Abstract
Background:
The objectives of this study were to investigate the dose of lamotrigine when prescribed with an enzyme inhibitor or enzyme inducer in patients discharged from a mental health trust and to determine the corresponding lamotrigine plasma concentrations and the factors that may affect these.
Methods:
All patients discharged on lamotrigine between October 2007 and September 2012 were identified using the pharmacy dispensing database. We recorded demographic details, lamotrigine dose and plasma levels and coprescribed medication.
Results:
During the designated period, 187 patients were discharged on lamotrigine of whom 117 had their plasma levels recorded. The mean lamotrigine daily dose was 226.1 mg (range 12.5–800 mg) and the mean plasma level 5.9 mg/l (range 0.8–18.1 mg/l). Gender, ethnicity, diagnosis and smoking status had no significant effect on dose or plasma levels. Patients taking an enzyme-inducing drug (n = 6) had significantly lower plasma levels [mean (SD) 3.40 (1.54) mg/l] than those not taking enzyme inducers [n = 111; 6.03 (3.13) mg/l; p = 0.043]. Patients taking an enzyme-inhibiting drug (n = 23) had significantly higher levels [7.47 (3.99) mg/l] than those not taking an inhibitor [n = 94; 5.52 (2.75) mg/l; p = 0.035]. No significant difference was found between the doses of lamotrigine in patients taking an enzyme inhibitor and those not taking one (p = 0.376). No significant difference was found between the doses of lamotrigine in patients taking an enzyme-inducing drug and those not taking any (p = 0.574).
Conclusions:
Current dosing recommendations indicate that lamotrigine doses should be halved in individuals taking enzyme inhibitors and doubled in those on enzyme inducers. In our survey these recommendations were rarely followed with the consequence that patients received too high or too low a dose of lamotrigine, respectively.
Keywords: drug interactions, lamotrigine, therapeutic drug monitoring
Introduction
Lamotrigine is an anticonvulsant which entered the market in 1994 as an add-on treatment for seizure disorders. Currently it is licensed for the adjunctive or monotherapy treatment of various seizure disorders in both adults and children. In adults it is also indicated for the prevention of depressive episodes in bipolar I disorder [GlaxoSmithKline UK, 2016]. Also not uncommonly in mental health, lamotrigine is used off-license to augment clozapine in patients who have refractory schizophrenia and have shown an inadequate response to clozapine monotherapy [Tiihonen et al. 2003]. It is also employed to act as prophylaxis against seizures in susceptible patients when clozapine plasma levels are above 500–600 mg/l [Varma et al. 2011].
Lamotrigine is metabolized in the liver primarily by conjugating with glucuronic acid to form the inactive metabolite 2-N-glucuronide [Rambeck and Wolf, 1993]. Uridine 5′-diphospho-glucuronosyltransferase 1A4 (UGT1A4) and UGT2B7 are the enzymes involved in this reaction [Magdalou et al. 1992; Rowland et al. 2006]. Other drugs and their metabolites that involve this metabolic route may show altered metabolism or affect the metabolism of lamotrigine due to competition for this pathway. As the metabolism of lamotrigine does not depend upon any cytochrome P450 enzymes, the possibility for drug interactions are generally limited [Rambeck and Wolf, 1993]. However, lamotrigine does have some clinically relevant interactions.
Upon starting lamotrigine for any indication, the dose needs to be titrated slowly to a maintenance dose to reduce the risk of developing a serious rash. The maintenance monotherapy dosage of lamotrigine in epilepsy ranges typically between 100 and 200 mg daily. However, when used with an adjunctive drug that inhibits lamotrigine glucuronidation (such as valproate) the titration of lamotrigine needs to be slower and lower maintenance doses of lamotrigine are required. Conversely, when lamotrigine is administered with a drug that can induce its glucuronidation (such as carbamazepine) the initial starting dose of lamotrigine is higher and the final maintenance dose of lamotrigine should also be higher than when an inducer is not administered.
