thus no ethical approval and patient consent are required.
2.2. Search strategy
This meta-analysis were performed in accordance with recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and was reported in compliance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement) guidelines.[9] We performed a systematic electronic search in PubMed for relevant studies of randomized controlled trials (RCTs) published before August 2016. We used the following Medical Subject Heading (MeSH) terms and corresponding keywords dexmedetomidine, general anesthesia, and postoperative nausea and vomiting. Hand searching techniques also were used to identify appropriate studies. Moreover, articles that met the following criteria were included: randomized and double-blind study design; the intervention was treatment with dexmedetomidine given systemically in any dose during the perioperative period; patient undergone general anesthesia experiencing PONV.
2.3. Data extraction and analysis
All data were extracted by 2 reviewers (SH-Jin and DD-Liang) and then independently reviewing every selection for accuracy and consistency. Any discrepancy was resolved by JL-Wang for discussion and consensus. The following outcome measures were extracted from the retrieved reports: perioperative fentanyl consumption, number of patients experiencing PONV, number of patients undergoing bradycardia or hypotension. Moreover, the subgroup analysis was performed for different dexmedetomidine administration modes.
The following data were also collected by S-H. Jing and confirmed by other authors (CY Chen and MY Zhang): first author, year of publication, participants, type of surgery, administration mode of dexmedetomidine, comparisons, number of patients. Extracted data were entered into a standardized Excel (Microsoft Corporation, The Redmond, Washington, US) file.
2.4. Risk of bias assessment
The risk of bias of included studies was assessed independently by 2 authors (SH-Jin and CY Chen) using the Cochrane risk-of-bias tool. We reviewed each trial and scored as “high,” “low,” or “unclear” risk of bias to the following criteria: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other bias (Fig. 1).
Figure 1.
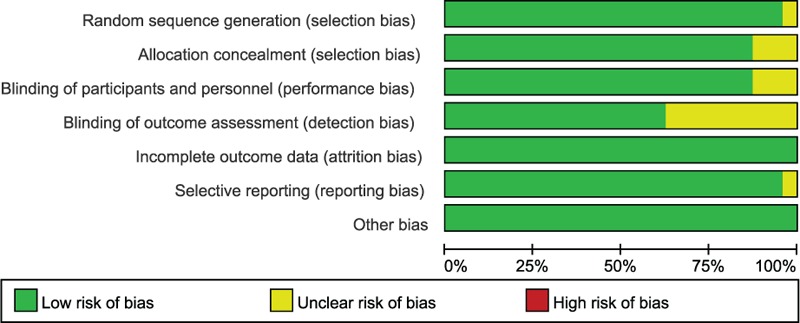
The risk of bias of included studies.
2.5. Statistical analysis
Statistical analysis was performed using the Review Manager 5.3 software. We calculated relative risks (RRs) with 95% CIs for dichotomous outcomes by the Mantel–Haenszel method (fixed or random models). Continuous outcomes measured were expressed as a mean value and standard deviation and were analyzed by using weighted mean differences (WMD). I-square (I2) test was performed to assess the impact of study heterogeneity on the results of the meta-analysis. According to the Cochrane review guidelines, if severe heterogeneity was present at I2 > 50%, the random effect models were chosen, otherwise the fixed effect models were used. The funnel plot was used to detect potential publication bias.
3. Results
3.1. Trial selection
The process of literature screening, study selection, and reasons for exclusion was shown in the flow diagram. Our initial search yielded 102 records. After removing duplicates and screening the titles and abstracts, 24 RCTs published during 2005 to 2016 met the criteria and were included in the analysis.
3.2. Trials characteristics
The main characteristics of the included trials are summarized in Table 1.
Table 1.
Characteristics of the included trials.
