Abstract
Background:
Previous studies indicated that the puerarin injection has been widely employed in China for the treatment of acute ischemic stroke. We aim to evaluate the efficacy and safety of the puerarin injection for the treatment of acute ischemic stroke.
Methods:
A systematic literature search was performed in PUBMED, EMBASE, SPRINGER LINK, Scopus, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP Journals Database, Wanfang database and the China Biological Medicine database before November 2016, randomized controlled clinical trials (RCTs) of puerarin injection treating acute ischemic stroke were included. In addition, we searched reference lists of relevant retrieved articles. Two authors extracted data independently. The effective rate, the neurologic deficit score, the blood rheology indexes, and fibrinogen were assessed and analyzed by the Review Manager 5.3 software. The continuous variables were expressed as MD with 95% CI and dichotomous data used RR or ORs. Adverse reactions related to the puerarin injection were also examined.
Results:
Thirty-five RCTs with a total of 3224 participants were identified in the meta-analysis. The combined results of 32 trials indicated that the puerarin injection was better than control drugs at the clinical effective rate (RR 1.22, 95% CI 1.17 to 1.28, P < 0.001) and 16 studies showed the neurological deficit was significantly improved (MD –3.69, 95% CI –4.67 to –2.71, P < 0.001); the hemorheology index and fibrinogen were much lower with the puerarin injection when compared with western conventional medicines (WCM) or other control drugs (the whole blood viscosity: MD –0.89, 95% CI –1.37 to –0.41, P < 0.001; the HCT: MD –0.04, 95% CI –0.06 to –0.02, P < 0.001; the fibrinogen: MD –0.64, 95% CI –0.96 to –0.31, P < 0.001). Eleven trials reported that the adverse reactions related to the puerarin injection included facial flushing, dizziness, vomiting, nausea, and other mild gastrointestinal discomfort and allergic reaction. No serious adverse drug reactions were reported.
Conclusions:
Puerarin injection may be more effective and relatively safe in clinic for treating acute ischemic stroke. However, the current evidence is insufficient due to the poor methodological quality and lack of adequate safety data. Further RCTs are required to examine its efficacy.
Keywords: acute cerebral infarction, acute ischemic stroke, meta-analysis, puerarin, randomized controlled trials
1. Introduction
Stroke is the main cause of death and disability in the world. Although various surveillance systems are used to assess stroke and its sequela, stroke still remains one of the top causes of mortality, disability, and affects the disability-adjusted life years.[1] In China, there are 1.5 to 2 million new cases of stroke each year. Stroke has been ranked as the first leading cause of mortality and long-term disability, which caused a heavy economic burden to the family and even the whole society.[2] The incidence of stroke due to ischemia accounts for 68%.[3] The ischemic stroke is caused by blockages or narrowing of the arteries that provide blood to the brain, resulting in ischemia severely and decreased blood flow.[4] Acute ischemic stroke and metabolic syndrome patients triggered a more intense immune-inflammatory activation, which results in a higher degree of immuno-inflammation and arterial stiffness.[5] Regrettably, so far, no routine effective specific therapy for ischemic stroke is generally accepted, except for aspirin and thrombolytic treatment with recombinant tissue plasminogen activator for highly selected patients.[6] Therefore, various kinds of complementary and/or alternative medicine are being developed worldwide. Traditional Chinese medicine has been widely used in the treatment of ischemic stroke such as rhizoma gastrodiae, radix astragali, radix puerariae, and other Chinese herbal medicine or non-medication therapies for many years.[7]
Gegen, the dried root of pueraria lobata, is one of the earliest and most important edible crude herbs used for various medical purposes in Chinese medicine. Puerarin (relative molecular weight 416.38, Fig. 1), the major bioactive component of the traditional Chinese medicine Radix puerariae (kudzu root), is a major isoflavonoid with polyhydroxy.[8] Puerariae radix has been reported to display anti-inflammatory effects,[9] antiplatelet aggregation,[10] antioxidant,[11] as well as decreasing plasma cholesterol.[12] Puerarin injection was a common dosage form of puerarin for curing microcirculation disturbance and cardio-cerebrovascular diseases as Chinese patent drug for more than 20 years.[13,14] Randomized controlled trials (RCTs) upon puerarin injection have exhibited to improve neurological deficit after cerebral ischemia in patients.[15] A previous review about puerarin treating ischemic stroke presented a positive conclusion;[16] however, the sample size was too small to draw a reliable conclusion. Therefore, in this paper, we included more trials and aimed to evaluate the clinical efficacy and safety of puerarin injection for treating acute ischemic stroke as well as to provide high-quality evidence for further clinical utilization.
Figure 1.
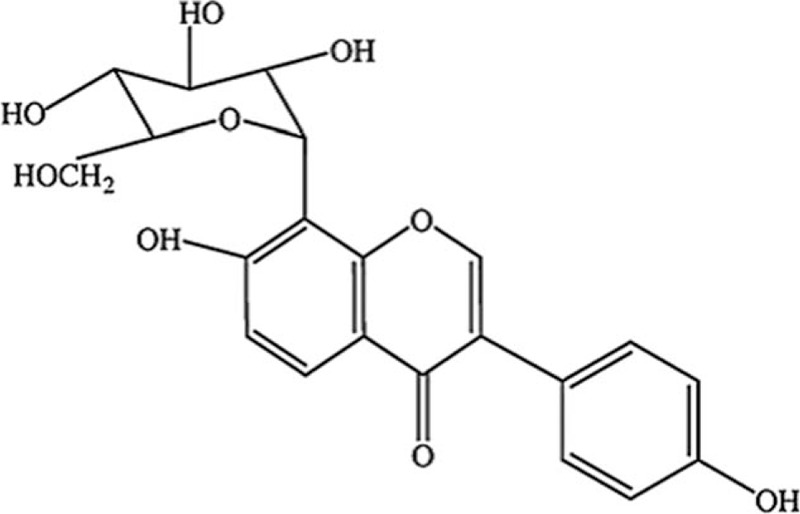
Chemical structure of puerarin.