For maintenance treatment of bipolar I disorder 200 mg is the recommended target dose for lamotrigine [GlaxoSmithKline UK, 2016]. Although licensed only for the prophylaxis of bipolar I depression it is also used off-label for the acute treatment of this disorder. The advantage over antidepressants is that lamotrigine does not induce switching or rapid cycling [Taylor et al. 2015]. In practice, it is also used as an adjunct to other mood stabilizers. For clozapine augmentation doses of lamotrigine 25–300 mg have been recommended [Taylor et al. 2015].
A therapeutic plasma concentration target range is not established for lamotrigine. However, a therapeutic range of 2.5–15 mg/l has been suggested in epilepsy [Cohen et al. 1987; Johannessen et al. 2003; Kilpatrick et al. 1996; Lardizabal et al. 2003]. It is believed that levels above 15 mg/l may be associated with toxicity [Morris et al. 1998].
Routine blood monitoring of lamotrigine is not recommended. However, if ineffectiveness, poor adherence or toxicity is suspected blood plasma level monitoring is recommended [National Institute for Health and Care Excellence, 2016]. Likewise, when prescribed with an enzyme inducer or enzyme inhibitor plasma level monitoring is also suggested [GlaxoSmithKline UK, 2016].
The aim of this survey was to determine in a mental health setting to what extent lamotrigine dose is reduced or increased when prescribed with an enzyme inhibitor or enzyme inducer, respectively, and to determine the resulting plasma levels and the factors that may affect these.
Methods
Patients discharged on lamotrigine between October 2007 and September 2012 were identified from the pharmacy electronic dispensing database system (JAC) of the South London and Maudsley NHS Foundation Trust (SLaM). SLaM is an inner city Trust serving a diverse population of 1.2 million people with areas of high unemployment and immigration. The dose of lamotrigine and any co-administered medication were noted. Patient details such as age, diagnosis, smoking status and reasons for taking lamotrigine were obtained from the patient electronic clinical notes (electronic patient journey system). Lamotrigine plasma level and the levels of any other available co-administered medication were obtained from the patient plasma level record system (PROL).
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences SPSS version 22. Normal Q–Q plot was used to assess normality of the outcome variables. For descriptive statistics, categorical variables were presented as number of cases (n) and percentage (%), continuous variables as mean and standard variation. Student’s t test (independent samples) was employed for the comparison of two groups. The analysis of variance (ANOVA) was used for the comparison of three groups or more for the continuous variables. The Pearson correlation test was employed to ascertain the association between the continuous variables. Significant results were shown by a p value < 0.05.
The Drugs and Therapeutics Committee of SLaM confirmed this as a service evaluation so ethics approval was not required. Data were collected by AB. All data were anonymized by PDH before analysis.
Results
The characteristics of study subjects are shown in Table 1. There were 189 patients discharged from SLaM on lamotrigine in the 5-year period from October 2007 and September 2012. Two patients were not included in the study because it was unclear whether lamotrigine was ever actually prescribed. Table 2 lists the ‘other’ diagnoses that were recorded.
Table 1.
Characteristics of the study sample.
| Demographics n = 187 |
|
|---|---|
| Characteristics | N (%) |
| Gender | |
| Male | 122 (65.2) |
| Female | 65 (34.8) |
| Ethnicity | |
| Asian | 8 (4.3) |
| Black | 23 (12.3) |
| White | 156 (83.4) |
| Age, years | |
| Mean | 46.6 |
| Range | 13–90 |
| Standard deviation | 17.4 |
| Smoking status | |
| Smoker | 99 (52.9) |
| Nonsmoker | 49 (26.2) |
| Don’t know | 39 (20.9) |
| Reason for lamotrigine | |
| Bipolar affective disorder/TRD | 52 (27.8) |
| Clozapine augmentation | 40 (21.4) |
| Other | 87 (46.5) |
| TRS AP augmentation | 8 (4.3) |
| Diagnosis | |
| Bipolar affective disorder | 46 (24.6) |
| Schizoaffective disorder | 15 (8.0) |
| Schizophrenia/TRS | 31 (16.6) |
| Other | 95 (50.8) |
| Taking an enzyme inducer | |
| Yes | 10 (5.3) |
| No | 177 (94.7) |
| Taking an enzyme inhibitor | |
| Yes | 27 (14.4) |
| No | 160 (85.6) |
TRD, treatment-resistant depression; TRS AP, treatment-resistant schizophrenia treated with an antipsychotic other than clozapine.
