Abstract
Flexible bronchoscopy has been more and more used for diagnosis and management diseases of respiratory system in pediatrics. Previous studies have reported that remifentanil (RF) and propofol are safe and effective for flexible bronchoscopy in adults, however, there have no trials evaluate the efficacy of DEX-RF versus dexmedetomidine-propofol in children undergoing flexible bronchoscopy.
We divided 123 children undergoing flexible bronchoscopy with DEX-RF or dexmedetomidine-propofol into 2 groups: Group DR (n = 63, DEX infusion at 1.0 μg kg−1 for 10 minutes, then adjusted to 0.5–0.7 μg kg−1 h−1; RF infusion at 1.0 μg kg−1 for 5 minutes, then adjusted to 0.05–0.2 μg kg−1 min−1), Group DP (n = 60, DEX infusion at 1.0 μg kg−1 for 10 minutes, then adjusted to 0.5–0.7 μg kg−1 h−1; propofol infusion at 10 μg kg−1 for 5 minutes, then adjusted to 0.05–0.1 μg kg−1 min−1). Ramsay sedation scale of the 2 groups was maintained at 3. Anesthesia onset time; total number of intraoperative patient movements; hemodynamics; total cumulative dose of DEX; amount of and time to first-dose rescue midazolam and lidocaine; postoperative recovery time; adverse events; and bronchoscopist satisfaction score were recorded.
Anesthesia onset time was significantly shorter in DP (8.22 ± 2.48 vs 12.25 ± 6.43 minutes, respectively, for DP, DR, P = 0.015). The perioperative hemodynamic profile was more stable in DR than DP group. More children moved during flexible bronchoscopy in DP group (P = 0.009). Total dose of rescue midazolam and lidocaine was significantly higher in DR than in DP (P < 0.001). Similarly, the time to first dose of rescue midazolam and lidocaine was significantly longer in DP than in DR (P < 0.001). Total cumulative dose of DEX was more in DR than DP group (P < 0.001). The time to recovery for discharge from the postanesthesia care unit (PACU) was significantly shorter in DP than in DR group (P < 0.001). The bronchoscopist-satisfaction scores were higher for DR than DP (P = 0.036). There were significant differences between the 2 groups in terms of the overall incidence of hypertension, tachycardia, and hypoxemia (P < 0.05).
Although underwent longer recovery time and more incidence of rescue scheme, DEX-RF resulted in more stable hemodynamic profiles and bronchoscopist-satisfaction scores, lesser patient movements, and can hence be more effectively used in children undergoing flexible bronchoscopy than dexmedetomidine-propofol.
Keywords: dexmedetomidine, flexible bronchoscopy, pediatric, propofol, remifentanil
1. Introduction
Bronchoscopic interventional procedures are performed under general anesthesia for a higher success rate, especially for children.[1–3] It has been the challenge for anesthesiologist to select appropriate degree of anesthesia to meet the procedural needs.[4] Short-acting opioids, propofol, midazolam, newer drugs such as dexmedetomidine (DEX) have facilitated the conduct of this procedure. Even so, more and more hospitals have been set up a technical team to decrease the increasing adverse events of flexible bronchoscopy.[5,6] Recently, the anesthetic technique of monitored anesthesia care (MAC) without respiratory depression has been widely used.[7] The most commonly used anesthetic agents include midazolam, propofol, etomidate, opioids, and inhalation anesthetics, however, each of these drugs has its limitations.[8–10]. Combination of these drugs can result in severe respiratory depression, which is the most common complication and the reason of flexible bronchoscopy failure.[11,12] Therefore, to seek the reasonable combination of drugs, that can be used effectively in children during flexible bronchoscopy, is urgent.
DEX, a highly selective agonist of the α2 adrenergic receptor, has a more favorable pharmacokinetic profile than clonidine.[13] Previous studies have reported that DEX combined with midazolam, propofol, or opioids could be safely and effectively used for flexible bronchoscopy.[14–16] However, hypotension, bradycardia, and excessive sedation have been reported during these articles. An independent search of MEDLINE, PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and Web of Science for English language articles between 2000 and 2015, with the key terms “dexmedetomidine,” “remifentanil,” “propofol,” “pediatric,” and “flexible bronchoscopy,” revealed no trials to compare the efficacy of Dexmedetomidine-remifentanil (RF) versus dexmedetomidine-propofol for pediatric flexible bronchoscopy.