2. Methods
2.1. Database searched
We used “puerarin,” “ischemic stroke,” or “cerebral infarction” as the search terms to search PUBMED, EMBASE, SPRINGER LINK, Scopus, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP Journals Database, Wanfang database, and the China Biological Medicine database before November 2016.
2.2. Inclusion criteria
Ischemic stroke was diagnosed clinically according to the World Health Organization definition or the diagnostic criteria issued at the Second and revised at the Fourth National Cerebrovascular Diseases Conference in China[17] and approved by CT scan or MRI. Patients with ischemic stroke within 7 days of onset and diagnosed without serious organic disease and complications were considered.[18] RCTs that evaluated efficacy and safety of puerarin for ischemic stroke patients were included.
2.3. Intervention measures
The experimental groups were given puerarin with sodium chloride or glucose injection, the intervention for treatment groups included only puerarin herbal without other Chinese medicine. The patients of the control group were given WCM such as aspirin or other medicine without puerarin. In some cases, 2 groups would be given basic treatment on the basis of the condition of the patient in the same time.
2.4. Outcomes
The total effective rate was the primary outcome. Secondary outcomes were the neurological deficit improvement after treatment. Third outcomes included hemorheology index with whole blood viscosity, hematocrit (HCT), and fibrinogen. The adverse events were recorded.
2.5. Data extraction and statistical analysis
For all studies included in the systematic review, data extraction and study quality assessment were independently conducted by 2 authors (Q-HZ and X-LL), with disagreement resolved by consensus. The following data were extracted from each primary study, if available, including study types, patient characteristics, and treatment. The Review Manager 5.3 software was used for data-analysis. A fixed-effect model or random-effect model was used across the trials, and risk ratios with their 95% confidence intervals (CI) were calculated for dichotomous data. If continuous data were available, weighted mean difference or standardized mean difference was to be calculated. I2 statistic showed the degree of heterogeneity. Groups were distributed to subgroups based on the different kinds of blood rheology indexes. The bias assessed through the Funnel plot or Egger tests in this study.
2.6. Quality assessment
We evaluated the risk of bias according to the Cochrane risk of bias tool, which included the following 7 domains, random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
3. Results
3.1. Assessment of quality
On the basis of search strategy, 523 potentially relevant articles were identified after duplicates removed. Then, 378 articles were excluded by reviewing the types and designs of trials, and another 110 articles were excluded by reviewing the inclusion criteria. Thus, there were 35 primary studies, with 3224 participants in total, included in the systematic review. All of these studies were conducted in China and published before November 2016, described as randomized, and did not report the method of random sequences generation.[19–53] The study screening procedure was summarized in a flow diagram (Fig. 2). Detailed characteristics of the 35 studies and puerarin dose in each study were described in Table 1. Based on the GRADE system, the evidence of effective rate and neurological deficit score were weak recommendation (Figs. 3 and 4). There was no significant publication bias, and no small study effects were found in the funnel plot (Fig. 5) or revealed by the Egger (P = 0.006).
Figure 2.
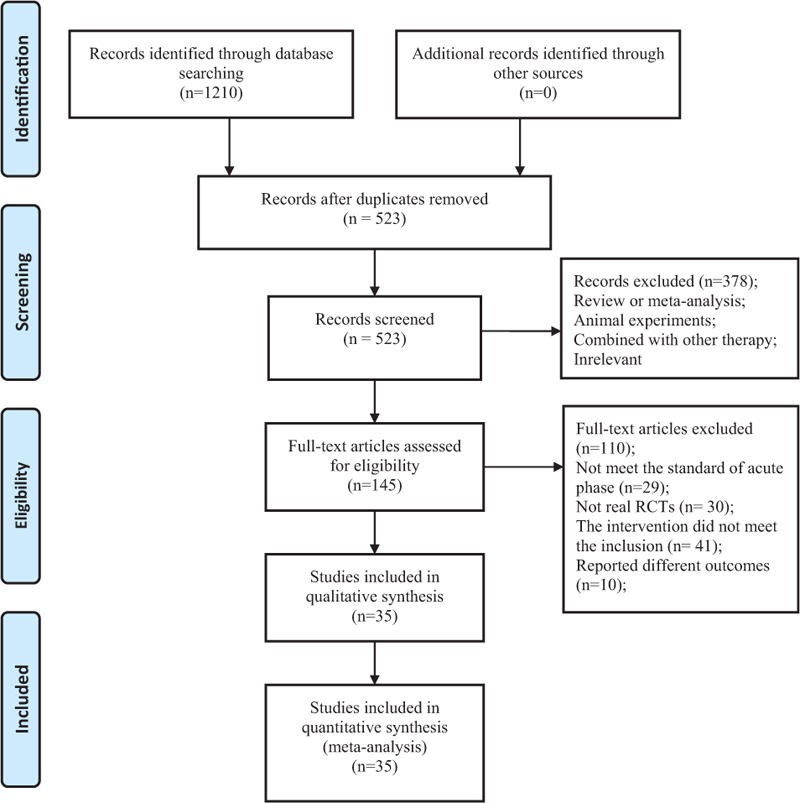
Flowchart summarizing the selection process of meta-analyses.
Table 1.
Characteristics of 35 included studies on the effect of puerarin for acute ischemic stroke.
