Abstract
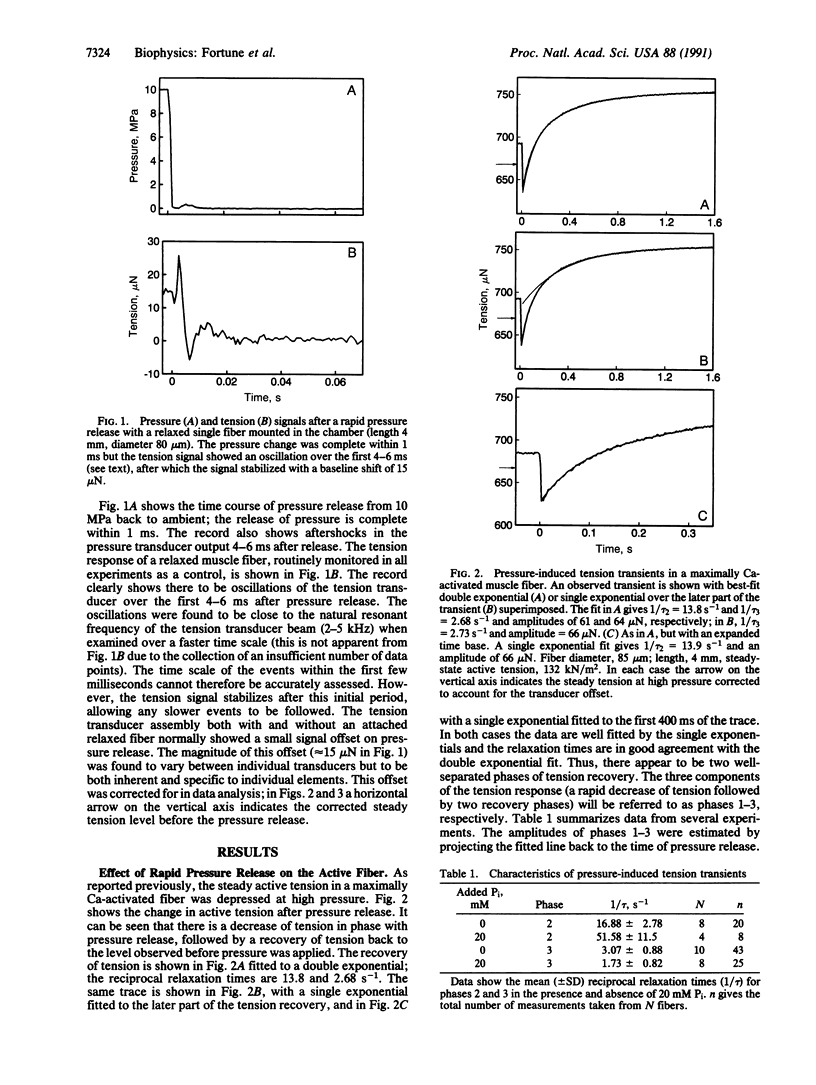
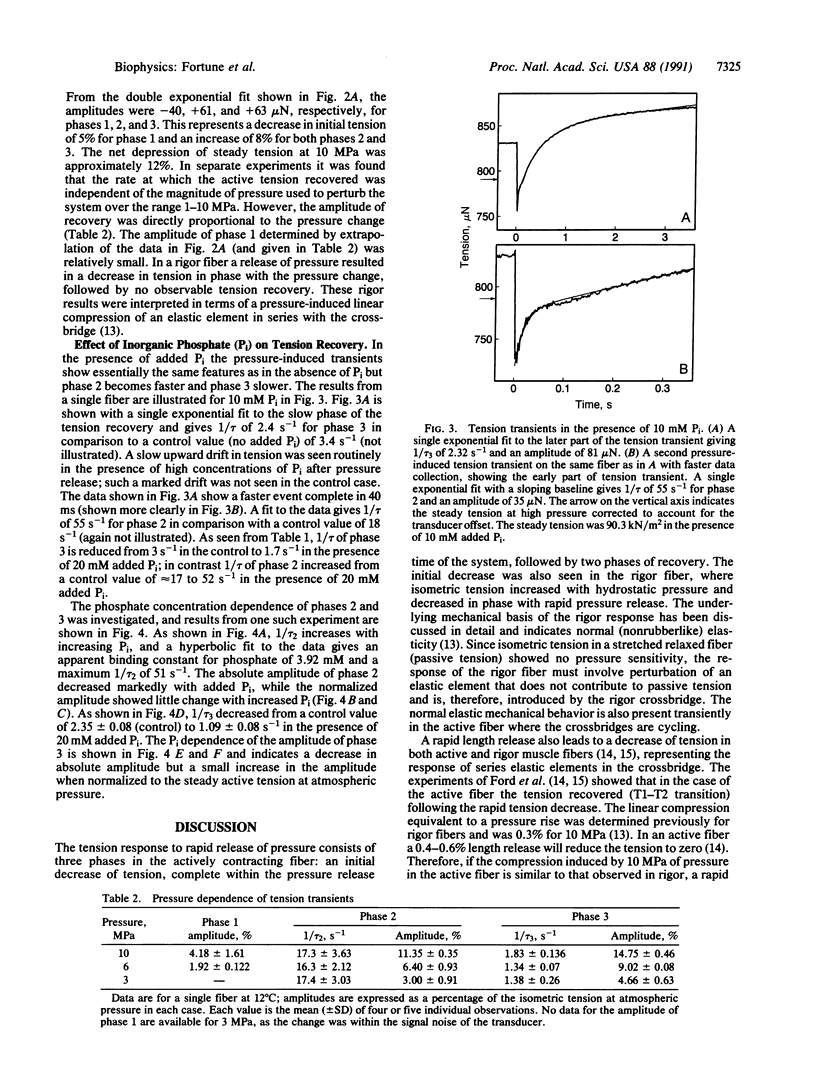
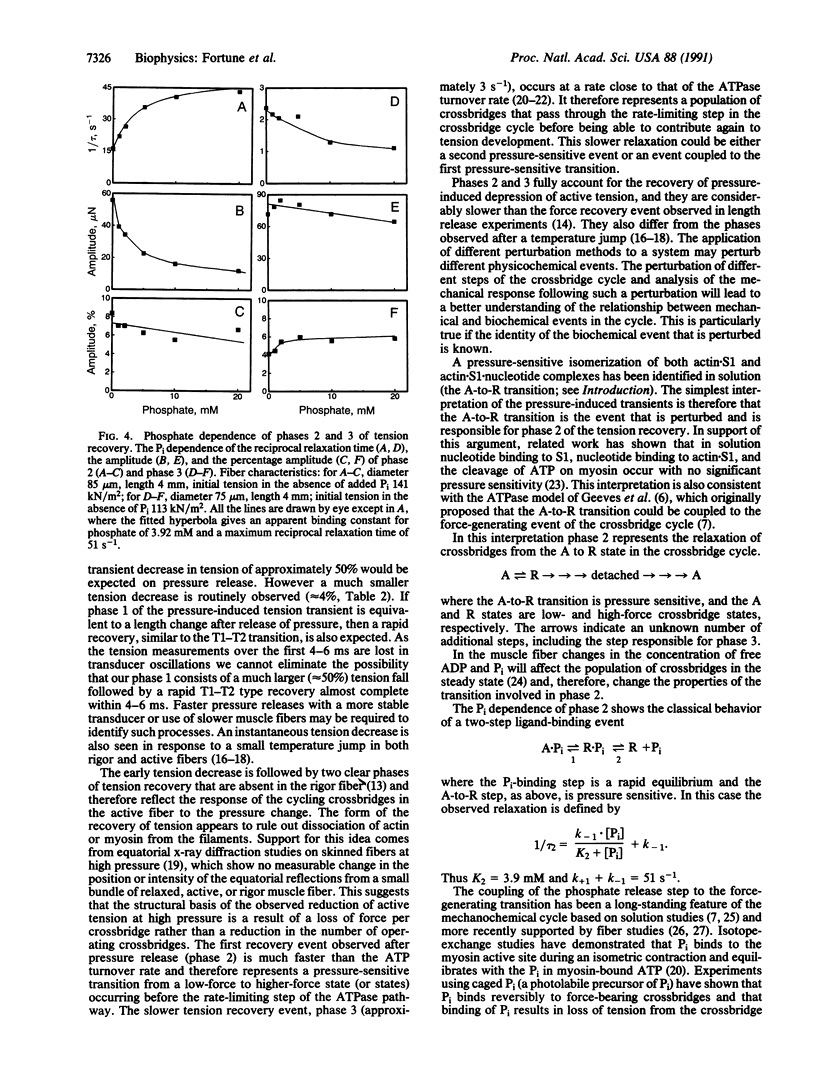
We have previously shown that the isometric tension of a fully calcium-activated skinned rabbit psoas muscle fiber is reversibly depressed by increased hydrostatic pressure. We report here the characterization of tension transients induced by a rapid (less than 1-ms) release of increased pressure at 12 degrees C. The tension transient consists of three clear phases, an initial further decrease of tension in phase with pressure change followed by two phases of tension increase back to the level recorded at ambient pressure. The mean reciprocal relaxation time for phase 2 (1/tau 2) was approximately 17 s-1 and that for phase 3 (1/tau 3) was 3 s-1. The presence of 20 mM inorganic phosphate markedly increased 1/tau 2 to approximately 52 s-1 and decreased 1/tau 3 to approximately 1.7 s-1. These observations are interpreted in terms of a pressure-sensitive transition between two attached crossbridge states of low (or zero) and higher force. This is compatible with the pressure-sensitive isomerization of actomyosin previously observed in solution. The results presented allow us to propose a coupling between a specific pressure-sensitive isomerization of purified actomyosin, the phosphate release step of the ATPase pathway, and the force-generating event of the cross-bridge cycle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bershitsky SYu, Tsaturyan A. K. Effect of joule temperature jump on tension and stiffness of skinned rabbit muscle fibers. Biophys J. 1989 Nov;56(5):809–816. doi: 10.1016/S0006-3495(89)82727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. C., Reese T. S. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys J. 1990 Jul;58(1):1–12. doi: 10.1016/S0006-3495(90)82348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates J. H., Criddle A. H., Geeves M. A. Pressure-relaxation studies of pyrene-labelled actin and myosin subfragment 1 from rabbit skeletal muscle. Evidence for two states of acto-subfragment 1. Biochem J. 1985 Dec 1;232(2):351–356. doi: 10.1042/bj2320351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. S., Harrington W. F. Force generation by muscle fibers in rigor: a laser temperature-jump study. Proc Natl Acad Sci U S A. 1987 Feb;84(4):975–979. doi: 10.1073/pnas.84.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. S. The influence of pressure on the self-assembly of the thick filament from the myosin of vertebrate skeletal muscle. Biochem J. 1981 Aug 1;197(2):301–308. doi: 10.1042/bj1970301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Eccleston J. F., Kanagasabai T. F., Geeves M. A. The kinetic mechanism of the release of nucleotide from elongation factor Tu promoted by elongation factor Ts determined by pressure relaxation studies. J Biol Chem. 1988 Apr 5;263(10):4668–4672. [PubMed] [Google Scholar]
- Eisenberg E., Greene L. E. The relation of muscle biochemistry to muscle physiology. Annu Rev Physiol. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Ferenczi M. A., Homsher E., Trentham D. R. The kinetics of magnesium adenosine triphosphate cleavage in skinned muscle fibres of the rabbit. J Physiol. 1984 Jul;352:575–599. doi: 10.1113/jphysiol.1984.sp015311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune N. S., Geeves M. A., Ranatunga K. W. Pressure sensitivity of active tension in glycerinated rabbit psoas muscle fibres: effects of ADP and phosphate. J Muscle Res Cell Motil. 1989 Apr;10(2):113–123. doi: 10.1007/BF01739967. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Goody R. S., Gutfreund H. Kinetics of acto-S1 interaction as a guide to a model for the crossbridge cycle. J Muscle Res Cell Motil. 1984 Aug;5(4):351–361. doi: 10.1007/BF00818255. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Ranatunga K. W. Tension responses to increased hydrostatic pressure in glycerinated rabbit psoas muscle fibres. Proc R Soc Lond B Biol Sci. 1987 Nov 23;232(1267):217–226. doi: 10.1098/rspb.1987.0070. [DOI] [PubMed] [Google Scholar]
- Geeves M. A. The dynamics of actin and myosin association and the crossbridge model of muscle contraction. Biochem J. 1991 Feb 15;274(Pt 1):1–14. doi: 10.1042/bj2740001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glyn H., Sleep J. Dependence of adenosine triphosphatase activity of rabbit psoas muscle fibres and myofibrils on substrate concentration. J Physiol. 1985 Aug;365:259–276. doi: 10.1113/jphysiol.1985.sp015770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., McCray J. A., Ranatunga K. W. Transient tension changes initiated by laser temperature jumps in rabbit psoas muscle fibres. J Physiol. 1987 Nov;392:71–95. doi: 10.1113/jphysiol.1987.sp016770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. Phosphate release and force generation in skeletal muscle fibers. Science. 1985 Jun 14;228(4705):1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Ikkai T., Ooi T. The effects of pressure on F-G transformation of actin. Biochemistry. 1966 May;5(5):1551–1560. doi: 10.1021/bi00869a015. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. A model of crossbridge action: the effects of ATP, ADP and Pi. J Muscle Res Cell Motil. 1989 Jun;10(3):181–196. doi: 10.1007/BF01739809. [DOI] [PubMed] [Google Scholar]
- Webb M. R., Hibberd M. G., Goldman Y. E., Trentham D. R. Oxygen exchange between Pi in the medium and water during ATP hydrolysis mediated by skinned fibers from rabbit skeletal muscle. Evidence for Pi binding to a force-generating state. J Biol Chem. 1986 Nov 25;261(33):15557–15564. [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]