2. Materials and methods
2.1. Patients
We obtained approval from the institutional review board of Liaocheng People's Hospital to conduct this retrospective trial. Children undergoing flexible bronchoscopy between January 2015 and July 2016 with written informed consent of their parents were enrolled in this study if they met the following inclusion criteria: age between 5 and 10 years and American Society of Anesthesiology (ASA) grade I to II. The exclusion criteria included congenital disease, second- or third-degree heart block, DEX, propofol and/or RF allergy, asthma, neuropsychiatric diseases, operation time longer than 1 hour, pulse oxygen saturation <90% before flexible bronchoscopy, body mass index (BMI) >30 kg m−1, and those children whose parents/legal guardians refused to provide informed consent.
Patients were divided into 2 groups: Group DR (n = 63, DEX infusion at 1.0 μg kg−1 for 10 minutes, then adjusted to 0.5–0.7 μg kg−1 h−1; RF infusion at 1.0 μg kg−1 for 5 minutes, then adjusted to 0.05–0.2 μg kg−1 min−1), Group DP (n = 60, DEX infusion at 1.0 μg kg−1 for 10 minutes, then adjusted to 0.5–0.7 μg kg−1 h−1; propofol infusion at 10 μg kg−1 for 5 minutes, then adjusted to 0.05–0.1 μg kg−1 min−1) DoCare Clinic electronic anesthesia recording system data were utilized during this trial. Flexible bronchoscopy was performed by the same bronchoscopist, who had 10 years of residency experience.
After baseline hemodynamic parameters were obtained, intravenous midazolam 0.03 mg kg−1 and atropine 0.01 mg kg−1 were administered. ASA standard monitoring 5-lead electrocardiography, noninvasive arterial blood pressure, peripheral pulse-oximetry (SpO2), respiratory rate (RR), and temperature (TEM) were continuously monitored using an automated system (Philips IntelliVue MP70) according to the previous studies.[17] All children received oxygen supplementation at 3 L minute−1 through a nasal cannula and forced-air warming device (EQUATOR Convective Warmer, EQ-5000) was used during the procedure to maintain normothermia.
2.2. Flexible bronchoscopy
After loading doses of DEX, RF, or propofol infusion, all steps were carried out in accordance with the operation of our center. Briefly, 2 mL of 1% lidocaine was used to spray in the oral cavity. Three milliliters of 1% lidocaine was delivered through the flexible bronchoscope channel to suppress the cough reflex while visualizing the vocal cords, trachea, and the right and left main bronchi. Flexible bronchoscopy was performed when the Ramsay sedation score reached 3 (children exhibit subject responds to commands). 0.02 mg kg−1 midazolam or 1 mL of 1% lidocaine was administered while the Ramsay sedation score >3. The amount of administered midazolam and lidocaine was recorded. DEX, RF, or propofol infusion was stopped when flexible bronchoscopy was completed. All patients were transferred to the postanesthesia care unit (PACU) after bronchoscopy. On arrival at the PACU, hemodynamic parameters were monitored every 5 minutes for the first 20 minutes, and then every 10 minutes for the rest of the time until the children were discharged (Aldrete Score ≥9).[18] The bronchoscopist's satisfaction was assessed (1, extremely dissatisfied; 2, not satisfied but able to manage; 3, satisfied; 4, extremely satisfied) directly after flexible bronchoscopy.
During the procedure, bradycardia and tachycardia were defined as 20% lesser or greater than the baseline and treated by intravenous atropine 0.2 mg or esmolol 10 mg, respectively. Hypertension and hypotension were defined as 20% greater or lesser than the baseline and treated by urapidil (10 mg) or ephedrine (6 mg), respectively. Hypoxemia was defined as SpO2 <90% for >30 seconds and treated with oxygen supplementation at 6 L minute−1 or verbal and tactile stimulation, chin lifts, jaw thrusts, a face mask, and/or manual ventilation.
2.3. Outcome variables
The intraoperative hemodynamic data (HR, mean arterial pressure (MAP), RR, SpO2, TEM) obtained from Phillips IntelVue monitor were recorded at the following time points: arrival at the operating room (T1); after bolus administration of drug (T2), at the initiation of flexible bronchoscopy (T3); at 1 minute (T4), 5 minutes (T5), 10 minutes (T6), and at the end of bronchoscopy (T7); and at arrival (T8), 5 minutes (T9), and 10 minutes (T10) at the PACU. Anesthesia onset time, total number of intraoperative patient movements, total cumulative dose of DEX, the amount of and time to first dose of rescue midazolam and lidocaine, postoperative recovery time (between withdrawal of flexible bronchoscope and discharge from PACU), adverse events, and bronchoscopist satisfaction scores were recorded.