3.3. PONV
PONV was reported in 16 studies. As between-study heterogeneity not existed (P = 0.33), a fixed-effects model was adopted. The combined MD was 0.53 (95% CI: 0.45, 0.62) and it was significant (Z = 5.60, P < 0.00001).[10–24] Thus the PONV of the dexmedetomidine group was significantly lower compared with the control group. Subgroup analysis showed that dexmedetomidine administration by loading dose plus continuous infusion or by loading dose or just by continuous infusion, the incidence of PONV during general anesthesia was decreased significantly (Fig. 2).[10–34]
Figure 2.
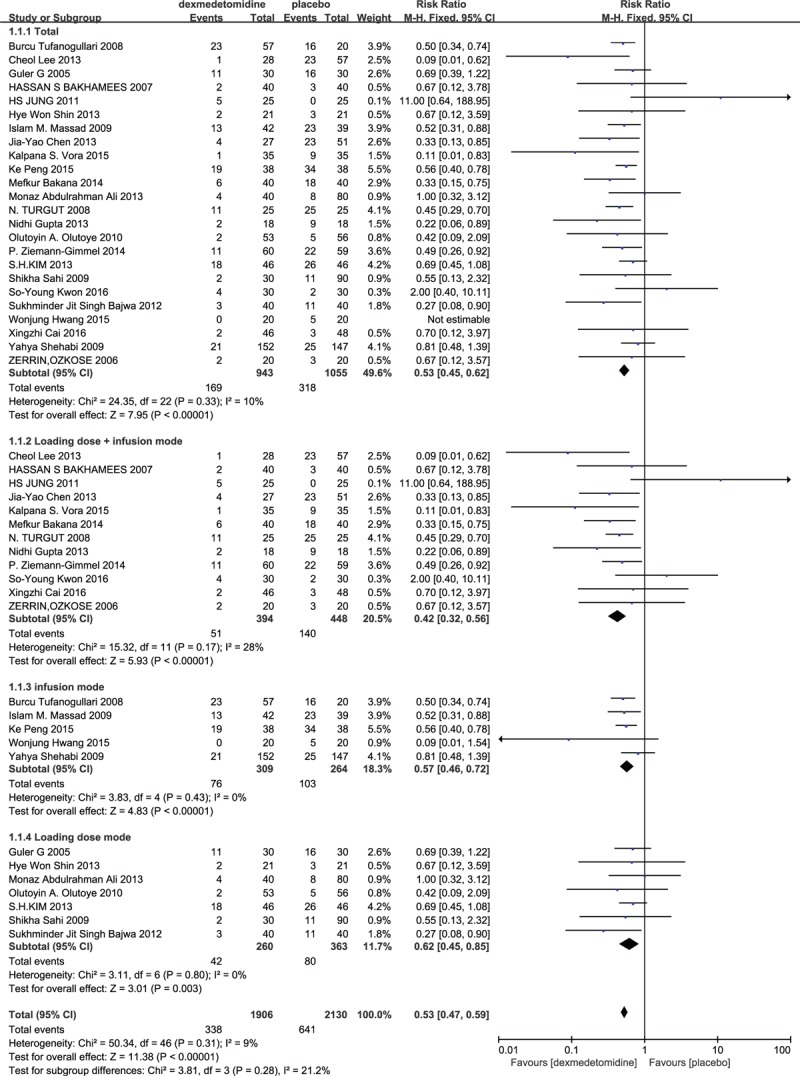
The total effect of dexmedetomidine and different infusion modes on PONV.
3.3.1. Perioperative fentanyl consumption
Four trials assessed the need for use of perioperative fentanyl. The pooled analysis shown a significant decrease in need for the use of fentanyl (SMD Std. Mean Difference −1.43 (95% CI: −2.39, −0.46), although the study heterogeneity was high[10,13,16,19] (Fig. 3).
Figure 3.
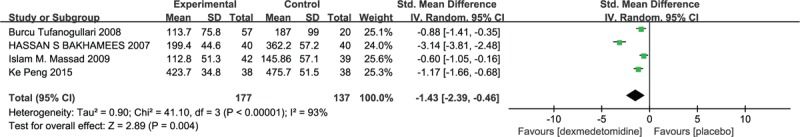
Perioperative fentanyl consumption in dexmedetomidine and placebo group.