Table 2.
List of ‘other’ diagnoses for patients who were discharged on lamotrigine.
| Other diagnoses n = 95 |
| Anorexia nervosa Asperger’s syndrome Cyclothymia Dementia in Alzheimer’s disease with late onset Emotionally unstable personality disorder Generalised anxiety disorder, agoraphobia, obsessive compulsive disorder Mental retardation, pervasive development disorder Mixed obsessional thoughts and acts Mental and behavioural disorders due to use of cannabinoids/opioids/alcohol Organic personality behavioural disorder Post-traumatic stress disorder Persistent delusional disorder Recurrent depressive disorder with or without psychotic symptoms Somatisation disorder |
The mean lamotrigine daily dose was 226.1 mg (range, 12.5–800 mg). Clozapine augmentation was associated with the largest mean dose of lamotrigine as plotted in Figure 1. Overall, 117 patients had their plasma levels recorded and the mean plasma level was 5.9 mg/l (range 0.8–18.1 mg/l). Table 3 records the mean lamotrigine daily dose and mean plasma levels for the different patient characteristics.
Figure 1.
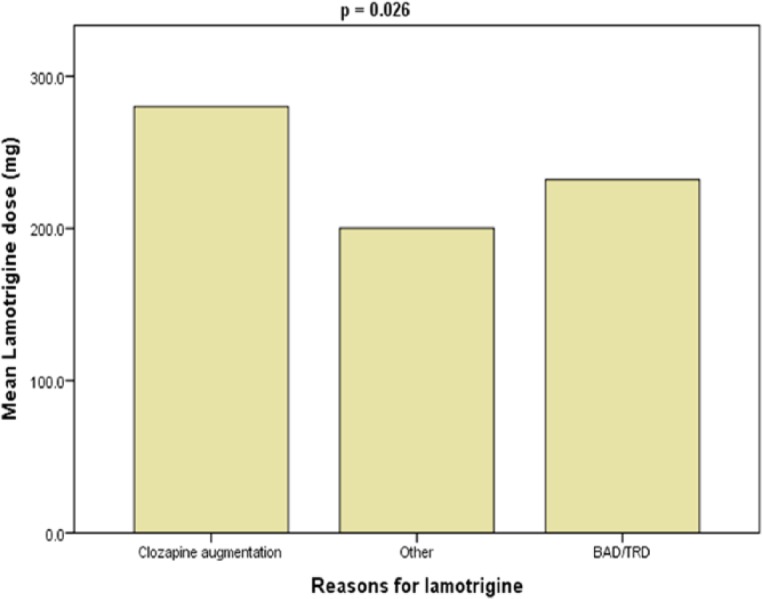
Mean daily dose of lamotrigine for the groups reason for taking lamotrigine.
Table 3.
Mean daily dose and mean plasma level of lamotrigine for different patient characteristics.