2.4. Statistical analysis
The Kolmogorov–Smirnov test was used to assess the distribution of variables. Homogeneity of variance was determined using Levene tests. Quantitative data was expressed as mean ± standard deviation or median and interquartile range (IQR). Intergroup comparisons were performed using repeated-measures analysis of variance. Categorical data was expressed as frequency and percentage and analyzed using Chi-squared tests or Fisher exact tests as appropriate. Probability (P) values <0.05 were considered statistically significant. Statistical analysis was performed with SPSS for Windows Version 16.0 (SPSS, Inc., Chicago, IL).
3. Results
3.1. Baseline characteristics
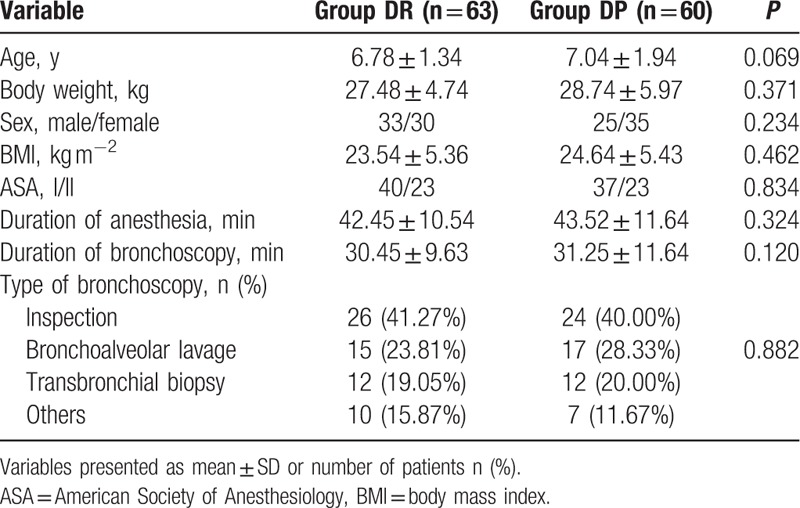
In all, 171 children undergoing flexible bronchoscopy were screened between January 2015 July 2016 (Fig. 1). Of these, 48 children were excluded as they did not meet the inclusion criteria: 2 children had congenital disease, 3 had second-degree heart block, 12 had a history of asthma, 3 had neuropsychiatric diseases, pulse oxygen saturation of 10 children was <90% before flexible bronchoscopy, BMI of 8 children was >30 kg m−1, and the parents of 10 children refused to give informed consent. Finally, 123 children were included in the primary analysis and divided into 3 groups: 63 children in the DR group, 60 children in the DP group. Demographic and baseline clinical parameters were not significantly different among the three groups (P > 0.05, Table 1).
Figure 1.
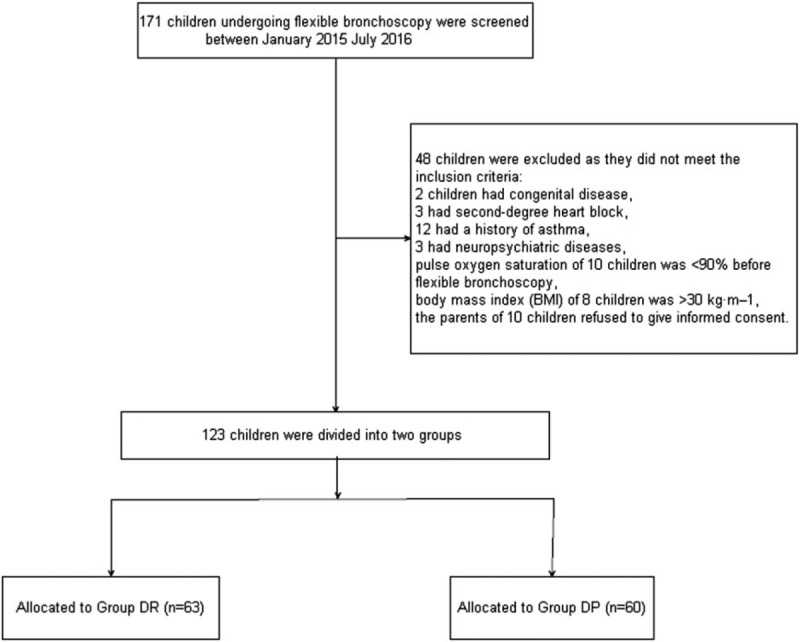
Patient enrolment flow diagram. This illustrates the flow of all patients screened and excluded.