3.3.2. The effect of dexmedetomidine on children and adult
Five trials reported the effect of dexmedetomidine on children and 19 trials about adult. The pooled analysis shown total incidence of PONV was 13.69% in dexmedetomidine group. The combined MD was 0.50 (95% CI: 0.33, 0.76) and it was significant (Z = 3.22, P = 0.001). However, the total incidence of PONV in placebo group was 25.96%. The combined MD was 0.54 (95% CI: 0.45, 0.64) and it was significant (Z = 7.27, P < 0.00001) (Supplemental Fig).
3.4. Side effects
3.4.1. Incidence of bradycardia
Six studies described the incidence of bradycardia.[14,18,20,22,23,28] A fixed-effect model was adopted since no between-study heterogeneity was found (P > 0.05). The pooled RR was determined as 5.0 (95% CI: 1.70, 14.72) and it was no difference according to the statistical result (Z = 2.92, P = 0.09) (Fig. 4).
Figure 4.
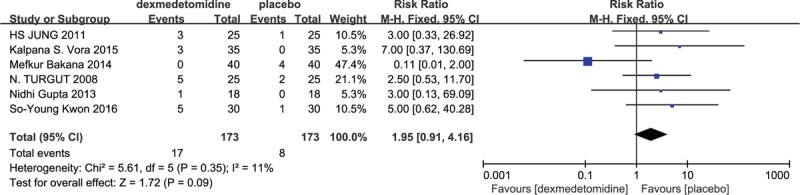
Incidence of bradycardia in dexmedetomidine and placebo group.
3.4.2. Incidence of hypotension
There were 5 studies reporting perioperative hypotension.[14,18,23,28,32] Compared with placebo, no difference was found between 2 groups (P = 0.82) (Fig. 5).
Figure 5.
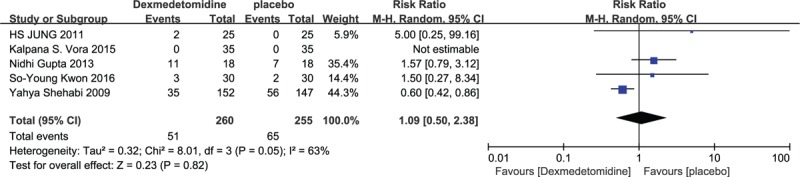
Incidence of hypotension in dexmedetomidine and placebo group.
3.5. Risk of bias
The funnel plot was applied for assessing publication bias of studies included in the incidence of PONV in this meta-analysis. No evident publication bias was obtained through the visual distribution (Fig. 6).
Figure 6.
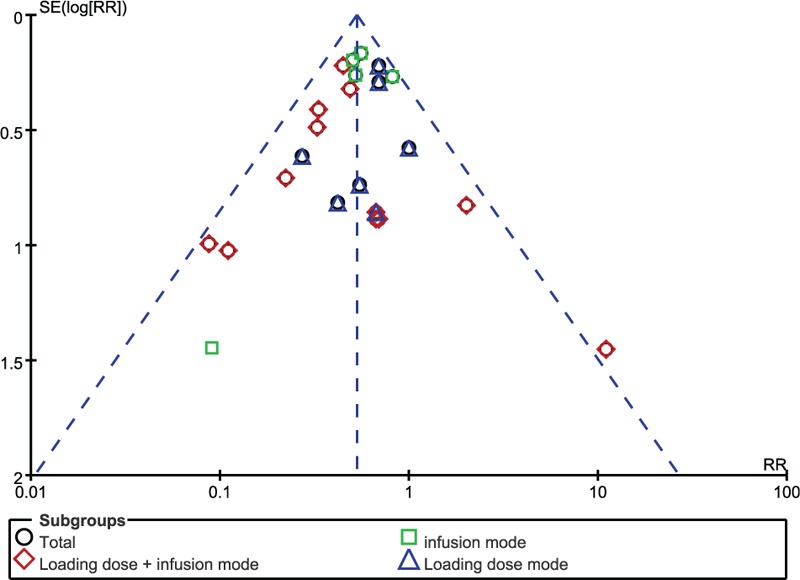
Test for publication bias of the studies included in the incidence of PONV. PONV = postoperative nausea and vomiting.