| Characteristic | Lamotrigine dose (mg) |
Lamotrigine concentration (mg/l) |
||||
|---|---|---|---|---|---|---|
| Mean (SD) | t test (df) | p value | Mean (SD) | t test (df) | p value | |
| Gender | ||||||
| Female | 225.39 (156.47) | −0.078 (185) | 0.938 | 5.93 (3.34) | 0.151 (115) | 0.093 |
| Male | 227.31 (164.69) | 5.84 (2.62) | ||||
| Ethnicity | ||||||
| Black and Asian | 210.49 (167.43) | −0.596 (185) | 0.552 | 6.83 (2.64) | 1.28 (185) | 0.552 |
| White | 229.15 (157.58) | 5.76 (3.17) | ||||
| Enzyme inducer | ||||||
| Yes | 270.00 (249.39) | 0.583 (9.386)* | 0.574 | 3.40 (1.54) | −2.047 (115) | 0.043 |
| No | 223.57 (152.97) | 6.03 (3.13) | ||||
| Enzyme inhibitor | ||||||
| Yes | 200.93 (138.59) | −0.888 (185) | 0.376 | 7.47 (3.99) | 2.218 (27.337)* | 0.035 |
| No | 230.30 (162.13) | 5.52 (2.75) | ||||
| Smoking status | ||||||
| Yes | 240.51 (170.58) | 1.692 (146) | 0.093 | 5.88 (2.91) | 0.282 (89) | 0.779 |
| No | 192.86 (140.22) | 5.70 (3.01) | ||||
| Diagnosis | ||||||
| Scz/SA | 250.27 (172.41) | 0.746 (2,184)# | 0.476 | 5.95 (2.59) | 2.641 (2,114)# | 0.076 |
| BAD | 212.72 (145.95) | 6.86 (3.97) | ||||
| Other | 226.06 (158.60) | 5.27 (2.71) | ||||
| Reasons for lamotrigine | ||||||
| BAD/TRD | 231.92 (160.18) | 3.708 (2,184)# | 0.026 | 6.57 (3.78) | 1.381 (2,114) | 0.256 |
| Clozapine augmentation | 280.00 (182.68) | 5.75 (2.46) | ||||
| Other | 200.13 (142.41) | 5.47 (2.87) | ||||
Significant results are in bold (p < 0.05).
Welch’s t test; #Analysis of variance (ANOVA) F test.
BAD, bipolar affective disorder; df, degrees of freedom; SA, schizoaffective disorder; Scz, schizophrenia; TRD, treatment-resistant depression.
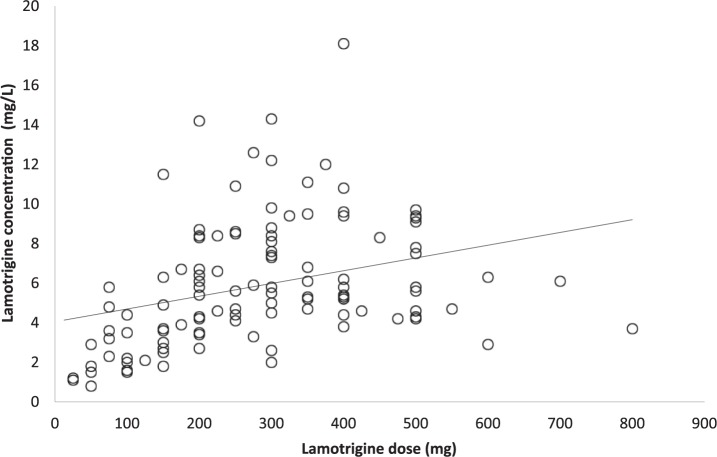
No association was found between patient’s age and dose of lamotrigine (Pearson correlation r = −0.063; p = 0.395; n = 187). Similarly, no association was discovered between age and plasma concentration of lamotrigine (r = −0.087; p = 0.350; n = 117). A weak association was determined between lamotrigine dose and lamotrigine concentration (r = 0.318; p = 0.001; n = 117; see Figure 2).
Figure 2.
Scatterplot for lamotrigine dose and plasma concentration (r = 0.318; p = 0.001).
Discussion
Main findings
The mean daily dose of lamotrigine was found to be 226.1 mg (range 12.5–800 mg) and the mean daily plasma level 5.9 mg/l (range 0.8–18.1 mg/l). A weak association was shown between lamotrigine daily dose and lamotrigine plasma levels. Our main finding was that patients taking an enzyme-inducing drug with lamotrigine had significantly lower lamotrigine plasma levels than those not taking one, despite receiving numerically higher doses. Conversely patients taking an enzyme-inhibiting drug had significantly higher levels than those not taking such drugs despite numerically lower doses (doses did not differ to a statistically significant degree). The reasons for being prescribed lamotrigine were significantly associated with the mean lamotrigine dose, with clozapine augmentation being associated with the highest mean dose. Gender, ethnicity and smoking status were found to have no significant effect on lamotrigine’s dose or plasma levels. This study also highlighted the range of use of lamotrigine in several psychiatric illnesses. In fact, its use in the large majority of cases was off-label; bipolar affective disorder accounted for 24.6%, schizoaffective disorder 8.0%, schizophrenia 16.6% and others 50.8%.