Table 1.
Demographic and baseline clinical parameters between the 2 groups.
3.2. Intraoperative variables
Baseline hemodynamic parameters were not statistically different between the 2 groups (P > 0.05, Fig. 2). Compared with group DR, both heart rate (HR) and MAP in groups DP group were significantly decreased from T2 to T10 (P < 0.05, Fig. 2). Comparing the 2 groups, we found that anesthesia onset time was significantly shorter in DP (8.22 ± 2.48 vs 12.25 ± 6.43 minutes, respectively, for DP, DR, P = 0.015). Total dose of rescue midazolam and lidocaine was significantly higher in DR than in DP (P < 0.001, Table 2). Similarly, the time to first dose of rescue midazolam and lidocaine was significantly longer in DP than in DR (P < 0.001, Table 2). Total cumulative dose of DEX was more in DR than DP group (P < 0.001).
Figure 2.
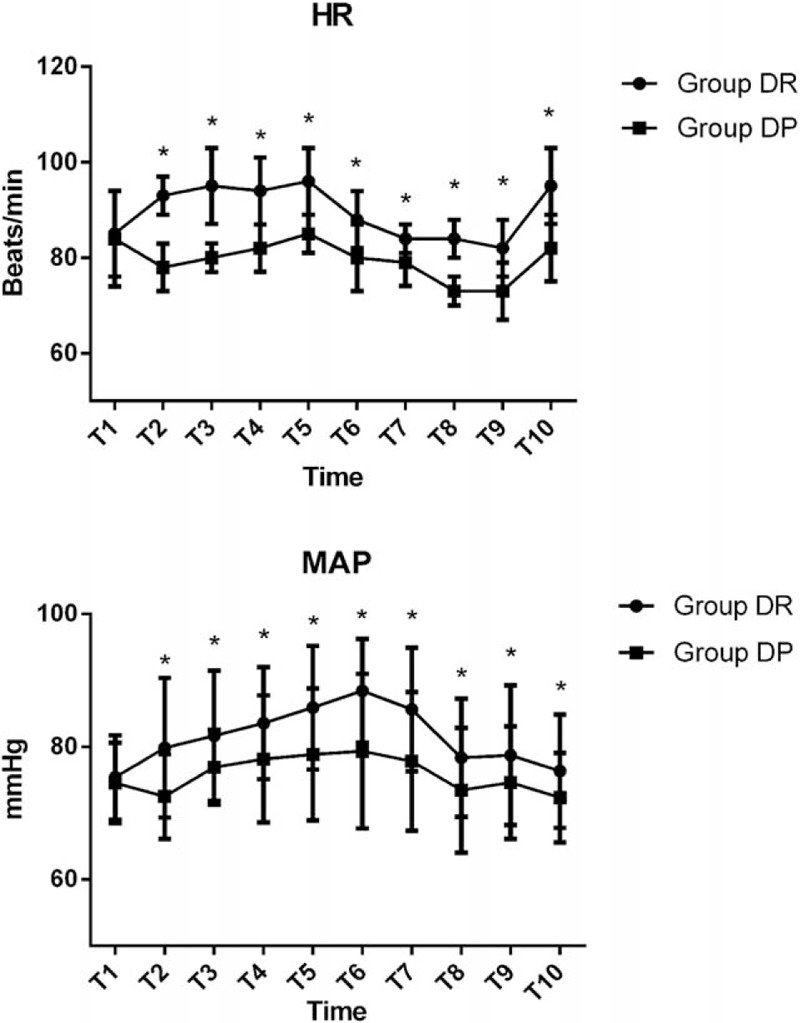
Hemodynamics were monitored in the 2 groups. T1, arrival at the operating room; T2, after bolus administration of drug; T3, at the initiation of flexible bronchoscopy; T4, 1 minute after initiation of bronchoscopy; T5, 5 minutes after initiation of bronchoscopy; T6, 10 minutes after initiation of bronchoscopy; T7, at the end of bronchoscopy; T8, arrival at PACU; T9, 5 minutes after arriving at PACU; T10, 10 minutes after arriving at PACU. ∗P < 0.05 versus Group DP.