4. Discussion
Through this meta-analysis we found that: dexmedetomidine, regardless of administration modes (by loading dose or loading dose plus continuous infusion or just infusion) significantly reduced the incidence of PONV in adult or children, compared to placebo, administration of dexmedetomidine diminished the perioperative fentanyl consumption, however, dexmedetomidine increased adverse events such as bradycardia and hypotension in loading dose or loading dose plus continuous infusion mode, indicating that dexmedetomidine in continuous infusion mode is superiority to prevent PONV.
Several meta-analyses regarding this topic have been published.[35,36] Although the main finding of our meta-analysis was consistent with previous meta-analyses. Differences between our meta-analysis and the previous ones should be noted. One meta-analysis included 15 trials with 899 patients and the other only included 11 trials with 692 patients, our present meta-analysis included 24 trials totaling 2046 patients with added statistical power of at least 1100 cases. Our present meta-analysis further reinforces earlier results of previous meta-analyses.
PONV is more common complication during general anesthesia than during spine anesthesia.[1,37,38] In clinical setting, PONV are treated effectively by antiemetics such as ondansetron. However, patients may experience headache, dizziness as well as drowsiness/sedation when ondansetron is used, which limit its wide application. Dexmedetomidine, as an anesthetic adjunct for general and regional anesthesia, has been demonstrated to reduce PONV. Our meta-analysis reached the same conclusion showing that dexmedetomidine reduced PONV significantly. Intriguing, there is no study report whether dexmedetomidine is superior to antiemetic like ondansetron for treatment of PONV. Herein we supposed that antiemetics combination with dexmedetomidine have more advantages to treat PONV. The antiemetic effect may be induced by direct antiemetic properties of α2 agonists through inhibition of catecholamine by parasympathetic tone. Also, administration of dexmedetomidine reduced the perioperative fentanyl consumption in this study may explain the decreased incidence of PONV.
In order to distinguish the effect of different administration modes of dexmedetomidine, we performed subgroup analysis and further exhibited the antiemetic effect of dexmedetomidine. In this analysis, we found 7 articles using loading dose mode (0.5–1 μg/kg), 12 articles using loading dose (0.5–1 μg/kg) and continuous infusion (0.1–0.7 μg/kg/h) mode, and 5 articles by continuous infusion mode (0.1–0.7 μg/kg/h). Moreover, the higher incidence of hypotension was found in loading dose and continuous infusion mode in this analysis. One study demonstrated satisfactory hemodynamic effects when administered without a loading infusion at doses between 0.2 and 0.4 μg/kg/h.[39] Therefore, many clinicians have decided to forego the administration of a loading dose. Based on the results from this report, we advocate to use continuous infusion mode of dexmedetomidine (0.1–0.7 μg/kg/h).
Dexmedetomidine has an onset of action after approximately 15 minutes and peaked at 1 hour after continuous infusion. Its distribution half-life (t½α) is 6 minutes in adults over the dose ranges of 0.2 to 0.7 μg/kg/h. While, its elimination half-life (t½β) range from 2.0 to 2.5 hours and a clearance of 39 L/h. The similar rates of infusion can be used in children and adults to produce a steady state plasma concentration.[40,41] It can prevent surgical stress response by decreasing blood pressure and heart rate.[42] Unfortunately, dexmedetomidine can cause hypotension and bradycardia in clinical, especially in patient with hypovolemia or atrioventricular block. In our study, the number of perioperative hypotension and bradycardia was increased in patients with dexmedetomidine, although no statistical significance was found between dexmedetomidine and placebo. The presynaptic α-2 receptors are stimulated by dexmedetomidine, then decreasing norepinephrine release may account for the hypotension and bradycardia.