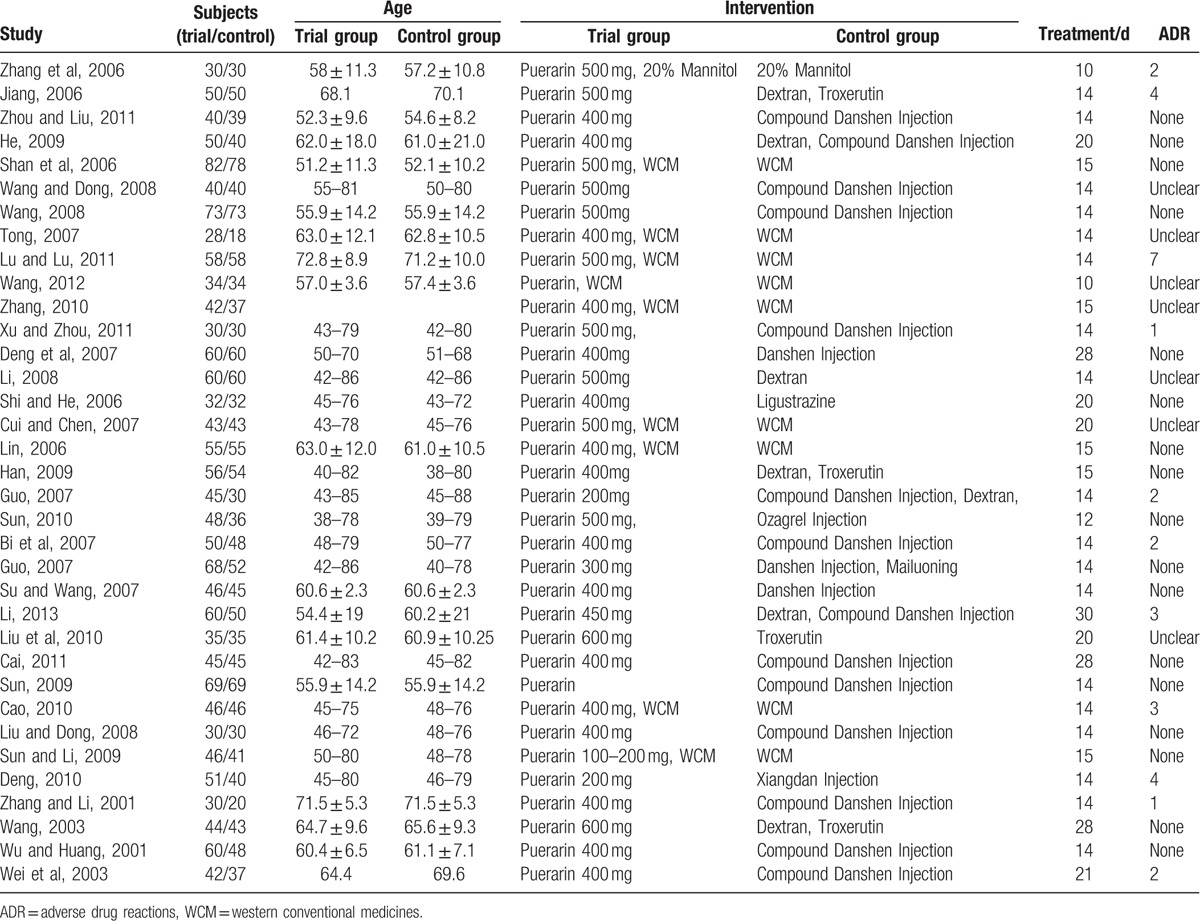
Figure 3.
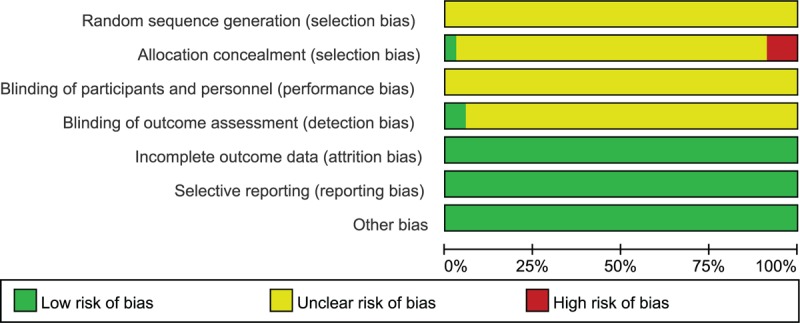
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Figure 4.
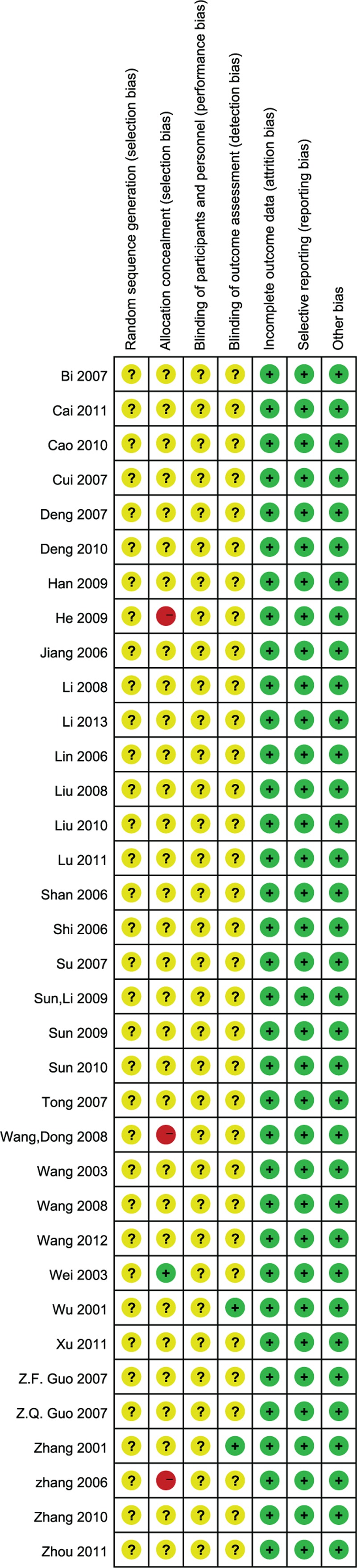
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
Figure 5.