Lamotrigine’s plasma concentrations
The Arbeitsgemeinschaft für Neuropsychop-harmakologie und Pharmakopsychiatrie guidelines [Hiemke et al. 2011] acknowledges that there are no established plasma level target range for lamotrigine’s mood-stabilizing effects. They do, however, recommend therapeutic drug monitoring and suggest a range of 3–14 mg/ml, the same as what is suggested for monitoring lamotrigine’s anticonvulsant effects. For the bulk of our patients who had had their lamotrigine levels measured, their levels fell within this suggested range. However, 17 patients had lamotrigine plasma levels below the suggested therapeutic threshold of 3 mg/l. The implications for having plasma levels this low is unknown.
Our study suggested a weak association between lamotrigine dose and lamotrigine plasma level but clearly the impact of enzyme inducers and inhibitors is important here.
Lamotrigine’s relevant clinical interactions potentially requiring dose adjustments
The interaction between lamotrigine and valproate is widely known. The dosing regimen for lamotrigine is changed substantially when these two drugs are prescribed together. Both drugs share glucuronidation via UGT as a major metabolite pathway and the interaction is thought to result from competitive inhibition of this enzyme group. The magnitude of the interaction was first quantified in a cross-over study of 18 volunteer subjects who received valproate (as divalproex) and three doses of lamotrigine 50, 100 and 150 mg/day [Anderson et al. 1996]. It was shown that when compared with previous studies where subjects received only lamotrigine, the co-administration of valproate increased lamotrigine’s half-life from 26.4 to 69.6 h and the clearance values were decreased from 0.48 to 0.20 ml/min/kg, respectively. The addition of lamotrigine to valproate, however, also resulted in the increased clearance of valproate with a 25% decrease in its plasma concentration. The nature of this bidirectional interaction cannot be explained by competitive inhibition alone. It was later studies by Rowland and coworkers that showed that glucuronidation of lamotrigine was metabolized by both UGT1A4 and UGT2B7 [Rowland et al. 2006]. Valproate is a known substrate of UGT2B7 but was shown not to affect the function of UGT1A4. The authors concluded that valproate decreased lamotrigine’s clearance via inhibition of UGT2B7 and lamotrigine increased valproate’s clearance possibly by induction of UGT1A4.
In practice, the coprescribing of valproate should provoke a halving of the lamotrigine dose. Our study found that there was no significant difference between the doses of lamotrigine in patients taking an enzyme inhibitor and those not taking one. The enzyme-inhibiting group’s mean lamotrigine dose was 200.93 mg daily which indicates that many patients were taking a higher dose than 200 mg daily. The Summary of Product Characteristics (SPC) for lamotrigine recommends a maximum dose of 200 mg daily of lamotrigine when taken with valproate [GlaxoSmithKline UK, 2016]. Valproate was the main inhibitor taken by patients in our study (two patients were taking an enzyme-inhibiting drug other than valproate). Our findings indicate that current guideline recommendations are not being followed. In one case a patient given valproate was also prescribed 400 mg daily of lamotrigine; this patient’s lamotrigine level was 15 mg/l.
When a known inducer of lamotrigine metabolism, such as carbamazepine, phenytoin or hormonal contraceptives is taken with lamotrigine the lamotrigine dose should be doubled [GlaxoSmithKline UK, 2016]. In our survey no significant difference was found between the doses of lamotrigine in patients taking an enzyme-inducing drug and those not taking any. The doses were numerically higher in the enzyme-inducing group but only for one patient was the dose doubled. Not doubling the dose of lamotrigine in these circumstances can be predicted to afford subtherapeutic plasma levels.
We found significantly lower lamotrigine plasma levels in patients taking an enzyme-inducing drug than those not taking an enzyme inducer. Conversely, in patients taking an enzyme-inhibiting drug with lamotrigine we found significantly higher plasma levels of lamotrigine than those not taking an enzyme inhibitor. These findings indicate that therapeutic drug monitoring of lamotrigine would be helpful before and after an enzyme-inducing or an enzyme-inhibiting drug is added to an established dose of lamotrigine. The dose of lamotrigine could then be adjusted accordingly after the enzyme-modifying drug is established. This in fact is what is currently recommended in lamotrigine’s SPC [GlaxoSmithKline UK, 2016].