The incidence of PONV in pediatric patients was reported as high as 34%. However, the incidence in adult appears to decrease with age.[2,43–45] In our meta analysis, the results are consistent with previous study showing that the incidence of PONV in pediatric patients is much higher than that in adult. Operations such as strabismus, adenotonsillectomy may partially explain the higher incidence of PONV in children, although the potential mechanism is complex.
Nausea and vomiting are 2 distinguishing phenomena. Previous report assess the variables independently.[46] However, nausea and vomiting are usually coexistence in a patient, the occurrence of postoperative nausea (PON) or postoperative vomiting (POV) is noticeably parallel to PONV, thus some researches do not try to distinguish the 2 variables. So, we regard the PONV variables as a substitute for PON or POV, if only PON or POV was reported in the trials. Therefore, in this study, we only analyzed the effect of dexmedetomidine on PONV.
Our meta-analysis had some limitations. First, the included studies in different clinical setting would complicate the results of our meta-analysis. Second, prior histories such as motion sickness and nonsmoker were not recorded and analyzed in our study. Third, the different surgical types and length of operation contributed to the heterogeneity in fentanyl consumption. Therefore, more RCTs about this kind of patients and various administration modes of dexmedetomidine during general anesthesia are required to detect the efficacy of dexmedetomidine on PONV.
In conclusion, this current meta-analysis suggested that administration of dexmedetomidine reduce the PONV, also reduced the perioperative fentanyl consumption. Moreover, when we use it in continuous infusion mode, the potential adverse events such as bradycardia and hypotension could reduce.
Supplementary Material
Footnotes
Abbreviations: DEX = dexmedetomidine, ICU = intensive care unit, MeSH = Medical Subject Heading, PON = postoperative nausea, PONV = postoperative nausea and vomiting, POV = postoperative vomiting, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, RCT = randomized controlled trial.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Borgeat A, Ekatodramis G, Schenker CA. Postoperative nausea and vomiting in regional anesthesia: a review. Anesthesiology 2003;98:530–47. [DOI] [PubMed] [Google Scholar]
- [2].Stadler M, Bardiau F, Seidel L, et al. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology 2003;98:46–52. [DOI] [PubMed] [Google Scholar]
- [3].Lin CJ, Williams BA. Postoperative nausea and vomiting in ambulatory regional anesthesia. Int Anesthesiol Clin 2011;49:134–43. [DOI] [PubMed] [Google Scholar]
- [4].McCracken G, Houston P, Lefebvre G. Guideline for the management of postoperative nausea and vomiting. J Obstet Gynaecol Can 2008;30:600–7. 608–16. [DOI] [PubMed] [Google Scholar]
- [5].Raphael JH, Norton AC. Antiemetic efficacy of prophylactic ondansetron in laparoscopic surgery: randomized, double-blind comparison with metoclopramide. Br J Anaesth 1993;71:845–8. [DOI] [PubMed] [Google Scholar]
- [6].Apfel CC, Läärä E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999;91:693–700. [DOI] [PubMed] [Google Scholar]
- [7].Afonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anestesiol 2012;62:118–33. [DOI] [PubMed] [Google Scholar]
- [8].Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med 2010;36:926–39. [DOI] [PubMed] [Google Scholar]
- [9].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tufanogullari B, White PF, Peixoto MP, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg 2008;106:1741–8. [DOI] [PubMed] [Google Scholar]