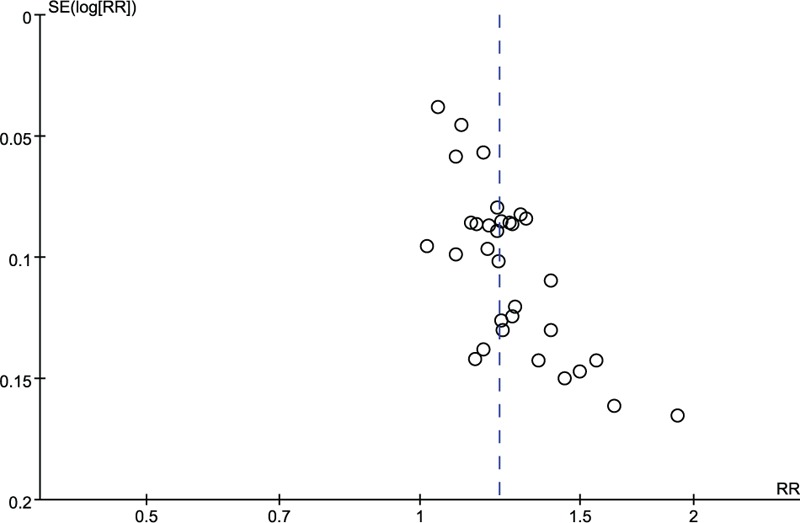
Funnel plot of the total effective rate.
3.2. Outcomes
3.2.1. The clinical effective rate
In total, 32 trials adopted the effective rate to assess the clinical improvement and the random-effective model was used for statistical analysis. The analysis showed favor of puerarin (n = 2967, RR 1.22, 95% CI 1.17 to 1.28, P < 0.001), heterogeneity χ2 = 58.69, P = 0.002, I2 = 47%, Fig. 6).
Figure 6.
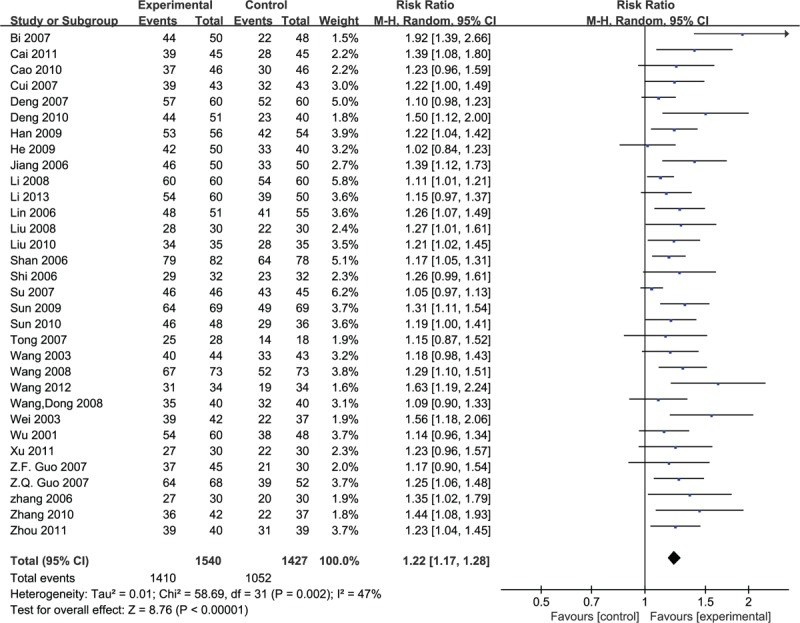
Meta-analyses of the total effective rate.
3.2.2. The scores of neurological deficits
However, 16 studies which used the neurologic deficit score were qualified to perform a meta-analysis, and the random effective model was used for statistical analysis because of the heterogeneity (n = 1358, MD –3.69, 95% CI –4.67 to –2.71, P < 0.001, heterogeneity χ2 = 49.43, P < 0.0001, I2 = 70%), and favored the puerarin group (Fig. 7).
Figure 7.
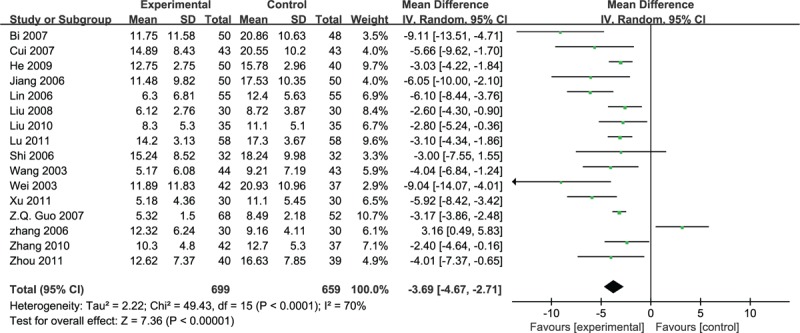
Meta-analyses of the scores of neurological deficits.
3.2.3. Blood rheology indexes and fibrinogen
Twelve studies involved whole blood viscosity, and the random effective model was used for statistical analysis because of the heterogeneity (n = 1036, MD –0.89, 95% CI –1.37 to –0.41, P < 0.001, heterogeneity χ2 = 359.22, P < 0.0001, I2 = 97%) and favored the puerarin group (Fig. 8).