Clozapine augmentation
In our study clozapine, augmentation was associated with the largest mean dose of lamotrigine. This was unexpected as guidelines recommend lower doses (50–200 mg) of lamotrigine for clozapine augmentation [Taylor et al. 2015]. Lamotrigine has been shown not to affect the plasma levels of clozapine significantly [Spina et al. 2006] and vice versa [Reimers et al. 2005].
Effect of age, ethnicity, sex and smoking status
Age, gender, ethnicity and smoking status were found not to have any significant effect on lamotrigine dose or plasma levels in our study.
Reimers and colleagues [Reimers et al. 2005] found male gender and being 70 or over to be associated with greater lamotrigine plasma concentrations. They state that this significant effect of age was not found in previous studies as low numbers of elderly subjects were included. In our study we had 17 patients aged 70 or over. In their study, they had 70 subjects aged 70 or over. With regards to gender even though a statistically significant effect was found the numerical value was low at 7%. The researchers postulate that this finding maybe coincidental or due to males tending to have a larger weight than females however acknowledging that evidence of weight effects and lamotrigine is lacking. They also suggest hormonal factors may explain this difference and call for more research on the effect of gender and body weight.
Reinsberger and colleagues [Reinsberger et al. 2008] found smoking to have a significant effect on lamotrigine’s plasma levels and propose induction of UGT2B7 by tobacco as a possible mechanism. Smokers had lower levels than nonsmokers. The clinical relevance of this interaction was not elucidated.
Limitations
This study had a number of limitations. No data were collected about clinical efficacy and adverse effects. Smoking status was underreported and not all patients had lamotrigine plasma levels done. The extent to which patients were adherent to all their prescribed medication was unknown. The duration of lamotrigine treatment was not determined; it is known that low lamotrigine levels could be observed in the first few weeks of taking due to lamotrigine auto-induction. This could be confounded with the small doses needed at the start of treatment or co-administration with an enzyme-inducing drug.
Conclusion
Current dosing recommendations in the formal Product Licence indicate that lamotrigine doses should be halved in individuals taking enzyme inhibitors and doubled in those on enzyme inducers. In our survey these recommendations were not always followed with the consequence that patients receive too high or too low a dose of lamotrigine, respectively. Therapeutic drug monitoring of lamotrigine’s plasma concentrations is recommended to assist in optimizing the dosing of lamotrigine particularly in patients taking co-administered enzyme-inducing or enzyme-inhibiting drugs. Prescribers used different doses of lamotrigine for different indications and lamotrigine was used almost entirely for off-label indications.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: DT has received payments for lectures and advisory boards from Eli Lilly, Lundbeck, Bristol Myers Squibb, Astra Zeneca, Sunovion and Otsuka. FG has received payments for lectures and advisory boards from Lundbeck, Roche, Sunovion, Bristol Myers Squibb and Otsuka and has a family member with professional links to Eli Lilly and GlaxoSmithKline. Other authors declare no potential conflicts of interest.
Contributor Information
Petrina Douglas-Hall, Clinical Pharmacist, South London and Maudsley NHS Foundation Trust, Pharmacy Department, Maudsley Hospital, Denmark Hill, London SE5 8AZ, UK.
Olubanke Dzahini, Research Pharmacist, South London and Maudsley NHS Foundation Trust, Pharmacy Department, Maudsley Hospital, London, UK.
Fiona Gaughran, Consultant Psychiatrist, South London and Maudsley NHS Foundation Trust, National Psychosis Service, Maudsley Hospital, London, UK.
Ahmed Bile, Pharmacist, Hounslow East Pharmacy, Middlesex, UK.
David Taylor, Director of Pharmacy and Pathology, Professor of Psychopharmacology, South London and Maudsley NHS Foundation Trust, Pharmacy Department, Maudsley Hospital, London, UK Institute of Pharmaceutical Science, King’s College, London, London, UK.