Table 2.
Comparison of intraoperative variables between the 2 groups.
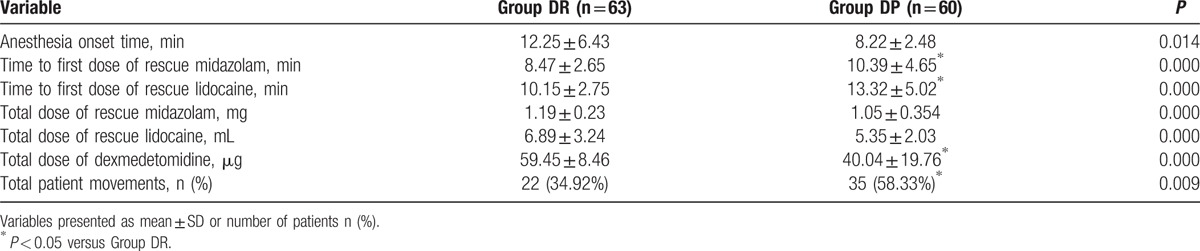
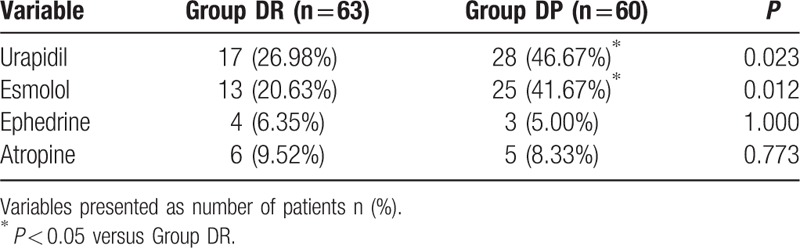
More children moved during flexible bronchoscopy in DP group (P = 0.009, Table 2). Although most of the patient movements could be controlled with the rescue drugs (midazolam or lidocaine), there were still 10 children in the DP group, 4 children in the DR group required alternative sedation to complete the flexible bronchoscopy. There were significant differences between the 2 groups in terms of the overall incidence of hypertension, tachycardia, and hypoxemia (P < 0.05, Table 3). More children in the DP group need urapidil and esmolol during the flexible bronchoscopy (P < 0.05, Table 4).
Table 3.
Adverse events of patients between the 2 groups.
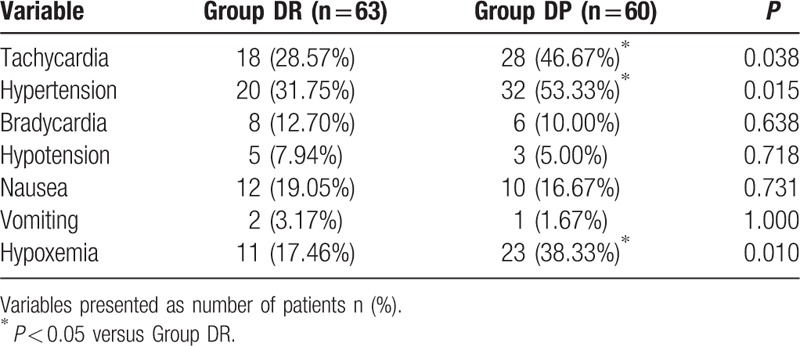
Table 4.
The vascular active drugs of 2 groups during bronchoscopy.
3.3. Postoperative variables
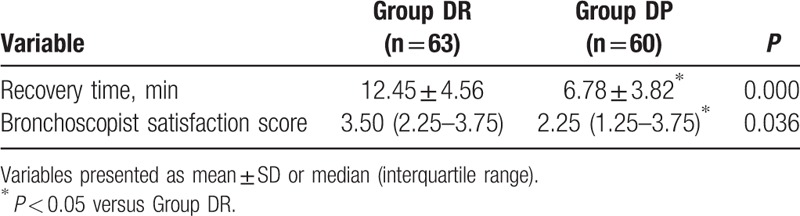
The time to recovery for discharge from the PACU was significantly shorter in DP than in DR group (P < 0.001). The bronchoscopist-satisfaction scores were higher for DR than DP (P = 0.036, Table 5).
Table 5.
Comparison of postoperative variables in the 3 groups.