Figure 8.
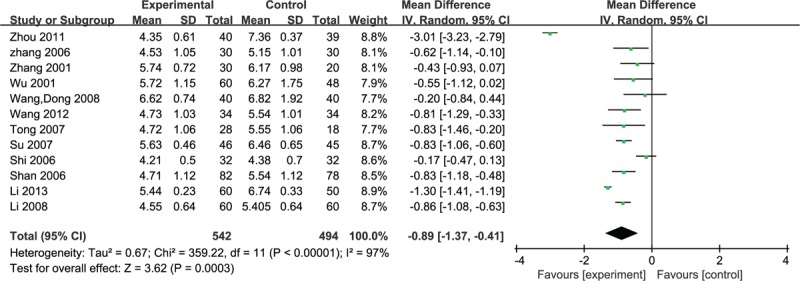
Meta-analyses of the whole blood viscosity scores.
Twelve studies adopted HCT to evaluate the clinical significance of puerarin for the ischemic stroke in hemocyte, due to the heterogeneity (n = 1070, MD –0.04, 95% CI –0.06 to –0.02, P < 0.001, heterogeneity χ2 = 245.98, P < 0.0001, I2 = 96%), the random effective model was used. The consequence showed the favor of experimental group (Fig. 9).
Figure 9.
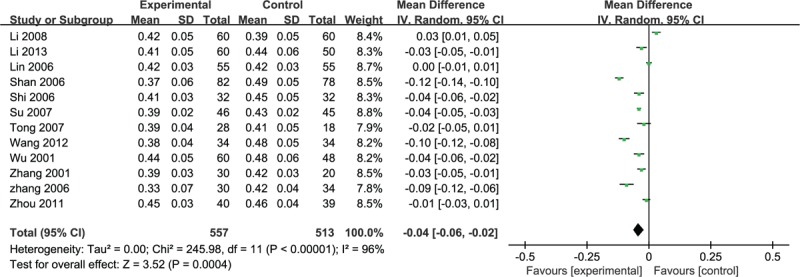
Meta-analyses of HCT. HCT = hematocrit.
Eleven of the studies adopted the fibrinogen to assess the clinical improvement and the random-effective model was used for statistical analysis (heterogeneity χ2 = 233.64, P < 0.0001, I2 = 96%). The puerarin group was significantly lower than the fibrinogen control group (n = 1011, MD –0.64, 95% CI –0.96 to –0.31, P < 0.001) (Fig. 10).
Figure 10.
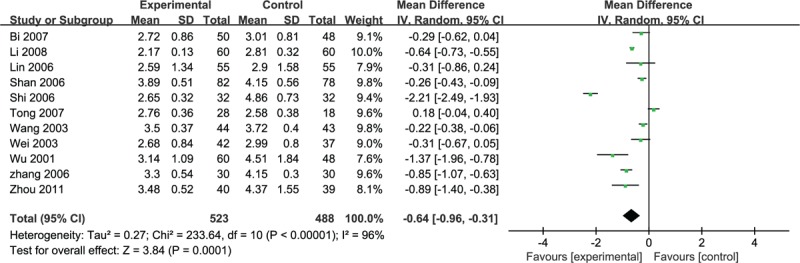
Meta-analyses of the fibrinogen.
3.3. Safety
Due to the variety of symptoms and the low number of adverse reactions reported, it was difficult to conduct a meta-analysis, so the adverse reactions were described. Eleven studies reported that patients might be temporary bloating, nausea and other gastrointestinal reactions, dizziness and facial flushing, but the symptoms were relieved after continued treatment,[19,20,27,30,37,39,42,46,49,50,53] rashes was reported in 2 trials,[37,49] whereas the left trials reported no adverse effects. No serious adverse drug reactions occurred.
4. Discussion
In our study, the efficacy and safety of puerarin injection in curing acute ischemic stroke were investigated. Thirty-five RCTs involving a total of 3224 participants with acute ischemic stroke were included. The results demonstrated that puerarin could improve the neurological deficit of acute ischemic stroke, lower blood viscosity, and reduce fibrinogen production. The outcomes were partially similar to the results of a previous review,[13] which just assessed the efficiency of puerarin for ischemic stroke and showed that puerarin improved neurological deficit significantly more than the control. However, the review neither evaluated the effect on blood rheology indexes nor fibrinogen, and its possible mechanism was not discussed. Actually, plasma fibrinogen played a major determinant in platelet aggregation and blood viscosity, whereas high blood viscosity led to blood stagnation and then promoted thrombosis, resulting in the development of ischemic stroke.[54,55] So evaluating the effect of puerarin injection on blood rheology indexes and fibrinogen was important. Moreover, our enrolled sample size was much larger and we focused on acute ischemic stroke treatment, whereas they included acute and chronic ischemic stroke. In summary, our study tried to offer a high-quality evidence-based approach upon puerarin injection for treating ischemic stroke.