References
- Anderson G., Yau M., Gidal B., Harris S., Levy R., Lai A., et al. (1996) Bidirectional interaction of valproate and lamotrigine in healthy subjects. Clin Pharmacol Ther 60: 145–156. [DOI] [PubMed] [Google Scholar]
- Cohen A., Land G., Breimer D., Yuen W., Winton C., Peck A. (1987) Lamotrigine, a new anticonvulsant: pharmacokinetics in normal humans. Clin Pharmacol Ther 42: 535–541. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline UK. (2016) Summary of Product Characteristics: Lamictal. Available at: https://www.medicines.org.uk/emc/medicine/4228
- Hiemke C., Baumann P., Bergemann N., Conca A., Dietmaier O., Egberts K., et al. (2011) AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 44: 195–235. [DOI] [PubMed] [Google Scholar]
- Johannessen S., Battino D., Berry D., Bialer M., Kramer G., Tomson T., et al. (2003) Therapeutic drug monitoring of the newer antiepileptic drugs. Ther Drug Monit 25: 347–363. [DOI] [PubMed] [Google Scholar]
- Kilpatrick E., Forrest G., Brodie M. (1996) Concentration-effect and concentration-toxicity relations with lamotrigine: a prospective study. Epilepsia 37: 534–538. [DOI] [PubMed] [Google Scholar]
- Lardizabal D., Morris H., Hovinga C., Del Mar Carreno M. (2003) Tolerability and pharmacokinetics of oral loading with lamotrigine in epilepsy monitoring units. Epilepsia 44:536–539. [DOI] [PubMed] [Google Scholar]
- Magdalou J., Herber R., Bidault R., Siest G. (1992) In vitro N-glucuronidation of a novel antiepileptic drug, lamotrigine, by human liver microsomes. J Pharmacol Exp Ther 260:1166–1173. [PubMed] [Google Scholar]
- Morris R., Black A., Harris A., Batty A., Sallustio B. (1998) Lamotrigine and therapeutic drug monitoring: retrospective survey following the introduction of a routine service. Br J Clin Pharmacol 46: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2016) Bipolar disorder: assessment and management: Clinical Guidance 185 (February 2016. update). Available at: https://www.nice.org.uk/guidance/cg185 [PubMed]
- Rambeck B., Wolf P. (1993) Lamotrigine clinical pharmacokinetics. Clin Pharmacokinet 25: 433–443. [DOI] [PubMed] [Google Scholar]
- Reimers A., Skogvoll E., Sund J., Spigset O. (2005) Drug interactions between lamotrigine and psychoactive drugs: evidence from a therapeutic drug monitoring service. J Clin Psychopharmacol 25: 342–348. [DOI] [PubMed] [Google Scholar]
- Reinsberger C., Dorn T., Kramer G. (2008) Smoking reduces serum levels of lamotrigine. Seizure 17: 651–653. [DOI] [PubMed] [Google Scholar]
- Rowland A., Elliot D., Williams J., Mackenzie P., Dickinson R., Miners J. (2006) In vitro characterization of lamotrigine N2-glucuronidation and the lamotrigine-valproic acid interaction. Drug Metab Dispos 34: 1055–1062. [DOI] [PubMed] [Google Scholar]
- Spina E., D’Arrigo C., Migliardi G., Santoro V., Muscatello M., Mico U., et al. (2006) Effect of adjunctive lamotrigine treatment on the plasma concentrations of clozapine, risperidone and olanzapine in patients with schizophrenia or bipolar disorder. Ther Drug Monit 28: 599–602. [DOI] [PubMed] [Google Scholar]
- Taylor D., Paton C., Kapur S. (2015) Maudsley Prescribing Guidelines in Psychiatry, 12th edn. Oxford: Wiley Blackwell. [Google Scholar]
- Tiihonen J., Hallikainen T., Ryynanen O., Repo-Tiihonen E., Kotilainen I., Eronen M., et al. (2003) Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled crossover trial. Biol Psychiatry 54: 1241–1248. [DOI] [PubMed] [Google Scholar]
- Varma S., Bishara D., Besag F., Taylor D. (2011) Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol 1: 47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]