4. Discussion
DEX combined with RF (i.e., DEX infusion at 1.0 μg kg−1 for 10 minutes, then adjusted to 0.5–0.7 μg kg−1 h−1; RF infusion at 1.0 μg kg−1 for 5 minutes, then adjusted to 0.05–0.2 μg kg−1 min−1) resulted in more stable hemodynamic profiles, bronchoscopist-satisfaction scores and lesser patient movements. However, compared with the DP group, the underwent longer recovery time was longer and the incidence of rescue scheme was higher in the DR group.
Flexible bronchoscopy is usually used for the diagnosis and treatment of respiratory diseases by respiratory physicians and pediatricians.[19] Though general anesthesia is the gold-standard technique for most bronchoscopies, especially in complex procedures of flexible bronchoscopy, MAC has recently been used in simple flexible bronchoscopy. It cannot only provide excellent operating conditions for the bronchoscopist but also accelerate children rapid recovery.[16,20] Previously, benzodiazepines are one of the most commonly used drugs during flexible bronchoscopy. They play the role of muscle relaxant besides sedative, hypnotic and anxiolytic though the γ-aminobutyric acid receptor. Midazolam, because of short elimination half-life, is the first-line agent among benzodiazepines. However, the respiratory depression of midazolam varies, especially in those with comorbidities or those on other concurrent respiratory depressant drugs.[21,22] Propofol has been widely used in gastrointestinal endoscopy, bronchoscopy, awake bronchoscopy intubation, interventional, or radiological procedures for its unique characteristics of pharmacology. However, because of the narrow therapeutic index of sedation and lack of analgesia, propofol is now allowed for use only by anesthesiologists with close monitoring.[23,24] Ketamine, as a bronchodilator and analgesic drug, has been increasingly used in pediatric flexible bronchoscopy. However, the increase of salivation and secretions limited the extensive application.[25,26] RF is the most frequently used opioids during bronchoscopy for its analgesic properties and short immediate half-life, however, its sedative effect is too weak to last through the procedure. Besides, rapid infusion may also lead to stiffness of chest wall and respiratory depression.[27,28]
Previous studies have reported that RF compared with propofol can be used for pediatric flexible bronchoscopy, however, the incidence of hypoxia during the procedure is still high.[12] DEX, has sedative, anxiolytic, and analgesic properties, could reduce secretions while without respiratory depression even at higher doses (1 μg kg−1 h−1).[29] The infusion of DEX is usually recommended by manufacturers as following, 1 μg kg−1 bolus for 10 minutes, infusion at the rate of 0.2–0.7 μg kg−1 h−1 during surgery, and RF infusion of 1 μg kg−1 bolus for 10 minutes, followed by a maintenance infusion at the rate of 0.1–0.2 μg kg−1 h−1. However, the pharmacokinetic age-dependent variability of DEX and RF may cause children to need larger initial doses than adults to reach similar steady-state plasma levels, as larger apparent volume of distribution in children, while the maintenance doses are similar.[30,31] Therefore, in this trial, we adopted DEX infusion at 1.0 μg kg−1 for 10 minutes, then adjusted to 0.5–0.7 μg kg−1 h−1; RF infusion at 1.0 μg kg−1 for 5 minutes, then adjusted to 0.05–0.2 μg kg−1 min−1. In our trial, the incidence of total patient movements among the 2 groups was 34.92% versus 58.33%, respectively, for DR and DP group, which is higher than that in previously reported studies.[32] The reason may be mainly because of the different combinations of drugs used in these studies.
Comparing the 2 groups, we found that anesthesia onset time was significantly shorter in Group DP. There was no difference between the 2 groups in the total dose of rescue lidocaine and midazolam, however, the time to first dose of rescue both lidocaine and midazolam were shorter in the DR group. The reason may be due to the synergistic sedative effect of DEX and propofol. At the same time, the incidence of tachycardia and hypertension was lower in the DR group as a result of better hemodynamic stability and synergistic sedative mechanism of DEX-RF than DEX-propofol. Although previous study have reported that DEX can provide dose-dependent hypotension and bradycardia during to reduction in the plasma levels of norepinephrine and epinephrine.[33] We did not observe any differences between the 2 groups. Because of the less consumption of DEX, the time to recovery for discharge from the PACU was significantly shorter in DP group. The bronchoscopist's satisfaction scores were significantly higher in DR group than in DP group, which may be because of fewer intraoperative patient movements in DR group.