As we all know, the ischemic stroke is a common cardiovascular disease involves death of brain tissue (cerebral infarction) resulting from an inadequate supply of blood and oxygen to the brain due to blockage of an artery.[56] Ischemic stroke causes a lot of damage to body and seriously affects the quality of life. Because the high morbidity and mortality of acute ischemic stroke patients increased with increasing age, which threatens the health of human beings.[57] Hypoxic ischemic brain injury often causes irreversible brain damage and the cascade of events leading to neuronal injury and death in ischemia includes the release of cytokines and free radicals, and induction of inflammation, apoptosis, and excitotoxicity.[58]
Many pharmacological interventions such as thrombolytic, antioxidant, cerebral vasodilator, Ca2+ channel blocker, and free radical scavenger have been observed to produce acute ischemia and cerebral ischemia-reperfusion protection.[7] Insufficiently, the usage criteria and administration time window are limited in thrombolytic,[59] and cerebral hemorrhagic complications occur more easily.[60] Therefore, it is necessary to seek some new alternative medicine with the characteristics of high safety, high efficiency, and synthetic therapeutic effects. In China, multiple kinds of Chinese medicinal herbs or effective constituents, such as Buyang Huanwu decoction[6], compound salvia injection[61], Xuesetong injection[62] and puerarin injection we discussed here, have been widely used to treat ischemic stroke for a long history. Puerarin is a major isoflavonoid derived from the Chinese medical herb radix puerariae (Gegen), which is the root of the familiar kudzu vine. In traditional Chinese medicine, radix puerariae has been widely used in the treatment of cerebrovascular disorders, cardiovascular diseases, cancer, Alzheimer's disease (AD), and diabetes and diabetic complications.[63] Puerarin is an isoflavone compound separated from the drying of kudzu root and its injection was purified and developed from the 1990s of the 20th century.[13]
By reviewing the recent pharmacological researches, we found that puerarin was a potent neuroprotective drug on MCAO-induced focal cerebral ischemia in vivo, by inhibiting both HIF-1α and TNF-α activation, followed by the inhibition of inflammatory responses (i.e., iNOS expression), apoptosis formation (active caspase-3), and neutrophil activation, resulting in a reduction of infarct volume in ischemia reperfusion brain injury.[64] Also, Liu et al[65] reported that puerarin reduced the ischemic infarct volume and improved neurological deficit after cerebra ischemia/reperfusion by activating the cholinergic anti-inflammatory pathway. In our meta-analysis, the neurologic deficit score of puerarin injection group did improve when compared with the control group (MD –3.69, 95% CI –4.67 to –2.71, P < 0.001). Yan et al[66] indicated that puerarin was relevant to triggering extracellular Ca2+ influx into endothelial cytosol, which involved the endothelial Ca2+–NO–cGMP pathway, prostacyclin, and opening of the 3 K+ channels and then effected the endothelium-dependent antivasoconstrictive; Pan et al[10] suggested that the puerarin injection could ameliorate the hemorheology and the abnormal augmentation of platelet aggregation, which these pharmacological researches met with the results of our meta-analysis that puerarin injection could reduce blood viscosity.
There are still some limitations in our study. For example, the period of most observation lasted for only 14 days and none of studies reported the record of results and dropout data, long-term observation, and flowing-up are required in further study. And there are several methodological limitations in the primary studies; all trials were RCTs but none of them reported the random method or allocation concealment, which may produce selection bias. So that, more randomized, double-blind, controlled, multicenter of clinical trials are needed. Though we made effort to find more clinical experiment trails, all the studies were from China mainland. All the adverse reactions were described due to only a few of the included trials reported adverse events and the cases, which was not enough for statistical analysis. In addition, the dose of included trials was different, which varied from 0.2 g to 0.6 g, and no standard dose delivered to the acute ischemic stroke target was obtained.
In conclusion, the meta-analysis indicates that the puerarin injection is more effective than WCM and provides evidence-based approach for treating ischemic stroke. However, more high quality-RCTs are needed to provide reliable evidence on the effectiveness of puerarin injection for treating acute ischemic stroke.
Footnotes
Abbreviations: CI = confidence interval, HCT = hematocrit, MCAO = middle cerebral artery occlusion, MD = mean differences, ORs = odds ratios, RCTs = randomized controlled clinical trials, RR = relative risk, WCM = Western conventional medicines.
Q-HZ and X-LL contributed equally to this study.
Authorship: Z-GM conceived and designed the study; Q-HZ, X-LL, Q-XM, and S-BY collected the data; Q-HZ, X-LL, and LX performed the analysis and prepared the manuscript; J-FW and L-JT made amendments to the manuscript; and Z-TF participated in designing the study and revised the manuscript. All authors read and approved the final version of the manuscript.
Funding: The present study was supported by Open Fund of Key Laboratory of Cardiovascular and Cerebrovascular Diseases Translational Medicine, China Three Gorges University (2016xnxg101).
The authors have no conflicts of interest to disclose.