- [11].Lee C, Kim YD, Kim JN. Antihyperalgesic effects of dexmedetomidine on high-dose remifentanil-induced hyperalgesia. Korean J Anesthesiol 2013;64:301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guler G, Akin A, Tosun Z, et al. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth 2005;15:762–6. [DOI] [PubMed] [Google Scholar]
- [13].Bakhamees HS, El-Halafawy YM, El-Kerdawy HM, et al. Effects of dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypass. Middle East J Anaesthesiol 2007;19:537–51. [PubMed] [Google Scholar]
- [14].Jung HS, Joo JD, Jeon YS, et al. Comparison of an intraoperative infusion of dexmedetomidine or remifentanil on perioperative haemodynamics, hypnosis and sedation, and postoperative pain control. J Int Med Res 2011;39:1890–9. [DOI] [PubMed] [Google Scholar]
- [15].Shin HW, Yoo HN, Kim DH, et al. Preanesthetic dexmedetomidine 1 μg/kg single infusion is a simple, easy, and economic adjuvant for general anesthesia. Korean J Anesthesiol 2013;65:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Massad IM, Mohsen WA, Basha AS, et al. A balanced anesthesia with dexmedetomidine decreases postoperative nausea and vomiting after laparoscopic surgery. Saudi Med J 2009;30:1537–41. [PubMed] [Google Scholar]
- [17].Chen JY, Jia JE, Liu TJ, et al. Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can J Anaesth 2013;60:385–92. [DOI] [PubMed] [Google Scholar]
- [18].Vora KS, Baranda U, Shah VR, et al. The effects of dexmedetomidine on attenuation of hemodynamic changes and there effects as adjuvant in anesthesia during laparoscopic surgeries. Saudi J Anaesth 2015;9:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Peng K, Jin XH, Liu SL, et al. Effect of intraoperative dexmedetomidine on post-craniotomy pain. Clin Ther 2015;37:1114.e1–21.e1. [DOI] [PubMed] [Google Scholar]
- [20].Bakan M, Umutoglu T, Topuz U, et al. Opioid-free total intravenous anesthesia with propofol, dexmedetomidine and lidocaine infusions for laparoscopic cholecystectomy: a prospective, randomized, double-blinded study. Rev Bras Anestesiol 2015;65:191–9. [DOI] [PubMed] [Google Scholar]
- [21].Ali MA, Abdellatif AA. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: a comparison of dexmedetomidine and propofol. Saudi J Anaesth 2013;7:296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Turgut N, Turkmen A, Gökkaya S, et al. Dexmedetomidine-based versus fentanyl-based total intravenous anesthesia for lumbar laminectomy. Minerva Anestesiol 2008;74:469–74. [PubMed] [Google Scholar]
- [23].Gupta N, Rath GP, Prabhakar H, et al. Effect of intraoperative dexmedetomidine on postoperative recovery profile of children undergoing surgery for spinal dysraphism. J Neurosurg Anesthesiol 2013;25:271–8. [DOI] [PubMed] [Google Scholar]
- [24].Olutoye OA, Glover CD, Diefenderfer JW, et al. The effect of intraoperative dexmedetomidine on postoperative analgesia and sedation in pediatric patients undergoing tonsillectomy and adenoidectomy. Anesth Analg 2010;111:490–5. [DOI] [PubMed] [Google Scholar]
- [25].Bindu B, Pasupuleti S, Gowd UP, et al. A double blind, randomized, controlled trial to study the effect of dexmedetomidine on hemodynamic and recovery responses during tracheal extubation. J Anaesthesiol Clin Pharmacol 2013;29:162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ziemann-Gimmel P, Goldfarb AA, Koppman J, et al. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br J Anaesth 2014;112:906–11. [DOI] [PubMed] [Google Scholar]
- [27].Kim SH, Oh YJ, Park BW, et al. Effects of single-dose dexmedetomidine on the quality of recovery after modified radical mastectomy: a randomised controlled trial. Minerva Anestesiol 2013;79:1248–58. [PubMed] [Google Scholar]