There are several limitations in our study. First, this study is a retrospective trial; a prospective randomized control trial is necessary to verify the feasibility of different dosage of DEX-RF in children undergoing flexible bronchoscopy. Second, we did not measure the serum concentrations of DEX, RF, or propofol in this study because of a short operation time and technical limitations. Finally, we did not perform transcutaneous capnography, which may be more accurate to assess the respiratory state of children undergoing flexible bronchoscopy.[34]
In summary, our study suggests that DEX-RF resulted in more stable hemodynamic profiles and bronchoscopist-satisfaction scores, lesser patient movements than dexmedetomidine-propofol though underwent longer recovery time and more incidence of rescue scheme. It can be more effectively used in children undergoing flexible bronchoscopy.
Footnotes
Abbreviations: ASA = American Society of Anesthesiology, BMI = body mass index, DEX = dexmedetomidine, HR = heart rate, IQR = interquartile range, MAC = monitored anesthesia care, MAP = mean arterial pressure, PACU = postanesthesia care unit, RF = remifentanil, RR = respiratory rate, SpO2 = pulse-oximetry, TEM = temperature.
Author contributions: HZ, BF, and WZ conceived and designed the trial; BF analyzed the data; HZ, WZ collected the data, HZ, BF, and WZ wrote this paper.
The authors have no conflicts of interest to disclose.
References
- [1].Gollu G, Ates U, Can OS, et al. Percutaneous tracheostomy by Griggs technique under rigid bronchoscopic guidance is safe and feasible in children. J Pediatr Surg 2016;51:1635–9. [DOI] [PubMed] [Google Scholar]
- [2].Hammer J, Trachsel D, Nicolai T, et al. Caution to use bronchoscopic CO2 cryotherapy for foreign body removal in children. Pediatr Pulmonol 2016;51:889–91. [DOI] [PubMed] [Google Scholar]
- [3].Goussard P, Gie RP. The need for bronchoscopic services for children in low and middle-income countries. Expert Rev Respir Med 2016;10:477–9. [DOI] [PubMed] [Google Scholar]
- [4].José RJ, Shaefi S, Navani N. Sedation for flexible bronchoscopy: current and emerging evidence. Eur Respir Rev 2013;22:106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hayama M, Sato S, Shiroyama T, et al. Endoscopic bronchial occlusion with silicone spigots under virtual bronchoscopic navigation. Respirol Case Rep 2016;4:e00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dhooria S, Sehgal IS, Aggarwal AN, et al. Diagnostic yield and safety of cryoprobe transbronchial lung biopsy in diffuse parenchymal lung diseases: systematic review and meta-analysis. Respir Care 2016;61:700–12. [DOI] [PubMed] [Google Scholar]
- [7].Sarkiss M. Anesthesia for bronchoscopy and interventional pulmonology: from moderate sedation to jet ventilation. Curr Opin Pulm Med 2011;17:274–8. [DOI] [PubMed] [Google Scholar]
- [8].Wang W, Feng L, Bai F, et al. The safety and efficacy of dexmedetomidine vs. sufentanil in monitored anesthesia care during burr-hole surgery for chronic subdural hematoma: a retrospective clinical trial. Front Pharmacol 2016;7:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cho S, Han JI, Baik HJ, et al. Monitored anesthesia care for great saphenous vein stripping surgery with target controlled infusion of propofol and remifentanil: a prospective study. Korean J Anesthesiol 2016;69:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee JM, Lee SK, Lee SJ, et al. Comparison of remifentanil with dexmedetomidine for monitored anaesthesia care in elderly patients during vertebroplasty and kyphoplasty. J Int Med Res 2016;44:307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dalar L, Karasulu L, Abul Y, et al. Bronchoscopic treatment in the management of benign tracheal stenosis: choices for simple and complex tracheal stenosis. Ann Thorac Surg 2016;101:1310–7. [DOI] [PubMed] [Google Scholar]
- [12].Chen L, Yu L, Fan Y, et al. A comparison between total intravenous anaesthesia using propofol plus remifentanil and volatile induction/maintenance of anaesthesia using sevoflurane in children undergoing flexible fibreoptic bronchoscopy. Anaesth Intensive Care 2013;41:742–9. [DOI] [PubMed] [Google Scholar]