References
- [1].Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg 2011;76:S85–90. [DOI] [PubMed] [Google Scholar]
- [2].Zhang X, Sun Z, Ding C, et al. Metabolic syndrome augments the risk of early neurological deterioration in acute ischemic stroke patients independent of inflammatory mediators: a hospital-based prospective study. Oxid Med Cell Longev 2016;2016:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health 2013;1:e259–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999;22:391–7. [DOI] [PubMed] [Google Scholar]
- [5].Tuttolomondo A, Pecoraro R, Di RD, et al. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke with and without metabolic syndrome. Diabetol Metab Syndr 2014;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu B, Liu M, Liu H, et al. Meta-analysis of traditional Chinese patent medicine for ischemic stroke. Stroke 2007;38:1973–9. [DOI] [PubMed] [Google Scholar]
- [7].Hao C, Wu F, Shen J, et al. Clinical efficacy and safety of buyang huanwu decoction for acute ischemic stroke: a systematic review and meta-analysis of 19 randomized controlled trials. Evid Based Compl Alternat Med 2012;2012:630124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pan HP, Li G. Protecting mechanism of puerarin on the brain neurocyte of rat in acute local ischemia brain injury and local cerebral ischemia-reperfusion injury. Yakugaku Zasshi 2008;128:1689–98. [DOI] [PubMed] [Google Scholar]
- [9].Lim DW, Lee C, Kim IH, et al. Anti-inflammatory effects of total isoflavones from pueraria lobata on cerebral ischemia in rats. Molecules 2013;18:10404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pan HP, Yang JZ, Li LL, et al. Effect of puerarin injection on platelet aggregation in acute blood-stasis model rats (Chinese). Chin Hosp Pharm 2005;25:6–8. [Google Scholar]
- [11].Jia XB, Cai Y, Chen Y, et al. Pharmacokinetics of pueraria flavonoids from Pueraria lobata by anti-oxidant pharmacodynamics effect method (Chinese). China J Chin Mater Med 2007;32:2370–3. [PubMed] [Google Scholar]
- [12].Yan LP, Chan SW, Chan SC, et al. Puerarin decreases serum total cholesterol and enhances thoracic aorta endothelial nitric oxide synthase expression in diet-induced hypercholesterolemic rats. Life Sci 2006;79:324–30. [DOI] [PubMed] [Google Scholar]
- [13].Zeng GY, Gu WZ, Lin M, et al. The research of puerarin injection (Chinese). Acta Academiae Medicinae Sinicae 1996;1:59. [Google Scholar]
- [14].Duan CG, Li HW, Xu LN. The effects of puerarin on the cerebral microcirculation in golden hamsters (Chinese). Natl Med J Chin 1991;71:516–7. [Google Scholar]
- [15].Wu HQ, Sun QG, Zhan SQ, et al. Effects of puerarin on blood rheology of patients with cerebral infarction (Chinese). Chin J Hemorh 2005;15:244–6. [Google Scholar]
- [16].Liu B, Tan Y, Wang D, et al. Puerarin for ischaemic stroke. Cochrane Database Syst Rev 2016;2:004955. [DOI] [PubMed] [Google Scholar]
- [17].Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 1976;54:541–53. [PMC free article] [PubMed] [Google Scholar]
- [18].The Second National Cerebrovascular Diseases Conference Recommendations on clinical research in stroke (Chinese). Chin J Neurol Psychiatry 1988;21:57–60. [Google Scholar]
- [19].Zhang YC, Huang LP. The observation of clinical efficacy of puerarin glucose injection therapy in 30 cases with brain infarction. J Jinggangshan Univ (Chinese) 2006;5:94–5. [Google Scholar]
- [20].Jiang GH. The observation of efficacy of puerarin glucose injection therapy with brain infarction (Chinese). J Youjiang Med Coll Nationalities 2006;13:900. [Google Scholar]
- [21].Zhou TZ, Liu JD. Effects of puerarin injection on blood rheology and neurological deficit of patients with cerebral infarction (Chinese). Chin J Pract Nervous Dis 2011;13:45–6. [Google Scholar]
- [22].He JB. The observation of clinical efficacy of puerarin injection with acute cerebral infarction (Chinese). Contemp Med 2009;3:93–4. [Google Scholar]
- [23].Shan YH, Zhang YG, Wang Y, et al. The efficacy of puerarin injection treatment for acute cerebral infarction (Chinese). J Sichuan Trad Chin Med 2006;7:47–8. [Google Scholar]
- [24].Wang JF, Dong XY. The observation of clinical efficacy of puerarin injection with acute ischemic stroke (Chinese). J Prac Trad Chin Internal Med 2008;9:66–7. [Google Scholar]
- [25].Wang FS. The observation of efficacy of puerarin glucose injection therapy with acute cerebral infarction (Chinese). Mod J Integr Trad Chin West Med 2008;22:3464–5. [Google Scholar]
- [26].Tong N. The observation of efficacy of puerarin in 46 cases with acute cerebral infarction (Chinese). Mod J Integr Trad Chin West Med 2007;5:612–3. [Google Scholar]
- [27].Lu JM, Lu YZ. The observation of efficacy of puerarin in 58 cases with acute cerebral infarction (Chinese). Chin J Gerontol 2011;13:2575–6. [Google Scholar]
- [28].Wang YC. The study of clinical efficacy of puerarin in 68 cases with acute cerebral infarction (Chinese). Med Rev 2012;11:1774–6. [Google Scholar]
- [29].Zhang AJ. To observe 79 cases clinical curative effects of the puerarin to treat acute cerebral infarction (Chinese). Chin Med Mod Dist Educ China 2010;24:33. [Google Scholar]
- [30].Xu P, Zhou YF. The observation of efficacy of puerarin with acute cerebral infarction (Chinese). China J Clin Rational Drug Use 2011;20:58–9. [Google Scholar]
- [31].Deng SQ, Zheng QP, Li JM. The observation of clinical efficacy of puerarin with acute cerebral infarction (Chinese). Appl J Gen Pract 2007;4:345. [Google Scholar]
- [32].Li YG. Clinical study of puerarin for cerebral infarction (Chinese). Chin J Mod Drug App 2008;23:87–8. [Google Scholar]
- [33].Shi SH, He CY. The observation of efficacy of puerarin with acute cerebral infarction (Chinese). J Emerg Trad Chin Med 2006;12:1335–51. [Google Scholar]