- [28].Kwon SY, Joo JD, Cheon GY, et al. Effects of dexmedetomidine infusion on the recovery profiles of patients undergoing transurethral resection. J Korean Med Sci 2016;31:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bajwa SJ, Gupta S, Kaur J, et al. Reduction in the incidence of shivering with perioperative dexmedetomidine: a randomized prospective study. J Anaesthesiol Clin Pharmacol 2012;28:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hwang W, Lee J, Park J, et al. Dexmedetomidine versus remifentanil in postoperative pain control after spinal surgery: a randomized controlled study. BMC Anesthesiol 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cai X, Zhang P, Lu S, et al. Effects of intraoperative dexmedetomidine on postoperative pain in highly nicotine-dependent patients after thoracic surgery: a prospective, randomized, controlled trial. Medicine (Baltimore) 2016;95:e3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shehabi Y, Grant P, Wolfenden H, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study). Anesthesiology 2009;111:1075–84. [DOI] [PubMed] [Google Scholar]
- [33].Ozkose Z, Demir FS, Pampal K, et al. Hemodynamic and anesthetic advantages of dexmedetomidine, an alpha 2-agonist, for surgery in prone position. Tohoku J Exp Med 2006;210:153–60. [DOI] [PubMed] [Google Scholar]
- [34].Sahi S, Singh MR, Katyal S. Comparative efficacy of intravenous dexmedetomidine, clonidine, and tramadol in postanesthesia shivering. J Anaesthesiol Clin Pharmacol 2016;32:240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang G, Zhang L, Lou S, et al. Effect of dexmedetomidine in preventing postoperative side effects for laparoscopic surgery: a meta-analysis of Randomized Controlled Trials and Trial Sequential Analysis (PRISMA). Medicine (Baltimore) 2016;95:e2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhong WG, Ge XY, Zhu H, et al. Dexmedetomidine for antiemesis in gynecologic surgery: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2015;8:14566–76. [PMC free article] [PubMed] [Google Scholar]
- [37].Gan TJ, Meyer T, Apfel CC, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg 2003;97:62–71. table of contents. [DOI] [PubMed] [Google Scholar]
- [38].Pittet V, Perret C, Moret V, et al. Evolution of anaesthesia care and related events between 1996 and 2010 in Switzerland. Acta Anaesthesiol Scand 2013;57:1275–86. [DOI] [PubMed] [Google Scholar]
- [39].Ickeringill M, Shehabi Y, Adamson H, et al. Dexmedetomidine infusion without loading dose in surgical patients requiring mechanical ventilation: haemodynamic effects and efficacy. Anaesth Intensive Care 2004;32:741–5. [DOI] [PubMed] [Google Scholar]
- [40].Vilo S, Rautiainen P, Kaisti K, et al. Pharmacokinetics of intravenous dexmedetomidine in children under 11 yr of age. Br J Anaesth 2008;100:697–700. [DOI] [PubMed] [Google Scholar]
- [41].Dyck JB, Maze M, Haack C, et al. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology 1993;78:813–20. [DOI] [PubMed] [Google Scholar]
- [42].El-Shmaa NS, El-Baradey GF. The efficacy of labetalol vs dexmedetomidine for attenuation of hemodynamic stress response to laryngoscopy and endotracheal intubation. J Clin Anesth 2016;31:267–73. [DOI] [PubMed] [Google Scholar]
- [43].Cohen MM, Duncan PG, DeBoer DP, et al. The postoperative interview: assessing risk factors for nausea and vomiting. Anesth Analg 1994;78:7–16. [DOI] [PubMed] [Google Scholar]
- [44].Apfel CC, Greim CA, Haubitz I, et al. A risk score to predict the probability of postoperative vomiting in adults. Acta Anaesthesiol Scand 1998;42:495–501. [DOI] [PubMed] [Google Scholar]
- [45].van den Bosch JE, Kalkman CJ, Vergouwe Y, et al. Assessing the applicability of scoring systems for predicting postoperative nausea and vomiting. Anaesthesia 2005;60:323–31. [DOI] [PubMed] [Google Scholar]
- [46].Apfel CC, Roewer N, Korttila K. How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand 2002;46:921–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