- [13].Jessen Lundorf L, Korvenius Nedergaard H, Moller AM. Perioperative dexmedetomidine for acute pain after abdominal surgery in adults. Cochrane Database Syst Rev 2016;18:CD010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liao W, Ma G, Su QG, et al. Dexmedetomidine versus midazolam for conscious sedation in postoperative patients undergoing flexible bronchoscopy: a randomized study. J Int Med Res 2012;40:1371–80. [DOI] [PubMed] [Google Scholar]
- [15].Ryu JH, Lee SW, Lee JH, et al. Randomized double-blind study of remifentanil and dexmedetomidine for flexible bronchoscopy. Br J Anaesth 2012;108:503–11. [DOI] [PubMed] [Google Scholar]
- [16].Chadha M, Kulshrestha M, Biyani A. Anaesthesia for bronchoscopy. Indian J Anaesth 2015;59:565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cook-Sather SD, Harris KA, Chiavacci R, et al. A liberalized fasting guideline for formula-fed infants does not increase average gastric fluid volume before elective surgery. Anesth Analg 2003;96:965–9. [DOI] [PubMed] [Google Scholar]
- [18].Ead H. From Aldrete to PADSS: reviewing discharge criteria after ambulatory surgery. J Perianesth Nurs 2006;21:259–67. [DOI] [PubMed] [Google Scholar]
- [19].Bauer TL, Berkheim DB. Bronchoscopy: diagnostic and therapeutic for non-small cell lung cancer. Surg Oncol Clin N Am 2016;25:481–91. [DOI] [PubMed] [Google Scholar]
- [20].Abdelmalak B, Khanna A, Tetzlaff J. Fospropofol, a new sedative anesthetic, and its utility in the perioperative period. Curr Pharm Des 2012;18:6241–52. [DOI] [PubMed] [Google Scholar]
- [21].Pacifici GM. Clinical pharmacology of midazolam in neonates and children: effect of disease—a review. Int J Pediatr 2014;309342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Garnock-Jones KP. Oromucosal midazolam: a review of its use in pediatric patients with prolonged acute convulsive seizures. Paediatr Drugs 2012;14:251–61. [DOI] [PubMed] [Google Scholar]
- [23].Boonmak P, Boonmak S, Laopaiboon M. Deliberate hypotension with propofol under anaesthesia for functional endoscopic sinus surgery (FESS). Cochrane Database Syst Rev 2016;10:CD006623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rex DK. Endoscopist-directed propofol. Gastrointest Endosc Clin N Am 2016;26:485–92. [DOI] [PubMed] [Google Scholar]
- [25].Michelet D, Hilly J, Skhiri A, et al. Opioid-sparing effect of ketamine in children: a meta-analysis and trial sequential analysis of published studies. Paediatr Drugs 2016;18:421–33. [DOI] [PubMed] [Google Scholar]
- [26].Han E, Kwon NJ, Feng LY, et al. Illegal use patterns, side effects, and analytical methods of ketamine. Forensic Sci Int 2016;268:25–34. [DOI] [PubMed] [Google Scholar]
- [27].Yu EH, Tran DH, Lam SW, et al. Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain? Anaesthesia 2016;71:1347–62. [DOI] [PubMed] [Google Scholar]
- [28].Kamata M, Tobias JD. Remifentanil: applications in neonates. J Anesth 2016;30:449–60. [DOI] [PubMed] [Google Scholar]
- [29].Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth 2015;115:171–82. [DOI] [PubMed] [Google Scholar]
- [30].Dewhirst E, Tobias JD, Martin DP. Propofol and remifentanil for rapid sequence intubation in a pediatric patient at risk for aspiration with elevated intracranial pressure. Pediatr Emerg Care 2013;29:1201–3. [DOI] [PubMed] [Google Scholar]
- [31].Fang H, Yang L, Wang X, et al. Clinical efficacy of dexmedetomidine versus propofol in children undergoing magnetic resonance imaging: a meta-analysis. Int J Clin Exp Med 2015;15:11881–9. [PMC free article] [PubMed] [Google Scholar]
- [32].Irwin MG, Wong GT. Remifentanil and opioid-induced cardioprotection. J Cardiothorac Vasc Anesth 2015;29:S23–6. [DOI] [PubMed] [Google Scholar]
- [33].Bajwa S, Kulshrestha A. Dexmedetomidine: an adjuvant making large inroads into clinical practice. Ann Med Health Sci Res 2013;3:475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tobias JD. Transcutaneous carbon dioxide monitoring in infants and children. Paediatr Anaesth 2009;19:434–44. [DOI] [PubMed] [Google Scholar]