- [34].Cui Q, Chen YP. Clinical analysis of puerarin for cerebral infarction (Chinese). Asia-Pacific Trad Med 2007;6:26–7. [Google Scholar]
- [35].Lin HL. Clinical analysis of puerarin for acute cerebral infarction (Chinese). Mod Med Health 2006;3:364. [Google Scholar]
- [36].Han XG. The observation of efficacy of puerarin with acute cerebral infarction (Chinese). Chin J Pract Nerv Dis 2009;11:98. [Google Scholar]
- [37].Guo ZF. The observation of efficacy of puerarin injection in 45 cases with acute cerebral infarction (Chinese). Public Med Forum Magazine 2007;8:310–1. [Google Scholar]
- [38].Sun LR. The observation of efficacy of puerarin injection in 48 cases with acute cerebral infarction (Chinese). Inner Mongolia Trad Chin Med 2010;17:16–7. [Google Scholar]
- [39].Bi SQ, Li GQ, Zhou HG, et al. The observation of efficacy of puerarin in 50 cases with acute cerebral infarction (Chinese). Chin J Pract Nerv Dis 2007;3:117–8. [Google Scholar]
- [40].Guo ZQ. The observation of efficacy of puerarin injection with acute cerebral infarction (Chinese). China Sci Technol Chin Med 2007;14:350. [Google Scholar]
- [41].Su YH, Wang XA. The observation of efficacy of puerarin injection with acute cerebral infarction (Chinese). Chin J Prac Nervous Dis 2007;10:94–5. [Google Scholar]
- [42].Li JW. The clinical study of puerarin injection with acute cerebral infarction (Chinese). Strait Pharm J 2013;4:67–9. [Google Scholar]
- [43].Liu C, Li YP, Zhang RH, et al. Analysis of puerarin injection for treatment of acute cerebral infarction (Chinese). Clin J Trad Chin Med 2010;2:128–9. [Google Scholar]
- [44].Cai LM. The observation of efficacy of puerarin injection with acute cerebral infarction (Chinese). Strait Pharm J 2011;2:145–6. [Google Scholar]
- [45].Sun JS. The observation of efficacy of puerarin injection with acute cerebral infarction (Chinese). Mod J Integr Trad Chin West Med 2009;31:3842–3. [Google Scholar]
- [46].Cao Z. Clinical observation of puerarin injection with acute cerebral infarction (Chinese). J Med Forum 2010;3:85–6. [Google Scholar]
- [47].Liu CJ, Dong LM. The observation of clinical efficacy of puerarin injection with acute cerebral infarction (Chinese). China Pract Med 2008;13:47–8. [Google Scholar]
- [48].Sun SG, Li CR. Clinical evaluation of pueraria in 87 patients for cerebral infarction (Chinese). China Mod Med 2009;12:35–6. [Google Scholar]
- [49].Deng JG. Curative effects and adverse reactions of treating cerebral infarction with puerarin injection (Chinese). Anhui Health Vocational Tech Coll 2010;3:57–8. [Google Scholar]
- [50].Zhang JX, Li ZG. Clinical observation on the treatment of acute cerebral infarction by Puerarin in 30 cases(Chinese). Acta Academiae Medicinae 2001;10:25–6. [Google Scholar]
- [51].Wang ZQ. Clinical study of puerarin for cerebral infarction (Chinese). Chin J Integr Med Cardio-/Cerebrovascuiar Dis 2003;1:212–3. [Google Scholar]
- [52].Wu GP, Huang DD. Clinical study of puerarin in 60 for cerebral infarction (Chinese). Mod J Integr Trad Chin West Med 2001;10:910–1. [Google Scholar]
- [53].Wei LL, Wu YB, Feng HY. Clinical observation of puerarin injection with acute cerebral infarction (Chinese). J Emerg Trad Chin Med 2003;12:204–5. [Google Scholar]
- [54].Furukawa K, Abumiya T, Sakai K, et al. Increased blood viscosity in ischemic stroke patients with small artery occlusion measured by an electromagnetic spinning sphere viscometer. J Stroke Cerebrovasc Dis 2016;25:2762–9. doi: 10.1016/j.jstrokecerebrovasdis. [DOI] [PubMed] [Google Scholar]
- [55].Maresca G, Di BA, Marchioli R, et al. Measuring plasma fibrinogen to predict stroke and myocardial infarction. Arteriosclerosis 1990;10:1–7. [DOI] [PubMed] [Google Scholar]
- [56].Emsley HCA, Smith CJ, Tyrrell PJ, et al. Inflammation in acute ischemic stroke and its relevance to stroke critical care. Neurocrit Care 2008;9:125–38. [DOI] [PubMed] [Google Scholar]
- [57].Wang H, Ren S, Liu C, et al. An overview of systematic reviews of danhong injection for ischemic stroke. Evid-Based Comp Alt 2016;2016:8949835.doi: 10.1155/2016/8949835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu Z, Li P, Dan Z, et al. Protective effect of extract of Cordyceps sinensis in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Behav Brain Funct 2010;6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hajjar K, Kerr DM, Lees KR. Thrombolysis for acute ischemic stroke. Geriatrics 2011;54:901–7. [DOI] [PubMed] [Google Scholar]
- [60].Masingue M, Alamowitch S. An update on limitations of intravenous thrombolysis to treat acute ischemic stroke. Presse Med 2015;44:515–25. [DOI] [PubMed] [Google Scholar]
- [61].Zhang RJ, You C, Cai BW. Effect of compound salvia injection on blood coagulation in patients with traumatic cerebral infarction (Chinese). Chin J Integr Trad West Med 2004;24:882–4. [PubMed] [Google Scholar]
- [62].Zhang X, Wu J, Zhang B. Xuesaitong injection as one adjuvant treatment of acute cerebral infarction: a systematic review and meta-analysis. BMC Complement Altern Med 2015;15:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects. Phytother Res 2014;28:961–75. [DOI] [PubMed] [Google Scholar]
- [64].Yi C, Hsieh CY, Peng ZA, et al. Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats. J Biosoc Sci 2009;16:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liu XJ, Mei ZG, Qian JP, et al. Puerarin partly counteracts the inflammatory response after cerebral ischemia/reperfusion via activating the cholinergic anti-inflammatory pathway. Neural Regen Res 2013;8:3203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yan LP, Zhuang YL, Chan SW, et al. Analysis of the mechanisms underlying the endothelium-dependent antivasoconstriction of puerarin in rat aorta. Naunyn Schmiedebergs Arch Pharmacol 2009;379:587–97. [DOI] [PubMed] [Google Scholar]


